Abstract
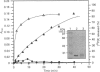
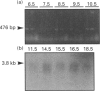
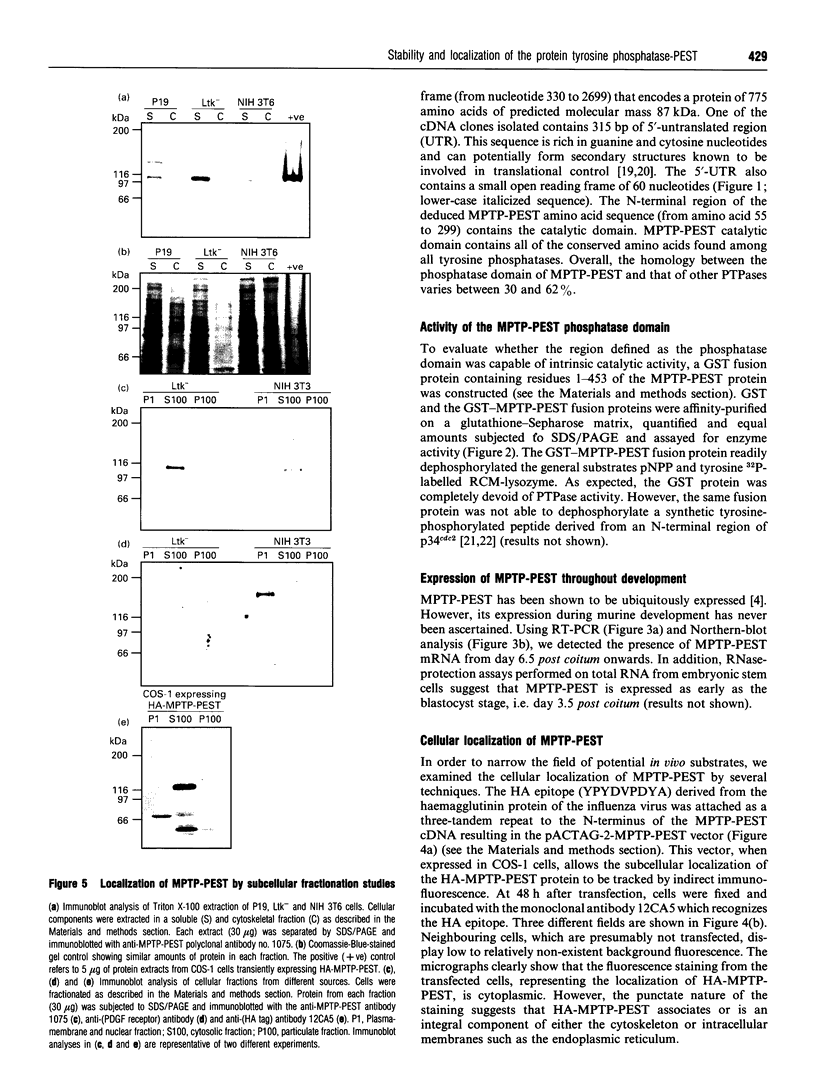
We have isolated the murine cDNA homologue of the human protein tyrosine phosphatase PTP-PEST (MPTP-PEST) from an 18.5-day mouse embryonic kidney library. The cDNA isolated has a single open reading frame predicting a protein of 775 amino acids. When expressed in vitro as a glutathione S-transferase fusion protein, the catalytic domain (residues 1-453) shows intrinsic phosphatase activity. Reverse transcriptase PCR and Northern-blot analysis show that MPTP-PEST mRNA is expressed throughout murine development. Indirect immunofluorescence in COS-1 cells against a heterologous epitope tag attached to the N-terminus of MPTP-PEST, together with cellular fractionation and Western-blot experiments from different murine cell lines, indicate that MPTP-PEST is a free cytosolic protein of 112 kDa. Finally, sequence analysis indicates that the C-terminal portion of the protein contains four regions rich in proline, glutamate, serine and threonine, otherwise known as PEST sequences. These are characteristic of proteins that display very short intracellular half-lives. Despite the presence of these motifs, pulse-chase labelling experiments demonstrate that MPTP-PEST has a half-life of more than 4 h.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulikas T. Putative nuclear localization signals (NLS) in protein transcription factors. J Cell Biochem. 1994 May;55(1):32–58. doi: 10.1002/jcb.240550106. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Litwin C. M., Hwang D. M., Wang J. H. Structural basis of specific and efficient phosphorylation of peptides derived from p34cdc2 by a pp60src-related protein tyrosine kinase. J Biol Chem. 1991 Sep 25;266(27):17919–17925. [PubMed] [Google Scholar]
- Cheng H. C., Nishio H., Hatase O., Ralph S., Wang J. H. A synthetic peptide derived from p34cdc2 is a specific and efficient substrate of src-family tyrosine kinases. J Biol Chem. 1992 May 5;267(13):9248–9256. [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. J., Gebbink M. F., Franza B. R., Jr, Hill D. E., Tonks N. K. Multi-site phosphorylation of the protein tyrosine phosphatase, PTP1B: identification of cell cycle regulated and phorbol ester stimulated sites of phosphorylation. EMBO J. 1993 May;12(5):1937–1946. doi: 10.1002/j.1460-2075.1993.tb05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E., Roy G., Patel D., Shaw A., Thomas M. L. Nuclear localization of the PEP protein tyrosine phosphatase. Mol Cell Biol. 1994 Jul;14(7):4938–4946. doi: 10.1128/mcb.14.7.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni J. V., Beahm P. H., Shifrin V., Jost C. A., Neel B. G. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992 Feb 7;68(3):545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Garton A. J., Tonks N. K. PTP-PEST: a protein tyrosine phosphatase regulated by serine phosphorylation. EMBO J. 1994 Aug 15;13(16):3763–3771. doi: 10.1002/j.1460-2075.1994.tb06687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib T., Herrera R., Decker S. J. Activators of protein kinase C stimulate association of Shc and the PEST tyrosine phosphatase. J Biol Chem. 1994 Oct 14;269(41):25243–25246. [PubMed] [Google Scholar]
- Harford J. B., Klausner R. D. Coordinate post-transcriptional regulation of ferritin and transferrin receptor expression: the role of regulated RNA-protein interaction. Enzyme. 1990;44(1-4):28–41. doi: 10.1159/000468745. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Schneikert J., Athanassiadis A., Moremen K. W. Isolation of a mouse Golgi mannosidase cDNA, a member of a gene family conserved from yeast to mammals. J Biol Chem. 1994 Apr 1;269(13):9864–9871. [PubMed] [Google Scholar]
- Hinnebusch A. G. Novel mechanisms of translational control in Saccharomyces cerevisiae. Trends Genet. 1988 Jun;4(6):169–174. doi: 10.1016/0168-9525(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Williams L. T. An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994 Dec 16;266(5192):1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- Kolodziej P., Young R. A. RNA polymerase II subunit RPB3 is an essential component of the mRNA transcription apparatus. Mol Cell Biol. 1989 Dec;9(12):5387–5394. doi: 10.1128/mcb.9.12.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejbkowicz F., Goyer C., Darveau A., Neron S., Lemieux R., Sonenberg N. A fraction of the mRNA 5' cap-binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9612–9616. doi: 10.1073/pnas.89.20.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P., Pratt G., Rechsteiner M. The C terminus of mouse ornithine decarboxylase confers rapid degradation on dihydrofolate reductase. Support for the pest hypothesis. J Biol Chem. 1991 Jun 15;266(17):11213–11220. [PubMed] [Google Scholar]
- Mathews M. B. Control of translation in adenovirus-infected cells. Enzyme. 1990;44(1-4):250–264. doi: 10.1159/000468763. [DOI] [PubMed] [Google Scholar]
- Mourey R. J., Dixon J. E. Protein tyrosine phosphatases: characterization of extracellular and intracellular domains. Curr Opin Genet Dev. 1994 Feb;4(1):31–39. doi: 10.1016/0959-437x(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Ostergaard H. L., Trowbridge I. S. Negative regulation of CD45 protein tyrosine phosphatase activity by ionomycin in T cells. Science. 1991 Sep 20;253(5026):1423–1425. doi: 10.1126/science.1654595. [DOI] [PubMed] [Google Scholar]
- Pakdel F., Le Goff P., Katzenellenbogen B. S. An assessment of the role of domain F and PEST sequences in estrogen receptor half-life and bioactivity. J Steroid Biochem Mol Biol. 1993 Dec;46(6):663–672. doi: 10.1016/0960-0760(93)90307-i. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Schalling M., Buckler A. J., Rogers A., Haber D. A., Housman D. Expression of the Wilms' tumor gene WT1 in the murine urogenital system. Genes Dev. 1991 Aug;5(8):1345–1356. doi: 10.1101/gad.5.8.1345. [DOI] [PubMed] [Google Scholar]
- Pot D. A., Dixon J. E. A thousand and two protein tyrosine phosphatases. Biochim Biophys Acta. 1992 Jul 22;1136(1):35–43. doi: 10.1016/0167-4889(92)90082-m. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Takekawa M., Itoh F., Hinoda Y., Adachi M., Ariyama T., Inazawa J., Imai K., Yachi A. Chromosomal localization of the protein tyrosine phosphatase G1 gene and characterization of the aberrant transcripts in human colon cancer cells. FEBS Lett. 1994 Feb 21;339(3):222–228. doi: 10.1016/0014-5793(94)80420-6. [DOI] [PubMed] [Google Scholar]
- Takekawa M., Itoh F., Hinoda Y., Arimura Y., Toyota M., Sekiya M., Adachi M., Imai K., Yachi A. Cloning and characterization of a human cDNA encoding a novel putative cytoplasmic protein-tyrosine-phosphatase. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1223–1230. doi: 10.1016/0006-291x(92)92335-u. [DOI] [PubMed] [Google Scholar]
- Tillmann U., Wagner J., Boerboom D., Westphal H., Tremblay M. L. Nuclear localization and cell cycle regulation of a murine protein tyrosine phosphatase. Mol Cell Biol. 1994 May;14(5):3030–3040. doi: 10.1128/mcb.14.5.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W., Lammers R., Huang J., Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993 Mar 12;259(5101):1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- Wilson R., Brophy P. J. Role for the oligodendrocyte cytoskeleton in myelination. J Neurosci Res. 1989 Apr;22(4):439–448. doi: 10.1002/jnr.490220409. [DOI] [PubMed] [Google Scholar]
- Yang Q., Co D., Sommercorn J., Tonks N. K. Cloning and expression of PTP-PEST. A novel, human, nontransmembrane protein tyrosine phosphatase. J Biol Chem. 1993 Mar 25;268(9):6622–6628. [PubMed] [Google Scholar]
- Yi T., Cleveland J. L., Ihle J. N. Identification of novel protein tyrosine phosphatases of hematopoietic cells by polymerase chain reaction amplification. Blood. 1991 Nov 1;78(9):2222–2228. [PubMed] [Google Scholar]
- den Hertog J., Pals C. E., Jonk L. J., Kruijer W. Differential expression of a novel murine non-receptor protein tyrosine phosphatase during differentiation of P19 embryonal carcinoma cells. Biochem Biophys Res Commun. 1992 May 15;184(3):1241–1249. doi: 10.1016/s0006-291x(05)80015-4. [DOI] [PubMed] [Google Scholar]