Summary
Background
There are limited data on the effectiveness of differentiated service delivery (DSD) for HIV care during sociopolitical turmoil. We assessed outcomes with a DSD model of care that includes patient choice between community-based antiretroviral therapy (ART) centres, home-based ART dispensing, or facility-based care at GHESKIO clinic during a period of severe civil unrest in Port-au-Prince, Haiti.
Methods
This retrospective analysis included data on patients with at least one HIV visit at GHESKIO between May 1, 2019, and December 31, 2021. Multivariable logistic regression models were used to assess predictors of attending ≥1 community visit during the study period, and failure to attend timely visits. HIV-1 RNA test results were reported among patients who had been ART for ≥3 months at last visit.
Findings
Of the 18,625 patients included in the analysis, 9659 (51.9%) attended at least one community visit. The proportion of community visits ranged from 0.3% (2019) to 44.1% (2021). Predictors of ≥1 community visit included male sex (aOR: 1.13; 95% CI: 1.06, 1.20), secondary education (aOR: 1.07; 95% CI: 1.01, 1.14), income > $USD 1.00/day (aOR: 1.24; 95% CI: 1.14, 1.35), longer duration on ART (aOR: 1.08 per additional year; 95% CI: 1.07, 1.09), and residence in Carrefour/Gressier (p < 0.0001 in comparisons with all other zones). Younger age and shorter time on ART were associated with late visits and loss to follow-up. Among 12,586 patients with an on-time final visit who had been on ART for ≥3 months, 11,131 (88.4%) received a viral load test and 9639 (86.6%) had HIV-1 RNA < 1000 copies/mL.
Interpretation
The socio-political situation in Haiti has presented extraordinary challenges to the health care system, but retention and viral suppression rates remain high with a model of community-based HIV care. Additional interventions are needed to improve outcomes for younger patients, and those with shorter time on ART.
Funding
No funding.
Keywords: Community-based HIV care, HIV service delivery in civil unrest, Differentiated service delivery models, Health service provision during conflict, Health systems resilience
Research in context.
Evidence before this study
Previous research has demonstrated that community-based care as a form of differentiated service delivery for patients with HIV can offer similar or superior outcomes for patients compared to facility-based care, particularly in low and middle-income countries. We searched PubMed and Google Scholar for articles evaluating community-based care models for the treatment of HIV using search terms “community-based” or “differentiated service delivery” and “HIV” and “antiretroviral therapy” published up to June 2023. These studies suggest that community-based care can improve patient satisfaction while maintaining comparable clinical outcomes to facility-based care.
However, evidence on the role of community-based care in settings of severe socio-political conflict is scarce. The unique challenges posed by unstable political instability, compromised healthcare infrastructure, and disrupted supply chains in conflict zones are likely to impact the implementation and effectiveness of community-based HIV care models. Limited research has been conducted to assess how these models can be adapted to maintain resilience under such circumstances.
Added value of this study
This research addresses a critical gap in our understanding of community-based HIV services in settings of severe socio-political instability. Our findings from Haiti, a country that has faced significant political instability and civil violence since 2019, demonstrate that community-based services can be successfully implemented while maintaining high levels of patient engagement even amidst extreme social unrest. This extends the known applicability of differentiated service delivery models to more challenging and volatile environments.
Implications of all the available evidence
These findings, in addition to existing literature, underscore the value of flexible and resilient healthcare delivery models that focus on improving access for patients, particularly in settings of conflict. The success of community-based services in Haiti suggest potential for replication in similar environments where healthcare systems are disrupted by instability. Moreover, this study contributes to the body of evidence supporting the integration of community-based services into broader HIV care strategies to strengthen health systems resilience and patient engagement.
Introduction
Provision of healthcare in Haiti has been integrally challenged by tumultuous socio-political conflict over the past five years. Indeed, it has been widely reported that Haiti’s healthcare system is near collapse.1, 2, 3 Previous studies in other settings have found that unstructured interruption to HIV services in the context of political conflict is associated with poor health outcomes including antiretroviral therapy (ART) resistance, HIV transmission, and mortality.4,5
Differentiated service delivery (DSD) models of care such as community-based distribution of ART have been implemented in a variety of settings to offer more adaptive and targeted care to meet the needs of the local population. Multiple studies have demonstrated that community-based ART distribution for patients who are stable on treatment can result in high rates of retention and viral suppression, which are comparable or superior to facility-based care.6, 7, 8, 9, 10 Community-based care may also increase patient access to services and reduce facility provider workloads.11, 12, 13, 14 Despite the salient nexus between political conflict and infectious disease, data are limited on the implementation of community-based ART care in situations of severe civil and political unrest.15, 16, 17, 18, 19, 20, 21
To decrease structural barriers to accessing HIV care during an extended period of severe civil unrest in Haiti, we implemented an innovative outreach strategy. Services were offered through a network of community sites and home ART distribution, as well as ongoing facility-based services, at the Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections (GHESKIO) in Port-au-Prince, Haiti. Challenges to healthcare delivery during the study period included prolonged protests resulting in multiple nationwide lockdowns, the onset of the coronavirus disease (COVID-19) pandemic, the assassination of President Jovenel Moise and ensuing unrest, and increasing gang violence throughout the capital city. At each visit, patients chose their site of care. To understand the potential impact of community centres on patient outcomes, we evaluated characteristics associated with the use of community-based care. We also report on rates of retention and viral suppression over a nearly three-year study period, and predictors of being late, lost to follow-up (LTFU), or deceased at the end of the study period.
Methods
Study design, setting, and participants
This retrospective cohort study included persons living with HIV (PLWH) of all ages who attended at least one HIV visit at GHESKIO between May 1, 2019 and December 31, 2021. An HIV visit was defined as a facility or community visit in which a patient was prescribed ART. Community visits included visits attended at a community site or home ART delivery by a community health worker (CHW). At each visit, patients were assessed for symptoms and received a focused physical examination. Adherence was also evaluated, and counselling was provided for patients with suboptimal adherence. Patients with clinical presentations requiring further evaluation were referred to the facility.
GHESKIO is a Haitian non-governmental organisation founded in 1982 that provides clinical care and training and conducts research focusing on infectious and chronic diseases. GHESKIO is the largest provider of HIV services in the Caribbean, and all services are provided free of charge. HIV care in Haiti is largely funded by the US President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. The adult HIV prevalence in Haiti is an estimated 1.7%.22 The majority of Haitians are Afro-Caribbean, and approximately 95% of the population identify as Black.23 Haiti is ranked 163 of 191 countries on the Human Development Index.24
For the past five years, Haiti has endured severe political turmoil, civil unrest and gang-related violence. Over 80% of the capital city of Port-au-Prince is controlled by armed gangs.25 GHESKIO’s central facilities are located adjacent to some of the country’s largest and most impoverished slums, where the gang violence is most intense. This has created major barriers to care-seeking for the majority of GHESKIO patients, who live in gang-controlled areas of the city. In addition to road blockages and violent protests, patients face the risk of kidnapping for ransom when they leave their homes.
Procedures
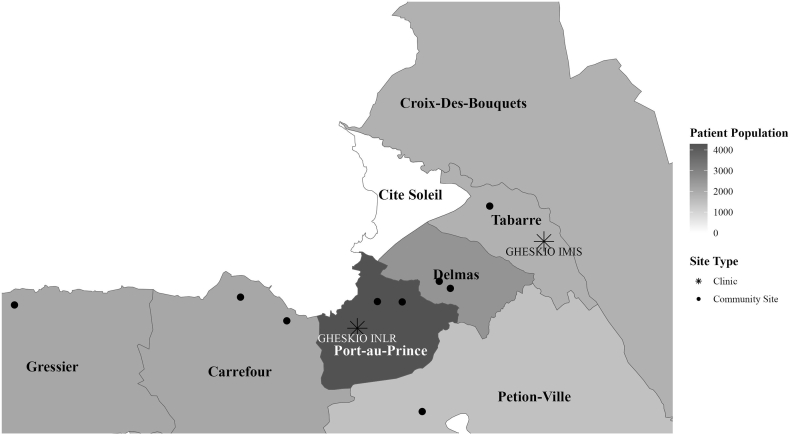
To facilitate access to services, GHESKIO developed a community-centred model of care. Beginning in 2019, a total of nine community centres were set up in the metropolitan Port-au-Prince area. These sites were established in collaboration with associations of PLWH and the Haitian Ministry of Health in areas with high HIV prevalence and/or violent gang activity. The locations of the community sites and two central facilities, Institut National de Laboratoire et Recherche (INLR) and Institut des Maladies Infectieuses et Sante de la Reproduction (IMIS), are plotted on the map in Fig. 1. The labelled zones coincide with five residence zones in the greater metropolitan area included in the analyses (Carrefour/Gressier, Delmas, Pétion-Ville, Croix-des-Bouquets, and downtown Port-au-Prince). We categorised patients living in Gressier with those living in Carrefour because of the low number of patients residing in Gressier (2% of the study population), and because the only major road connecting the two areas with the rest of Port-au-Prince, and the downtown GHESKIO facilities, was persistently blockaded by gangs.
Fig. 1.
Map of residence zones in Port-au-Prince. Note: Patients living in Tabarre were classified as living in Croix-Des-Bouquets in the EMR.
Each community centre was staffed with at least one nurse and one CHW who were trained and employed by GHESKIO. Each community centre was open six days per week, including Saturdays (facility sites were open only on weekdays). Community centres were equipped with basic medical equipment, including thermometers, sphygmomanometers, scales, a small pharmacy, and a computer with internet access and a secure connection to the GHESKIO electronic medical record (EMR). The following services were offered at each centre: checking vital signs; dispensing ART, isoniazid for TB prophylaxis, and co-trimoxazole for the prevention of opportunistic infections (OIs); provision of adherence support; and collection of dried blood spots for viral load testing.
All patients were given the option to attend a facility visit or a community site visit for routine HIV appointments. Community visits took place either at a community site or, for patients with severe challenges in seeking care at either a GHESKIO facility or community site, home delivery of ART was offered. Visit appointments were given for a date, but not a time. Patients were able to present at any site without prior arrangement. They received phone call reminders for each visit prior to their scheduled appointment. If a patient presented to a community site with symptoms of active disease, they were referred to a GHESKIO facility for evaluation. Visits were routinely scheduled every three to six months, depending on duration of ART and clinical status. When feasible, patients were asked to attend one routine evaluation visit per year at one of the GHESKIO facilities. First-line ART included once-daily tenofovir disoproxil fumarate, lamivudine, and dolutegravir (TLD) with multi-month prescriptions, which is standard of care in Haiti.
Outcomes
Study outcomes included attending at least one community-site visit (vs. all facility-based visits) and final status at database closure. The final status was defined as on-time if the patient attended their final visit within 30 days of the scheduled appointment date, late if they attended their final visit from 31 to 365 days after the scheduled appointment date, and LTFU if they were more than 365 days late, and not known to have died. Patients were classified as deceased or transferred based on their known status at the end of the study period.
Final HIV-1 RNA test results were reported among patients who had been on ART for ≥3 months at the last visit. The proportion of patients who received an HIV-1 RNA test in the previous 12 months, and the proportion with <1000 copies/mL were reported. The threshold of 1000 copies/mL was selected in accordance with World Health Organization guidelines, because community sites collected dried blood spots for viral load testing, and this was the lower limit of detection.26 Viral load results were reported by final status (on-time, late, LTFU, deceased, transferred).
Statistical analysis
Demographic, clinical, and laboratory data were extracted from GHESKIO’s EMR. Baseline demographic characteristics were summarized for the overall cohort, for those who attended at least one community visit, and for those who attended only facility visits, using medians and interquartile ranges (IQRs) for continuous variables and counts and percentages for categorical variables.
Bar plots were used to summarise the frequency of HIV visits by month, stratified by location of visit (i.e., facility, community centre, or home ART delivery). Additionally, the total number of monthly visits were normalised to 100 to evaluate the proportion of visits that were late or on-time, and where on-time visits took place. Patients who failed to attend their HIV visit within 30 days of the scheduled date were marked as late for that month.
Univariable and multivariable logistic regression models were used to assess predictors of attending at least one community visit, and of being late, LTFU, or deceased at the end of the study period. All covariates of interest, including age, sex at birth, education, income, civil status, residence area, and time on ART, were included in the regression models. Age and time on ART were continuous variables, residence zone was a categorical variable, and sex, education, income, and status were binary variables. Results are reported as adjusted odds ratios and corresponding 95% confidence intervals (CIs). Due to the high prevalence of patients who attended at least one community visit during the study period, we also modelled this outcome using robust Poisson regression and calculate prevalence ratios with their 95% CIs as sensitivity analyses. We conducted additional sensitivity analyses to assess the impact of varying the cutoff window for late visits (21–365 days and 41–365 days after the scheduled appointment date) and evaluate predictors of the number of days late as a continuous variable using ordinary least squares regression. All analyses were completed in R version 4.3.2.
Ethical considerations
Due to the retrospective study design, it was not feasible to obtain informed consent. This study was reviewed and approved by the institutional review boards of GHESKIO, Weill Cornell Medical College, and Brigham and Women’s Hospital.
Role of funding source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Results
A total of 18,625 patients attended at least one HIV visit between May 1, 2019 and December 31, 2021 and were included in the analysis. Median age was 44 years (IQR: 35, 43), and 10,940 (58.7%) patients were female, 9401 (50.5%) had no schooling or primary education only, 15,046 (80.8%) reported income ≤ $1.00 US dollar (USD) per day, and 8755 (47.0%) were married/cohabitating. The median time on ART at study baseline was 6.6 years (see Table 1). A total of 4150 (22.3%) of patients resided in Carrefour/Gressier, 2551 (13.7%) in Delmas, 1435 (7.7%) in Petion-ville, 3678 (19.8%) in Croix-Des-Bouquets, 4288 (23.0%) in downtown Port-au-Prince, and 2523 (13.6%) outside of the Port-au-Prince metropolitan area (note that the other residence zones are all included in the Port-au-Prince metropolitan area). The neighbourhood zones are plotted on a map in Fig. 1.
Table 1.
Demographic characteristics at study baseline.
| All patients |
Attended ≥1 community visit |
Only attended facility visits |
|
|---|---|---|---|
| (n = 18,625) | (n = 9659) | (n = 8966) | |
| Median age (IQR), years | 44 (35, 53) | 45 (36, 54) | 43 (34, 52) |
| Sex at birth | |||
| Female | 10,940 (58.7%) | 5515 (57.1%) | 5425 (60.5%) |
| Male | 7685 (41.3%) | 4144 (42.9%) | 3541 (39.5%) |
| Education level | |||
| None/Preschool | 3539 (19.0%) | 1777 (18.0%) | 1762 (19.7%) |
| Primary | 5862 (31.5%) | 2963 (30.7%) | 2899 (32.3%) |
| At least some secondary school | 8519 (45.7%) | 4522 (46.8%) | 3997 (44.6%) |
| Data unavailable | 705 (3.8%) | 397 (4.1%) | 308 (3.4%) |
| Income level | |||
| <$1USD/day | 15,046 (80.8%) | 7642 (79.1%) | 7404 (82.6%) |
| ≥$1 USD/day | 2902 (15.6%) | 1627 (16.8%) | 1275 (14.2%) |
| Data unavailable | 677 (3.6%) | 390 (4.0%) | 287 (3.2%) |
| Civil status | |||
| Single | 6051 (32.5%) | 3040 (31.5%) | 3011 (33.6%) |
| Divorced/Separated/Widowed | 3168 (17.0%) | 1652 (17.1%) | 1516 (16.9%) |
| Married/Cohabitating | 8755 (47.0%) | 4593 (47.6%) | 4162 (46.4%) |
| Data unavailable | 651 (3.5%) | 374 (3.9%) | 277 (3.1%) |
| Residence area | |||
| Carrefour/Gressier | 4150 (22.3%) | 2707 (28.0%) | 1443 (16.1%) |
| Delmas | 2551 (13.7%) | 1416 (14.7%) | 1135 (12.7%) |
| Pétion-Ville | 1435 (7.7%) | 773 (8.0%) | 662 (7.4%) |
| Croix-Des-Bouquets | 3678 (19.8%) | 1717 (17.8%) | 1961 (21.9%) |
| Downtown PAP | 4288 (23.0%) | 2252 (23.3%) | 2036 (22.7%) |
| Outside PAP metropolitan area | 2523 (13.6%) | 794 (8.2%) | 1729 (19.3%) |
| Median time on ART (IQR), years | 6.57 (3.5, 10.3) | 7.23 (4.4, 11.2) | 5.62 (2.7, 9.5) |
ART, antiretroviral therapy; IQR, interquartile range; PAP, Port-au-Prince; USD, United States Dollar.
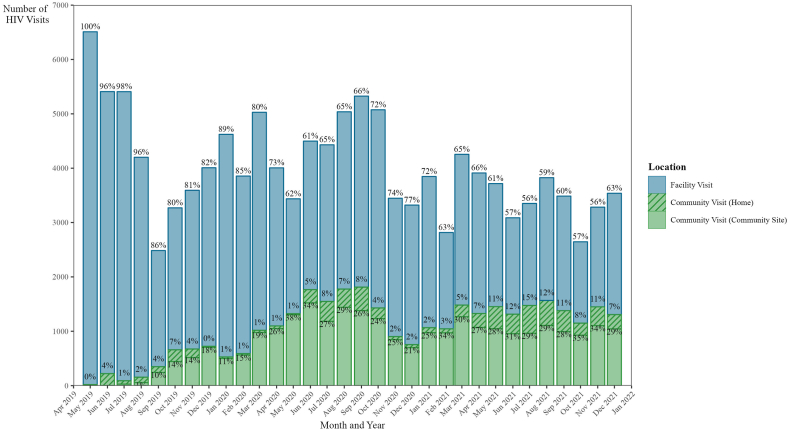
Over the study period, 9659 (51.9%) patients attended at least one community visit (defined as either occurring at a community centre or at home), and 8966 patients (48.1%) attended all their HIV visits at either the GHESKIO-INLR or GHESKIO-IMIS facilities. A total of 128,740 HIV visits were completed during the study period. Clinic visits occurring at the GHESKIO-INLR or GHESKIO-IMIS facilities accounted for 95,295 (74.0%) of the visits, while 27,110 (21.1%) visits occurred at community sites, and 6335 (4.9%) visits occurred at home. The number of monthly HIV visits fluctuated throughout the study period, ranging from 2485 to 6510 visits, with a median of 3851 (IQR: 3395, 4560) visits per month. The proportion of visits which occurred in the community ranged from 0.3% during May 2019, the first month of the community centre rollout, to 44.1% in November 2021 (see Fig. 2). A median of 1123 (IQR: 666, 1451) community-based visits and 2752 (IQR: 2185, 3400) facility-based visits occurred per month over the study period.
Fig. 2.
HIV visits by month and location. Note: Bar heights represent the number of HIV visits attended and data labels represent the proportion of visits per month that occurred either at a facility, community center, or home.
In univariable logistic analyses, predictors of receiving at least one community visit included older age, male sex, higher education, higher income, and longer time on ART. Patients who lived in residence zones other than Carrefour/Gressier were less likely to attend a community visit, compared to those who lived in Carrefour/Gressier (see Table 2). In the multivariable logistic analysis, predictors of receiving at least one community visit included male sex (aOR: 1.13; 95% CI: 1.06, 1.20), receipt of at least some secondary education (aOR: 1.07; 95% CI: 1.01, 1.14), income > $USD 1.00/day (aOR: 1.24; 95% CI: 1.14, 1.35), and longer duration on ART at the start of the study period (aOR: 1.08 per additional year; 95% CI: 1.07, 1.09). Although the prevalence of our dependent variable was high, the adjusted odds ratios were largely similar to prevalence ratios modelled using Poisson regression (Supplementary Table S1).
Table 2.
Predictors of attending at least one community visit.
| Variable | DV: attended ≥1 community visit (n = 9659) |
|||||
|---|---|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
|||||
| OR | 95% CI | p-value | aOR | 95% CI | p-value | |
| Age, decades | 1.08 | (1.05, 1.10) | <0.0001 | 0.99 | (0.96, 1.01) | 0.34 |
| Female sex at birth | 0.87 | (0.82, 0.92) | <0.0001 | 0.89 | (0.84, 0.95) | 0.0002 |
| Secondary education or higher | 1.09 | (1.03, 1.16) | 0.002 | 1.07 | (1.01, 1.14) | 0.023 |
| Income > $1 USD/day | 1.22 | (1.13, 1.32) | <0.0001 | 1.24 | (1.14, 1.35) | <0.0001 |
| Married/cohabitating | 1.05 | (0.99, 1.11) | 0.12 | 0.98 | (0.93, 1.05) | 0.63 |
| Residence area | ||||||
| Carrefour/Gressier | Reference | Reference | ||||
| Delmas | 0.67 | (0.60, 0.74) | <0.0001 | 0.66 | (0.60, 0.74) | <0.0001 |
| Pétion-Ville | 0.62 | (0.55, 0.70) | <0.0001 | 0.62 | (0.54, 0.70) | <0.0001 |
| Croix-Des-Bouquets | 0.47 | (0.43, 0.51) | <0.0001 | 0.48 | (0.43, 0.52) | <0.0001 |
| Downtown PAP | 0.59 | (0.54, 0.64) | <0.0001 | 0.62 | (0.57, 0.68) | <0.0001 |
| Outside PAP metropolitan area | 0.24 | (0.22, 0.27) | <0.0001 | 0.22 | (0.19, 0.24) | <0.0001 |
| Time on ART, years | 1.07 | (1.06, 1.08) | <0.0001 | 1.08 | (1.07, 1.09) | <0.0001 |
| N | 18,625 | 18,625 | ||||
aOR: adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; DV: dependent variable; PAP, Port-au-Prince; USD, United States Dollar.
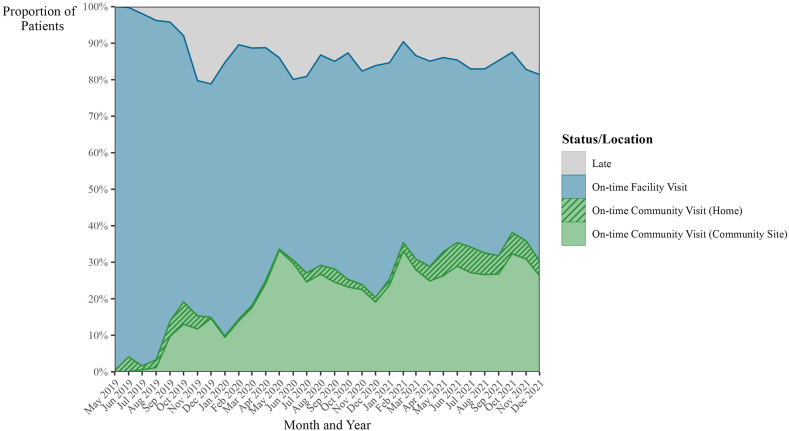
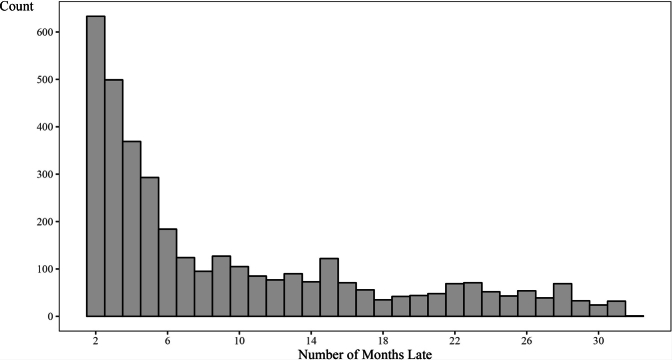
Among the total cohort (n = 18,625), 12,894 (69.2%) patients attended an on-time final visit (within 30 days of the scheduled date), 2223 (11.9%) were late to their final visit (31–365 days after scheduled date), 1499 (8.0%) were transferred, 1436 (7.7%) were LTFU, and 573 (3.1%) were known to have died. Among the 17,126 non-transferred patients, 75.3% had an on-time final visit, 13.0% were late to their final visit, 8.4% were LTFU, and 3.3% died. For patients classified as late, we also evaluated the time elapsed from their last visit, censored by study closure in Supplementary Fig. S1. Among the 2223 patients with late final visit, 1978 (89.0%) were less than 6 months late to their last scheduled visit as of the study closure in December 2021. Among all visits which were attended each month during the study period, a median of 85.4% (IQR: 83.0%, 88.8%) were on-time visits (see Fig. 3).
Fig. 3.
Proportion of on-time visits by month and location. Note: Shading for each month represents the proportion of monthly HIV visits that were completed on-time (within 30 days of the scheduled date), and where the on-time visits took place.
The median duration of follow-up was 27.5 months (IQR: 24.2, 29.4) for patients who were on-time for their final visit, 21.9 months (IQR: 17.8, 24.3) for those with late final visit, 8.7 months (IQR: 0.9, 15.5) for those who died, 7.7 months (IQR: 0.6, 14.0) for those LTFU, and 7.2 months (IQR: 0.6, 15.5) for those who were transferred.
Among the 12,894 patients with an on-time final visit, 12,586 (97.6%) had been on ART for ≥3 months at the date of database closure, and of these, 11,131 (88.4%) patients received a viral load test in the preceding 12 months; 9639 patients (86.6% of those tested), had HIV-1 RNA <1000 copies/mL. Among the 2223 patients who were late to their final visit, 2010 (90.4%) had been on ART ≥3 months, and of these, 1728 (86.0%) received a viral load test in the preceding 12 months, and 1186 (68.6% of those tested) had HIV-1 RNA <1000 copies/mL. Among the 1499 patients who were transferred, 1091 (72.8%) had been on ART ≥3 months, and of these, 921 (84.4%) received a viral load test in the preceding 12 months, and 627 (68.1% of those tested) had HIV-1 RNA <1000 copies/mL. Among the 1436 patients who were LTFU, 1264 (88.0%) had been on ART ≥3 months, and of these, 1096 (86.7%) received a viral load test in the preceding 12 months, and 686 (62.6% of those tested) had HIV-1 RNA <1000 copies/mL. Among the 573 patients who were known to be deceased, 462 (80.6%) had been on ART ≥3 months, and of these, 404 (87.4%) received a viral load test in the preceding 12 months, and 213 (52.7% of those tested) had HIV-1 RNA <1000 copies/mL.
Results of the multivariable analysis evaluating predictors of failure to attend an on-time final visit (late, LTFU, and deceased) among non-transferred patients are presented in Table 3. Younger age (per decade) was associated with late final visit (aOR: 0.91; 95% CI: 0.87, 0.94) and LTFU (aOR: 0.92; 95% CI: 0.88, 0.96), but older age was associated with mortality (aOR: 1.15; 95% CI: 1.07, 1.22). Lower education was associated with mortality (aOR: 0.82 for at least some secondary education; 95% CI: 0.68, 0.97). Married/cohabiting status was associated with late final visit (aOR: 1.22; 95% CI: 1.11, 1.34). Shorter time on ART at the start of the study period was associated with late final visit (aOR [per year on ART]: 0.95; 95% CI: 0.94, 0.96), LTFU (aOR: 0.96; 95% CI: 0.95, 0.97), and mortality (aOR: 0.97; 95% CI: 0.95, 0.99). With the Carrefour/Gressier residence zone as the reference category, living in Delmas was associated with lower risk of LTFU (aOR: 0.79; 95% CI: 0.69, 0.97) and living in downtown Port-au-Prince was associated with higher mortality (aOR: 1.33; 95% CI: 1.06, 1.66). Living outside the Port-au-Prince metropolitan area was associated with late final visit (aOR 1.91; 95% CI: 1.66, 2.20) and LTFU (aOR: 1.64; 95% CI: 1.38, 1.95), but lower mortality (aOR: 0.52; 95% CI: 0.36, 0.74).
Table 3.
Predictors of being late, lost to follow-up, or deceased at study closure.
| Variable | DV: late (n = 2223) |
DV: lost to follow-up (n = 1436) |
DV: deceased (n = 573) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | p-value | aOR | CI | p-value | aOR | 95% CI | p-value | |
| Age, decades | 0.91 | (0.87, 0.94) | <0.0001 | 0.92 | (0.88, 0.96) | 0.0001 | 1.15 | (1.07, 1.22) | <0.0001 |
| Female sex at birth | 1.01 | (0.92, 1.11) | 0.79 | 1.09 | (0.97, 1.20) | 0.14 | 0.85 | (0.72, 1.01) | 0.06 |
| Secondary education or higher | 0.98 | (0.90, 1.08) | 0.74 | 0.90 | (0.80, 1.00) | 0.05 | 0.82 | (0.68, 0.97) | 0.024 |
| Income > $1 USD/day | 0.97 | (0.85, 1.10) | 0.59 | 0.92 | (0.79, 1.08) | 0.30 | 0.80 | (0.62, 1.03) | 0.08 |
| Married/cohabitating | 1.22 | (1.11, 1.34) | <0.0001 | 1.10 | (0.99, 1.24) | 0.09 | 0.81 | (0.68, 0.96) | 0.015 |
| Residence area | |||||||||
| Carrefour/Gressier | Reference | Reference | Reference | ||||||
| Delmas | 0.99 | (0.84, 1.16) | 0.87 | 0.79 | (0.69, 0.97) | 0.022 | 0.77 | (0.57, 1.05) | 0.09 |
| Pétion-Ville | 0.88 | (0.72, 1.07) | 0.20 | 0.81 | (0.64, 1.04) | 0.09 | 0.76 | (0.53, 1.11) | 0.16 |
| Croix-Des-Bouquets | 1.02 | (0.88, 1.17) | 0.82 | 0.84 | (0.71, 1.00) | 0.05 | 0.85 | (0.65, 1.10) | 0.21 |
| Downtown PAP | 0.92 | (0.80, 1.06) | 0.24 | 1.01 | (0.86, 1.19) | 0.86 | 1.33 | (1.06, 1.66) | 0.014 |
| Outside PAP metropolitan area | 1.91 | (1.66, 2.20) | <0.0001 | 1.64 | (1.38, 1.95) | <0.0001 | 0.52 | (0.36, 0.74) | 0.0003 |
| Time on ART, years | 0.95 | (0.94, 0.96) | <0.0001 | 0.96 | (0.95, 0.97) | <0.0001 | 0.97 | (0.95, 0.99) | 0.0005 |
| N | 17,126 | 17,126 | 17,126 | ||||||
aOR: adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; DV: dependent variable; PAP, Port-au-Prince; USD, United States Dollar.
The results of sensitivity analyses in which we varied the cutoffs for late visits and evaluated predictors of the number of days late are reported in Supplementary Table S2. Associations were largely similar across different definitions for late visits, with younger age, married/cohabitating status, living outside of the Port-au-Prince metropolitan area, and shorter time on ART as statistically significant predictors of late status. When modelling the number of days late as a continuous outcome, patients living outside the downtown Port-au-Prince metropolitan area were approximately 28 days later on average compared with patients living in Carrefour/Gressier.
Discussion
The socio-political situation in Haiti has presented extraordinary challenges to the country, with particular stress placed on healthcare providers including GHESKIO. To reduce barriers to accessing care, patients were given the choice of attending community-based or facility-based visits. Over half of the patients in the cohort attended a community-based visit at least once during the study period, and about one-third of HIV visits in the final 12 months of the study period were conducted in a community site or through a home visit. Predictors of attending community-site visits included male sex, higher education, higher income, longer time on ART, and residence in the Carrefour/Gressier residence zone. Despite the challenges in travelling around Port-au-Prince, nearly 90% of non-transferred patients were retained in care for the study period, and a median of 85% of attended visits were on time each month. Viral suppression rates were nearly 90% among patients with on-time final visits.
In order to achieve the 95-95-95 targets of Joint United Nations Programme on HIV/AIDS (UNAIDS) at the global level, it will be essential to provide HIV services in settings of civil and political unrest.27 Our findings demonstrate that community sites may facilitate care in these settings. Community-based HIV services, and in particular community ART dispensing, complement traditional facility-based care by transferring basic services for clinically-stable patients out to community sites, reducing the burden on clinicians and facilitating more convenient and less costly access for patients.7,11,12 A study of stable patients on ART in the Democratic Republic of the Congo found similar results, with 90% retention at 24-months after decentralization to community-based ART refill centres.28 Tukei et al. conducted a cluster-randomized study in Lesotho among stable adults living with HIV and found that patients receiving community care interventions yielded comparable results in retention and viral load suppression compared to patients receiving facility-based care.6
PLWH who live in conflict-affected settings face particular structural barriers to accessing health services, and innovative strategies are necessary to ensure they are able to access ART in a timely manner. Ferreyra et al. described the development of contingency plans for continued HIV care during acute conflict in Central African Republic and Yemen, noting the provision of multi-month ART dispensing and health information cards as specific interventions. In South Sudan, Ferreyra et al. found high rates of acceptance and support for community-based HIV care in a qualitative study conducted in a conflict-affected population.16 Holt has reported on the importance of mobile care services to sustain HIV care during the ongoing war in Ukraine.29 Mekolle et al. and Omam et al. evaluate community-based care models implemented in conflict-affected regions in Cameroon and note their importance in maintaining health systems resilience.15,17
We believe that by expanding access to HIV services and bringing care closer to patients, community-based care strengthened patient autonomy and engagement with healthcare providers, which have been shown to improve retention in care.30,31 This engagement has proven crucial amidst sustained civil conflict in Haiti, which has severely disrupted the ability for Haitians to access basic services. Patients and providers alike face persistent threats of kidnapping and violence by gangs. Many roads throughout the country’s capital have been blockaded, rendering travel between neighbourhoods dangerous or impossible. Shortages of essential resources including food, fuel, and clean water further exacerbate challenges in maintaining healthcare services. This pervasive insecurity has led to the displacement of hundreds of thousands of Haitians, including over half of GHESKIO’s staff in the past three years.32 Despite these challenges, GHESKIO has continued to adapt its package of services to maintain patient care. With GHESKIO’s community-centred model of care, HIV outcomes are similar or superior to many other sites in low and middle-income countries, and to Haiti several years ago, prior to the period of severe civil unrest.33, 34, 35
It is noteworthy that males were more likely than females to attend care at community sites in our study. Other community-based care models have also found beneficial outcomes for community-based care for males. The SEARCH study, which offered streamlined ART delivery, decreased the gap in HIV-related outcomes in males in Uganda and Kenya, though disparities in rates of HIV diagnosis, treatment initiation, and viral suppression remained.36 The DO-ART study found higher rates of viral suppression in a model of community ART delivery vs. clinic-based care in South Africa and Uganda, especially for men; the community intervention offered convenient location, extended hours, and streamlined monitoring and resupply, and viral suppression was similar for men and women.37 The community sites at GHESKIO were open six days per week, which may have accommodated men with inflexible work schedules. The ability to receive care closer to their homes may have also facilitated visit attendance for men. Furthermore, prior studies have found that stigma surrounding clinical environments can deter men from engaging in health services.38 It is possible that men find community sites less stigmatizing than facility-based visits. This is of great importance because we did not find that male sex was associated with poorer outcomes, in contrast to many other studies.39, 40, 41, 42
Younger age and shorter time on ART were associated with late visits and LTFU, as found in other studies.41 These characteristics were also associated with failure to attend a community visit, indicating that other strategies may be necessary to improve outcomes for younger patients, and for those in the early periods of treatment initiation. We found that older age was associated with a higher rate of mortality. This has also been reported in other studies, highlighting the need for more specialised approaches, including diagnosis and treatment of non-communicable diseases, in older patients.43
Escalating gang violence in Haiti caused numerous instances of road blockages, fuel shortages, and nationwide security lockdowns, limiting patient access to healthcare. Patients who lived in Carrefour/Gressier, who were anticipated to have the greatest challenges in travelling to a GHESKIO facility, were more likely to use community services than those living in other residence zones. As the gang-related violence in the Carrefour/Gressier area is among the most severe in the country, with road blockages severely restricting transportation, we believe that the community site which was opened in the area made it possible for many patients to receive timely ART refills. Living in Carrefour/Gressier was not associated with being late for visits, and was only associated with higher rates of LTFU and mortality compared with one other residence zone. We noted that patients living outside of the Port-au-Prince metropolitan area were more likely to be late and LTFU. GHESKIO connects these patients with external healthcare networks closer to their area of residence that can provide more accessible care.
The addition of community sites to facility-based services also facilitated HIV continuity of care during the COVID-19 pandemic, which occurred in two major waves, from March to July 2020, and from November 2020 to March 2021 in Haiti. The option to attend community visits reduced the need for patients to travel to the main GHESKIO facilities, decreasing clinic congestion and waiting times. Community visits also facilitated care for COVID-19, as patients were routinely screened for COVID-19 symptoms at both facility- and community-site visits, and symptomatic patients were offered COVID-19 testing and care.
Our study is limited by being conducted in one HIV treatment centre in an urban setting in Haiti, which may limit the generalisability of our findings to other contexts. Furthermore, because GHESKIO provides comprehensive care with a focus on reaching vulnerable populations, it is possible these results may not be duplicated in other settings. The reliance on patient self-reported demographic data may introduce reporting biases. Data on race and ethnicity are not collected in electronic medical records as nearly all patients GHESKIO provides care for are Black and Afro-Caribbean. Additional variables such as sexual orientation and substance use, which may be correlated with HIV infection and care-seeking behaviours, were not collected at the time of this study. We cannot rule out whether patients at the highest risk of poor outcomes may have transferred to other sites, which may in turn inflate our outcomes for those remaining in care at GHESKIO. Additionally, our mortality data are likely underreported because vital status could not be confirmed among patients who were LTFU. Moreover, there still may be unmeasured confounders in our results such as the intensity of local conflict, which may impact accessibility to services as well as eventual health outcomes.
In conclusion, the socio-political situation in Haiti has presented extraordinary challenges to the country, with particular stress placed on the health care system. Despite these major challenges, retention and viral suppression rates have remained high with GHESKIO’s community-based model of care. Our study findings underscore the potential benefits of community-based care in sustaining HIV health service provision in settings of civil and political unrest.
Contributors
Conceptualization and Methodology, PJ, RS1, HCC, SPK. Formal Analysis, RS1, HCC, and SPK. Data curation: PJ, CG, MAJJ, ND, SV, RS2, KS, PR, FC, WJB, GF, SC, AM, MMD, MLM, JWP. Access to raw data: PJ, RS1, CG, MAJJ, ND, SV, RS2, KS, PR, FC, WJB, GF, SC, AM, MMD, MLM, AD, HCC, JWP, SPK. Data verification: PJ, RS1, AM, AD, HCC, SPK. Writing (original draft): RS1, SPK. Writing (review and editing): PJ, RS1, CG, MAJJ, ND, SV, RS2, KS, PR, FC, WJB, GF, SC, AM, MMD, MLM, AD, HCC, JWP, SPK. Visualization: RS1, HCC. Supervision: PJ, MMD, JWP, SPK. Decision to submit: PJ, RS1, CG, MAJJ, ND, SV, RS2, KS, PR, FC, WJB, GF, SC, AM, MMD, MLM, AD, HCC, JWP, SPK.
Data sharing statement
Anonymized participant data used in this analysis will be made available upon request to the corresponding author.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
We declare no competing interests.
Acknowledgements
PEPFAR and the Global Fund to Fight AIDS, Tuberculosis and Malaria provide funding for HIV service delivery at GHESKIO, but they had no role in the study design, data collection, data analysis, interpretation, or writing of this report. We recognize the contributions of community health workers, the Haitian Ministry of Health, and PLWH associations in Haiti, including Association de la Solidarite Nationale (ASON), Association des Femmes Haïtiennes Infectées et Affectées par le VIH (AFHIAVIH) and Grande Implication des Personnes Affectées et Infectées par le VIH/SIDA (GIPA), for their support in the care provision continuum, including the housing of patients and staff during periods of severe civil unrest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100847.
Appendix A Supplementary data
Supplementary Fig. S1.
Supplementary Fig. S1. Patients Late or Lost as of Study Close. Note: Analysis evaluates time elapsed from last visit, censored by study closure in December 2021.
References
- 1.WFP and UNICEF Executive Directors visit Haiti to galvanize international support amid record humanitarian needs. 2023. [press release] [Google Scholar]
- 2.Das M. Fuel shortage affects cancer care in Haiti. Lancet Oncol. 2021;22(12):1660. doi: 10.1016/S1470-2045(21)00646-X. [DOI] [PubMed] [Google Scholar]
- 3.Taylor L. Women are left vulnerable as Haiti’s spiralling gang violence and healthcare crisis intensifies. BMJ. 2022;378 doi: 10.1136/bmj.o2065. [DOI] [PubMed] [Google Scholar]
- 4.Mann M., Lurie M.N., Kimaiyo S., Kantor R. Effects of political conflict-induced treatment interruptions on HIV drug resistance. AIDS Rev. 2013;15(1):15–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen D.N., Unger H.W., Bjerregaard-Andersen M., et al. Political instability and supply-side barriers undermine the potential for high participation in HIV testing for the prevention of mother-to-child transmission in Guinea-Bissau: a retrospective cross-sectional study. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0199819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tukei B.B., Fatti G., Tiam A., et al. Twelve-month outcomes of community-based differentiated models of multimonth dispensing of ART among stable HIV-infected adults in Lesotho: a cluster-randomized noninferiority trial. JAIDS J Acq Immune Deficiency Syndr. 2020;85(3):280–291. doi: 10.1097/QAI.0000000000002439. [DOI] [PubMed] [Google Scholar]
- 7.Bemelmans M., Baert S., Goemaere E., et al. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health. 2014;19(8):968–977. doi: 10.1111/tmi.12332. [DOI] [PubMed] [Google Scholar]
- 8.Okoboi S., Ding E., Persuad S., et al. Community-based ART distribution system can effectively facilitate long-term program retention and low-rates of death and virologic failure in rural Uganda. AIDS Res Ther. 2015;12(1):37. doi: 10.1186/s12981-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limbada M., Zijlstra G., Macleod D., Ayles H., Fidler S. A systematic review of the effectiveness of non- health facility based care delivery of antiretroviral therapy for people living with HIV in sub-Saharan Africa measured by viral suppression, mortality and retention on ART. BMC Public Health. 2021;21(1):1110. doi: 10.1186/s12889-021-11053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auld A.F., Shiraishi R.W., Couto A., et al. A decade of antiretroviral therapy scale-up in Mozambique: evaluation of outcome trends and new models of service delivery among more than 300,000 patients enrolled during 2004—2013. JAIDS J Acq Immune Deficiency Syndr. 2016;73(2):e11–e22. doi: 10.1097/QAI.0000000000001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis N., Kanagat N., Sharer M., Eagan S., Pearson J., Amanyeiwe U.U. Review of differentiated approaches to antiretroviral therapy distribution. AIDS Care. 2018;30(8):1010–1016. doi: 10.1080/09540121.2018.1441970. [DOI] [PubMed] [Google Scholar]
- 12.Duffy M., Sharer M., Davis N., et al. Differentiated antiretroviral therapy distribution models: enablers and barriers to universal HIV treatment in South Africa, Uganda, and Zimbabwe. J Assoc Nurses AIDS Care. 2019;30(5):e132–e143. doi: 10.1097/JNC.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols B.E., Cele R., Lekodeba N., et al. Economic evaluation of differentiated service delivery models for HIV treatment in Lesotho: costs to providers and patients. J Int AIDS Soc. 2021;24(4) doi: 10.1002/jia2.25692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert H.N., Wyatt M.A., Pisarski E.E., et al. How community ART delivery may improve HIV treatment outcomes: qualitative inquiry into mechanisms of effect in a randomized trial of community-based ART initiation, monitoring and re-supply (DO ART) in South Africa and Uganda. J Int AIDS Soc. 2021;24(10) doi: 10.1002/jia2.25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekolle J.E., Tshimwanga K.E., Ongeh N.J., et al. Political instability and hiv/aids response in the south west and north west regions of Cameroon: a qualitative study. BMC Public Health. 2023;23(1):2155. doi: 10.1186/s12889-023-16994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreyra C., Moreto-Planas L., Wagbo Temessadouno F., et al. Evaluation of a community-based HIV test and start program in a conflict affected rural area of Yambio County, South Sudan. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omam L.A., Jarman E., Ekokobe W., Evon A., Omam E.N. Mobile clinics in conflict-affected communities of North West and South West regions of Cameroon: an alternative option for differentiated delivery service for internally displaced persons during COVID-19. Conflict Health. 2021;15(1):90. doi: 10.1186/s13031-021-00427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreyra C., O’Brien D., Alonso B., Al-Zomour A., Ford N. Provision and continuation of antiretroviral therapy during acute conflict: the experience of MSF in Central African Republic and Yemen. Conflict Health. 2018;12:30. doi: 10.1186/s13031-018-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagozzi B.E. On Malaria and the duration of civil war. J Conflict Resolut. 2016;60(5):813–839. [Google Scholar]
- 20.Koehnlein B., Koren O. COVID-19, state capacity, and political violence by non-state actors. J Peace Res. 2022;59(1):90–104. [Google Scholar]
- 21.Kustra T. HIV/AIDS, life expectancy, and the opportunity cost model of civil war. J Conflict Resolut. 2016;61(10):2130–2157. [Google Scholar]
- 22.UNAIDS, editor. Global data on HIV epidemiology and Response. 2019. AIDSinfo. [Google Scholar]
- 23.Haiti at a glance Washington, DC: embassy of the republic of Haiti. 2023. https://www.haiti.org/haiti-at-a-glance/ Available from:
- 24.Human Development Reports . Program UND. 2022. [Google Scholar]
- 25.Romero S., Paultre A., Dahir A.L. New York Times (Digital Edition); 2023. Can Kenya bring order to Haiti? Doubts are swirling. [Google Scholar]
- 26.Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. [PubMed] [Google Scholar]
- 27.Fast-track: ending the AIDS epidemic by 2030. Joint United Nations Programme on HIV/AIDS; Geneva: 2014. [Google Scholar]
- 28.Vogt F., Kalenga L., Lukela J., et al. Decentralizing ART supply for stable HIV patients to community-based distribution centers: program outcomes from an urban context in Kinshasa, DRC. J Acquir Immune Defic Syndr. 2017;74(3):326–331. doi: 10.1097/QAI.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holt E. Ukraine adapts its HIV response. Lancet HIV. 2022;9(11):e747–e748. doi: 10.1016/S2352-3018(22)00257-0. [DOI] [PubMed] [Google Scholar]
- 30.Gardner E.M., McLees M.P., Steiner J.F., del Rio C., Burman W.J. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinlivan E.B., Messer L.C., Adimora A.A., et al. Experiences with HIV testing, entry, and engagement in care by HIV-infected women of color, and the need for autonomy, competency, and relatedness. AIDS Patient Care STDS. 2013;27(7):408–415. doi: 10.1089/apc.2012.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.As displacement soars, Haiti requires USD 21 million for emergency shelter, protection services geneva/port-au-prince. International Organization for Migration; 2023. https://www.iom.int/news/displacement-soars-haiti-requires-usd-21-million-emergency-shelter-protection-services Available from: [Google Scholar]
- 33.Koenig S.P., Dorvil N., Devieux J.G., et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med. 2017;14(7) doi: 10.1371/journal.pmed.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boender T.S., Sigaloff K.C., McMahon J.H., et al. Long-term virological outcomes of first-line antiretroviral therapy for HIV-1 in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2015;61(9):1453–1461. doi: 10.1093/cid/civ556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jean Louis F., Buteau J., Francois K., et al. Virologic outcome among patients receiving antiretroviral therapy at five hospitals in Haiti. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0192077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hickey M.D., Ayieko J., Kwarisiima D., et al. Improved viral suppression with streamlined care in the SEARCH study. J Acquir Immune Defic Syndr. 2020;85(5):571–578. doi: 10.1097/QAI.0000000000002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnabas R.V., Szpiro A.A., van Rooyen H., et al. Community-based antiretroviral therapy versus standard clinic-based services for HIV in South Africa and Uganda (DO ART): a randomised trial. Lancet Glob Health. 2020;8(10):e1305–e1315. doi: 10.1016/S2214-109X(20)30313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colvin C.J. Strategies for engaging men in HIV services. Lancet HIV. 2019;6(3):e191–e200. doi: 10.1016/S2352-3018(19)30032-3. [DOI] [PubMed] [Google Scholar]
- 39.Koenig S.P., Bernard D., Devieux J.G., et al. Trends in CD4 count testing, retention in pre-ART care, and ART initiation rates over the first decade of expansion of HIV services in Haiti. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNairy M.L., Joseph P., Unterbrink M., et al. Outcomes after antiretroviral therapy during the expansion of HIV services in Haiti. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornell M., Majola M., Johnson L.F., Dubula-Majola V. HIV services in sub-Saharan Africa: the greatest gap is men. Lancet. 2021;397(10290):2130–2132. doi: 10.1016/S0140-6736(21)01163-6. [DOI] [PubMed] [Google Scholar]
- 42.Jarrin I., Geskus R., Bhaskaran K., et al. Gender differences in HIV progression to AIDS and death in industrialized countries: slower disease progression following HIV seroconversion in women. Am J Epidemiol. 2008;168(5):532–540. doi: 10.1093/aje/kwn179. [DOI] [PubMed] [Google Scholar]
- 43.Cornell M., Johnson L.F., Schomaker M., et al. Age in antiretroviral therapy programmes in South Africa: a retrospective, multicentre, observational cohort study. Lancet HIV. 2015;2(9):e368–e375. doi: 10.1016/S2352-3018(15)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.