Highlights
-
•
Prospective data regarding antibiotic prophylaxis in the surgical management of vulvar premalignant lesions is needed.
-
•
Informs power calculations for a definitive randomized trial addressing this question.
-
•
Highlights important potential covariates to consider for future trial design on this topic.
Keywords: Premalignant vulvar lesions, Antibiotic prophylaxis, Wound complications, Surgical excision
Abstract
No prospective data have been described to inform guidelines on antibiotic prophylaxis for partial vulvectomies. Thus, we conducted a single-center, pilot, double-blind randomized controlled trial to assess the effectiveness of prophylactic antibiotics to prevent wound complications after partial vulvectomies. Patients were randomly assigned 1:1 to preoperative antibiotics or no preoperative antibiotics. The primary outcome of 30-day postoperative wound complications occurred in 31 (62 %) of all patients, with no differences between groups. The most common wound complications were superficial separation (54.2 % antibiotic prophylaxis vs. 65.3 % no prophylaxis, p = 0.37) and surgical site infection (0 % antibiotic prophylaxis vs 7.7 % no prophylaxis, p = 0.49). However, this study was limited by differences in patient characteristics between the groups. This study provides data to perform power calculations for a trial examining the effect of preoperative antibiotics on surgical site infection.
1. Introduction
In the year 2000, the incidence of premalignant vulvar lesions in the United States was 2.86 per 100,000, a 411 % increase since 1973 (Judson et al., 2006). Surgical excision is recommended for numerous reasons, including failure of conservative measures or when invasive carcinoma cannot be excluded. Patients undergoing vulvar excision are at high risk for wound complications including surgical site infection and wound separation (ACOG Committee Opinion, 2011). However, given the lack of prospective trials, the American Congress of Obstetrics and Gynecologists offers no guidance regarding antibiotic prophylaxis for patients undergoing wide local excision/partial vulvectomy. As such, there is inconsistent use of antibiotic prophylaxis for these cases, and surgeons may favor no administration. For example, one academic institution reported that, over a 3.5-year period, as few as 9.5 % of patients who underwent partial vulvectomy received preoperative antibiotics (Boyles et al., 2021).
Most studies evaluating vulvar surgery have largely focused on wound complications after radical vulvectomy and inguinal lymphadenectomy to treat vulvar cancer (Gaarenstroom et al., 2003, Wills and Obermair, 2013, Janda et al., 2005). In cancer populations, wound complications are one of the most common causes of postoperative morbidity and result in increased healthcare costs and decreased quality of life (Gaarenstroom et al., 2003, Wills and Obermair, 2013, Janda et al., 2005). However, data from such studies cannot be extrapolated to premalignant lesions given the differences in aggressiveness of surgical technique, including both depth and width of the excision. Determining whether to administer prophylactic antibiotics is important because indiscriminate antibiotic use is inefficient and could lead to development of antibiotic-resistant bacteria. Moreover, 11–15 % of patients have adverse reactions to antibiotics, including skin rashes, diarrhea, and anaphylaxis (Idsoe et al., 1968, Jourdan et al., 2020). To provide evidence-based practice standards for patients undergoing wide local excision/partial vulvectomy for premalignant lesions, we need a large randomized controlled trial. To inform the power calculation of such a trial, we conducted this pilot randomized controlled trial to assess the rates of wound complications – including wound separation, surgical site infection, hematomas, and seromas – in patients undergoing partial vulvectomy with or without prophylactic antibiotics.
2. Methods
This was a single-center, pilot, double-blind, randomized controlled trial evaluating wound complications with and without prophylactic antibiotics in patients undergoing wide local excision/partial vulvectomy for vulvar premalignant lesions. Patients were recruited from outpatient gynecologic oncology offices from July 2018 to October 2021. All surgeries were performed under the supervision of faculty within the Division of Gynecologic Oncology at our institution. Patients were eligible if they were 18–85 years old and undergoing surgical management for a biopsy-proven benign or premalignant vulvar lesion. Patients were excluded if they were pregnant or breast feeding, had prior vulvar radiation, had evidence of active infection at the time of surgery, were undergoing concomitant radical vulvectomy or vulvar graft or flap, were unable to provide informed consent, or were non-English speaking. For this pilot study, a total enrollment of 50 was based on the annual number of wide local excisions/partial vulvectomies performed at our institution. Before initiating the study, all procedures were reviewed and approved by our institution’s Human Research Protection Office (Institutional Review Board, Project #201804136), and the trial was registered on ClinicalTrials.gov (NCT03578965). The full trial protocol is available upon request.
Eligible patients were told about the study and asked to consent at either the preoperative outpatient visit or in the preoperative holding area on the day of surgery. All patients received preoperative counseling by clinical nurse coordinators regarding postoperative vulvar care and hygiene. A computer-generated 1:1 simple randomization scheme was used on the day of surgery. Groups were centrally assigned by the study coordinator after the Principal Investigator confirmed that the patients met inclusion criteria. The study coordinator disclosed the randomization group to the anesthesia team, who screened the patient for allergies and administered antibiotics as described below. The patients, the principal investigator, the surgeons, and all members of the study team involved in data analysis were masked to the randomization group.
Incision sites were clipped only if there was concern about interference of hair with the procedure. All patients were screened for diabetes pre-operatively and were encouraged to complete a pre-operative chlorhexidine shower. Patients randomized to antibiotics received 2 g of intravenous (IV) cefazolin if ≤120 kg or 3 g if >120 kg within one hour of the procedure. In the case of anaphylactic penicillin allergy, 900 mg IV Clindamycin was administered. Pre-operative skin and vaginal prep was done with an alcohol-based agent. The skin and subcutaneous tissues were opened with a scalpel or with Bovie electrocautery on cutting current. At the time of surgery, the surgeon completed an operative data collection sheet describing method of skin closure, suture used, whether the subcutaneous tissue was closed, operation start time, length and depth of incision, whether a drain was placed, and whether the patient had a previous vulvar surgery. Patients were discharged on the day of surgery per standard clinical practice.
Patient demographic and clinical information were collected via chart review. At the two-week postoperative visit, a healthcare provider performed a standardized physical examination of the wound and filled out a data collection form describing primary and secondary outcomes. Also at this visit, the patient completed a survey regarding postoperative vulvar hygiene.
The primary outcome was wound complication rate within 30 days postoperatively. Wound complication was defined as a composite outcome including wound breakdown (wound separation ≥3 mm in depth), surgical site infection (Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN definitions), hematoma, or seroma. All surgical site infections met criteria for superficial infection, as they were defined within 30 (±10) days after surgery and involved only skin and subcutaneous tissue of the incision and at least one of the following: 1) purulent drainage, 2) cellulitis, abscess, or 3) required drainage, debridement, or antibiotics. Outcome measures were obtained from post-operative follow-up forms and chart review of the electronic medical record. Secondary outcomes included adverse events related to antibiotic use and clinical risk factors that correlate with vulvar wound complications, including demographic variables that predispose patients to infection and adherence to recommended vulvar hygiene.
Data were analyzed according to the intention-to-treat principle. Descriptive statistics were used to compare baseline clinical and surgical characteristics between groups and evaluate differences in adverse events. Categorical factors were compared between groups by using the Chi-squared or Fisher’s exact test as appropriate. Independent t-test and Mann-Whitney U test were used to compare normally and non-normally distributed continuous variables, respectively. The primary outcome was compared between groups by using a simple t-test. SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
3. Results
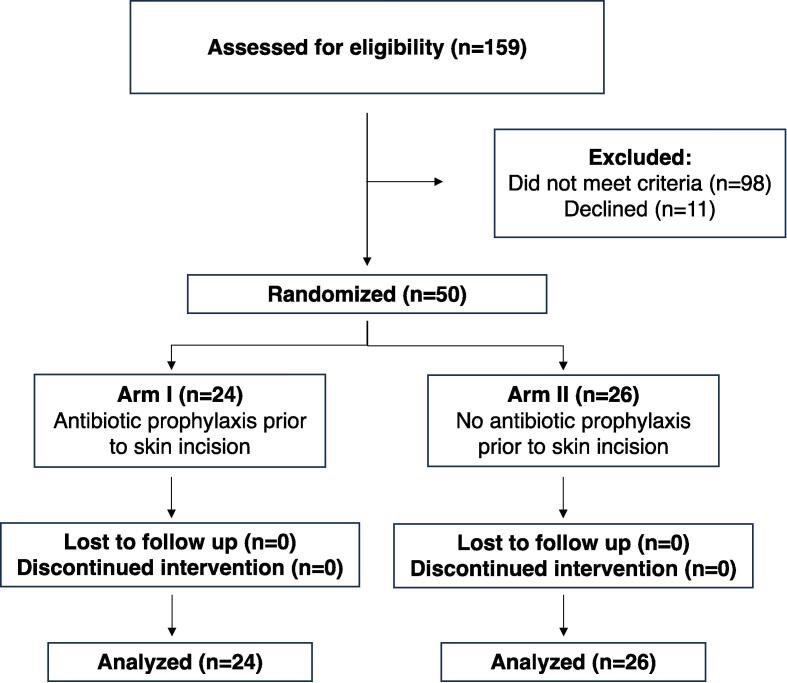
A total of 159 patients were screened, 61 met inclusion criteria and were approached, and 50 consented to the study and were randomized; 24 received prophylactic antibiotics and 26 received no antibiotics. No patients were lost to follow-up after surgery (Fig. 1).
Fig. 1.
Consort Flow Diagram.
Several statistically significant differences were noted between the patients in the two arms. Compared to those who received prophylactic antibiotics, patients who did not receive antibiotic prophylaxis were more likely to be Black; have diabetes mellitus, hypertension, or cardiac valvular disease; and be classified as American Society of Anesthesiology score 3–4 (Table 1). There were no significant differences in surgical characteristics between groups (Supplementary Table 1).
Table 1.
Patient Demographics and Clinical Characteristics (N=50)Ɨ
| Total | Antibiotics (N=24) | No Antibiotics (N=26) | P | |
|---|---|---|---|---|
| Age (years) mean (SD) | 53.7 + 12.7 | 51.1 + 10.3 | 56.1 + 14.4 | 0.17 |
| Body mass index (kg/m2) | 30.5 + 7.4 | 30.1 + 7.0 | 30.9 + 7.9 | 0.73 |
| Race | 0.04 | |||
| American Indian | 1 (2.0%) | 1 (4.2%) | 0 | |
| Black | 11 (22.0%) | 2 (8.3%) | 9 (34.6%) | |
| White | 38 (76.0%) | 21 (87.5%) | 17 (65.4%) | |
| Tobacco Use | 0.59 | |||
| Never | 16 (32.0%) | 7 (29.2%) | 9 (34.6%) | |
| Former | 13 (26.0%) | 5 (20.8%) | 8 (30.8%) | |
| Current | 21 (42.0%) | 12 (50.0%) | 9 (34.6%) | |
| HIV | 1 (2.0%) | 0 | 1 (3.9%) | 1 |
| Immunosuppressive Medications | 1 (2.0%) | 1 | 0 | |
| Steroid Use | 2 (4.0%) | 2 (8.3%) | 0 | 0.22 |
| Diabetes Mellitus | 9 (18.0%) | 1 (4.2%) | 8 (30.8%) | 0.02 |
| Hypertension | 24 (48.0%) | 6 (25.0%) | 18 (69.3%) | 0 |
| Congestive Heart Failure | 1 (2.0%) | 0 | 1 (4%) | 1 |
| Cardiac Valvular Disease | 6 (12.0%) | 0 | 6 (23.1%) | 0.02 |
| Coronary Artery Disease | 5 (10.0%) | 1 (4.2%) | 4 (15.4%) | 0.35 |
| Chronic Kidney Disease | 9 (18.0%) | 3 (12.5%) | 6 (23.1%) | 0.46 |
| Pulmonary Disease | 17 (34.0%) | 5 (20.8%) | 12 (46.2%) | 0.07 |
| ASA score | 0.04 | |||
| 1-2 | 32 (64.0%) | 19 (79.2%) | 13 (50.0%) | |
| 3-4 | 18 (36.0%) | 5 (20.8%) | 13 (50.0%) | |
| Prior Vulvar Surgery | 1.0 | |||
| Yes | 10 (21.3%) | 5 (21.7%) | 5 (20.8%) | |
| No | 37 (78.8%) | 18 (78.3%) | 19 (79.2%) | |
| Previous Treatment for Vulvar Disease | ||||
| Topical Imiquimod (yes) | 4 (8.0%) | 3 (12.5%) | 1 (4.0%) | 0.33 |
| Surgery (yes) | 9 (18.0%) | 4 (16.7%) | 5 (19.2%) | 1.0 |
| Steroids (yes) | 0 | 0 | 0 | |
| Laser Ablation (yes) | 3 (6.0%) | 1 (4.2%) | 2 (7.7%) | 1.0 |
| Pathology | ||||
| VIN1 | 1 (2.0%) | 1 (4.2%) | 0 | 0.48 |
| VIN2 | 5 (10.0%) | 5 (20.8%) | 0 | 0.02 |
| VIN3 | 40 (80.0%) | 18 (75.0%) | 22 (84.6%) | 0.48 |
| Paget’s | 2 (4.0%) | 0 | 2 (7.7%) | 0.49 |
| Lichen Sclerosis | 0 | 0 | 0 | - |
| Melanoma | 0 | 0 | 0 | - |
| HPV (warts) | 0 | 0 | 0 | - |
| Other | 5 (10.0%) | 3 (12.5%) | 2 (7.7%) | 0.66 |
Analyses were performed by Fisher exact test or Chi-square test for categorical variables; and independent t-tests for continuous variables.
ASA, American Society of Anesthesiology; HIV, human immunodeficiency virus; HPV, human papillomavirus; SD, standard deviation; VIN, vulvar intraepithelial neoplasia.
Data are n (%) unless otherwise specified.
Postoperative outcomes are summarized in Table 2. Overall wound complications occurred in 31 (62 %) of the 50 patients, with no difference between groups (54.2 % in antibiotic group vs. 69.2 % in no antibiotic group, p = 0.38). In the antibiotic group, all 13 complications were wound separations; there were zero surgical site infections, seromas, or hematomas. In the no antibiotic group, the most common wound complication was wound separation (65.3 %). However, this group experienced 2 (7.7 %) surgical site infections; both required antibiotics, and one required wound debridement. There were no seromas or hematomas. All wound separations in both groups were superficial (median depth of separation was 7.5 mm in the antibiotic group and 8.9 mm in the no antibiotics group) and healed by secondary intention. There were no grade 2–5 adverse events in either group, including allergic reaction or anaphylactic reaction to antibiotics. Self-reported adherence to postoperative vulvar hygiene practices was >80 % in both groups, with no significant differences in survey responses between groups (Supplementary Table 2).
Table 2.
30-Day Postoperative Outcomes.
| Outcome | TotalƗ |
AntibioticsƗ (N=24) |
No AntibioticsƗ (N=26) |
P |
|---|---|---|---|---|
| Primary composite outcome* | 31 (62 %) | 13 (54.2 %) | 18 (69.2 %) | 0.38 |
| Surgical site infection | 2 (4.0 %) | 0 | 2 (7.7 %) | 0.37 |
| Wound separation | 30 (62.5 %) | 13 (54.2 %) | 17 (65.3 %) | 0.37 |
| Length (mm)** | 20 (10–30) | 20 (12.5–35) | 10 (9.3–30) | 0.83 |
| Width (mm)** | 10 (5–20) | 10 (5–20) | 10 (5–20) | 0.65 |
| Depth (mm)** | 2 (1–5) | 2 (1–4.5) | 3 (0.5–5) | 0.69 |
| Seroma | 0 | 0 | 0 | − |
| Hematoma | 0 | 0 | 0 | − |
| Postoperative antibiotics | 2 (4.2 %) | 0 | 2 (7.7 %) | 0.49 |
| Need for debridement | 1 (2.1 %) | 0 | 1 (3.8 %) | 1.0 |
Analyses were performed by Fisher exact test or Chi-square test for categorical variables; and independent t-tests for continuous variables.
Surgical site breakdown, infection, hematoma, or seroma within 30 days post-procedure.
Data are median and interquartile range.
Data are n (%) unless otherwise specified.
4. Discussion
The data from this pilot randomized controlled trial provides prospectively collected data regarding rates of wound complications in patients undergoing vulvar excision for premalignant lesions. Rates of surgical site infection were low in both groups. No patients in the antibiotic prophylaxis group and only 2 (7.7 %) in the no antibiotics group experienced surgical site infections, although due to the imbalance between the two cohorts, a definitive conclusion cannot be drawn regarding the role of antibiotic prophylaxis in this difference. This study provides data that can be used to design a larger trial powered to definitively assess the effectiveness of prophylactic antibiotics to prevent surgical site infection after vulvar excision for premalignant lesions. The data from this study indicate that 648 patients would need to be enrolled to detect a 5 % decrease (from 7.7 % to 2.7 %) with 80 % power and a two-sided α = 0.05, assuming a 5 % loss to follow-up. A study of this size would require a multi-site design, which would also help ensure racial and ethnic diversity among participants. Additionally, given that these lesions are non-malignant, a future trial could include surgeries performed by both generalist obstetrician/gynecology and gynecologic oncology providers. Including enrollment from both provider groups would likely aid in trial accrual. Finally, as we did here, future trials should provide standardized education material regarding postoperative vulvar care.
A strength of our study is that, to our knowledge, it is the first prospective study to evaluate the use of antibiotics for wide local excision/simple vulvectomy. It thus strengthens the literature describing retrospective cohort studies. Another strength is that we implemented standardized patient counseling on postoperative vulvar care and hygiene to reduce confounding effects. Furthermore, we used data collection forms to prospectively capture postoperative wound complications, minimize reporting bias, and allow for accurate, objective reporting of our clinical outcomes.
A key weakness of our study is that, likely due to the small sample size, the two arms were imbalanced regarding several important covariates including race, diabetes, hypertension, and cardiovascular disease. All of these variables may have had a meaningful impact on differences in wound healing and should be considered in planning stratification in a future randomized controlled trial. Notably, our rate of wound complications (62 %) was much higher than those retrospectively reported by Mullen et al.(Mullen et al., 2019) (29 %) and Boyles et al.(Boyles et al., 2021) (42.3 %). Nevertheless, our surgical site infection rate (4 %) is consistent with the rates reported previously: 6.9 % (Mullen et al.) and 6.5 % (Boyles et al.).
In conclusion, our pilot data can be used to design a multi-site randomized controlled trial to determine whether or not prophylactic antibiotics reduce rates of surgical site infection after vulvar excision for premalignant lesions. Data from such a well-powered and rigorously performed trial could then be used to develop risk-based guidelines.
CRediT authorship contribution statement
Mary M. Mullen: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Whitney R. Grither: Writing – review & editing, Writing – original draft, Data curation. Hannah Millimet: Writing – review & editing, Writing – original draft. David G. Mutch: Writing – review & editing, Resources. Andrea R. Hagemann: Writing – review & editing, Resources. Carolyn K. McCourt: Writing – review & editing, Resources. Matthew A. Powell: Writing – review & editing, Resources. Premal H.Thaker: Writing – review & editing, Resources. Dineo Khabele: Writing – review & editing, Resources. Lindsay M. Kuroki: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Methodology, Investigation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. Debbie Frank for her helpful discussion, comments, and scientific editing. Financial support of this trial was provided with funding from the Washington University Department of Obstetrics and Gynecology. Support also provided by the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Clinical Trials Core who provided statistical services. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant #P30 CA91842
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.gore.2024.101476.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
References
- ACOG Committee Opinion No. 509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. Nov 2011;118(5):1192-1194. doi:10.1097/AOG.0b013e31823b17c2. [DOI] [PubMed]
- Boyles G.P., Weaver A.M., Cohn D.E., et al. Wound complications following vulvar excision for nonmalignant lesions. AJOG Glob Rep. 2021;1(4) doi: 10.1016/j.xagr.2021.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National Healthcare Safety Network Surgical Site Infection Event Report. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- Gaarenstroom K.N., Kenter G.G., Trimbos J.B., et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int. J. Gynecol. Cancer. 2003;13(4):522–527. doi: 10.1046/j.1525-1438.2003.13304.x. [DOI] [PubMed] [Google Scholar]
- Idsoe O., Guthe T., Willcox R.R., de Weck A.L. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38(2):159–188. [PMC free article] [PubMed] [Google Scholar]
- Janda M., Obermair A., Cella D., et al. The functional assessment of cancer-vulvar: reliability and validity. Gynecol Oncol. 2005;97(2):568–575. doi: 10.1016/j.ygyno.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Jourdan A., Sangha B., Kim E., et al. Antibiotic hypersensitivity and adverse reactions: management and implications in clinical practice. Allergy Asthma Clin. Immunol. 2020;16:6. doi: 10.1186/s13223-020-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson P.L., Habermann E.B., Baxter N.N., Durham S.B., Virnig B.A. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018–1022. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- Mullen M.M., Merfeld E.C., Palisoul M.L., et al. Wound complication rates after vulvar excisions for premalignant lesions. Obstet Gynecol. 2019;133(4):658–665. doi: 10.1097/AOG.0000000000003185. [DOI] [PubMed] [Google Scholar]
- Wills A., Obermair A. A review of complications associated with the surgical treatment of vulvar cancer. Gynecol Oncol. 2013;131(2):467–479. doi: 10.1016/j.ygyno.2013.07.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.