Abstract
The manganese-containing superoxide dismutase (MnSOD) is a major component of the cellular defence mechanisms against the toxic effects of the superoxide radical. Within the framework of studies on anti-oxidant enzymes and their protective role in the human parasitic nematode Onchocerca volvulus, sequences encoding the MnSOD were isolated and examined in this study. Degenerate primers were designed based upon conserved regions of MnSOD sequences from other organisms, and were used in PCR on reverse-transcribed O. volvulus total RNA and genomic DNA to identify partial cDNA and genomic DNA fragments encoding the O. volvulus MnSOD (OvMnSOD). The genomic DNA PCR product was used to screen an O. volvulus adult worm lambda unizap II cDNA library and the nucleotide sequence of the longest clone determined. The complete 5'-end of the OvMnSOD cDNA was obtained using the rapid amplification of cDNA ends (RACE) procedure with O. volvulus total RNA and was found to possess a spliced leader sequence at the 5'-terminus. The deduced primary sequence encodes a 25 kDa protein, which has the conserved residues required for enzyme activity and metal binding. The 24 N-terminal amino acids encoded by the OvMnSOD cDNA comprise a putative mitochondrial transit peptide. The OvMnSOD gene was also isolated from an O. volvulus adult worm lambda fix II genomic library, a restriction map was constructed and the nucleotide sequence determined. The OvMnSOD gene was found to possess five exons and four introns with consensus splice-site junctions. Potential regulatory elements were identified in the 5' genomic flanking sequence. Southern-blot analysis with total worm genomic DNA indicates a single-copy gene, with a restriction pattern consistent with that of the isolated gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R., Saul R. L., Ames B. N. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S., Chatterjee R. K., Srivastava V. M. Antioxidant enzymes in Acanthocheilonema viteae and effect of antifilarial agents. Biochem Pharmacol. 1990 Nov 15;40(10):2363–2369. doi: 10.1016/0006-2952(90)90734-3. [DOI] [PubMed] [Google Scholar]
- Borgstahl G. E., Parge H. E., Hickey M. J., Beyer W. F., Jr, Hallewell R. A., Tainer J. A. The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell. 1992 Oct 2;71(1):107–118. doi: 10.1016/0092-8674(92)90270-m. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988 Aug;4(8):218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Ludwig M. L., Stallings W. C., Fee J. A., Steinman H. M., Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988 Jan 25;263(3):1555–1562. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church S. L. Manganese superoxide dismutase: nucleotide and deduced amino acid sequence of a cDNA encoding a new human transcript. Biochim Biophys Acta. 1990 Oct 23;1087(2):250–252. doi: 10.1016/0167-4781(90)90213-l. [DOI] [PubMed] [Google Scholar]
- Cookson E., Blaxter M. L., Selkirk M. E. Identification of the major soluble cuticular glycoprotein of lymphatic filarial nematode parasites (gp29) as a secretory homolog of glutathione peroxidase. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5837–5841. doi: 10.1073/pnas.89.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. Oxidative damage to behavior during aging. Science. 1991 Dec 13;254(5038):1597–1597. doi: 10.1126/science.1684251. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Sun H. C. Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3217–3221. doi: 10.1073/pnas.89.8.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkle-Dührsen K., Liebau E., Walter R. D. The Onchocerca volvulus mRNAs for a hsp70, a collagen-like protein and a ribosomal protein possess a 5' spliced leader sequence. Trop Med Parasitol. 1993 Dec;44(4):337–339. [PubMed] [Google Scholar]
- Henkle-Dührsen K., Warnecke C., Brattig N., Liebau E., Walter R. D. Characterization of enzymatically active Onchocerca volvulus Cu/Zn superoxide dismutase expressed in Escherichia coli. Mol Biochem Parasitol. 1994 Sep;67(1):41–47. doi: 10.1016/0166-6851(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Henkle K. J., Liebau E., Müller S., Bergmann B., Walter R. D. Characterization and molecular cloning of a Cu/Zn superoxide dismutase from the human parasite Onchocerca volvulus. Infect Immun. 1991 Jun;59(6):2063–2069. doi: 10.1128/iai.59.6.2063-2069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
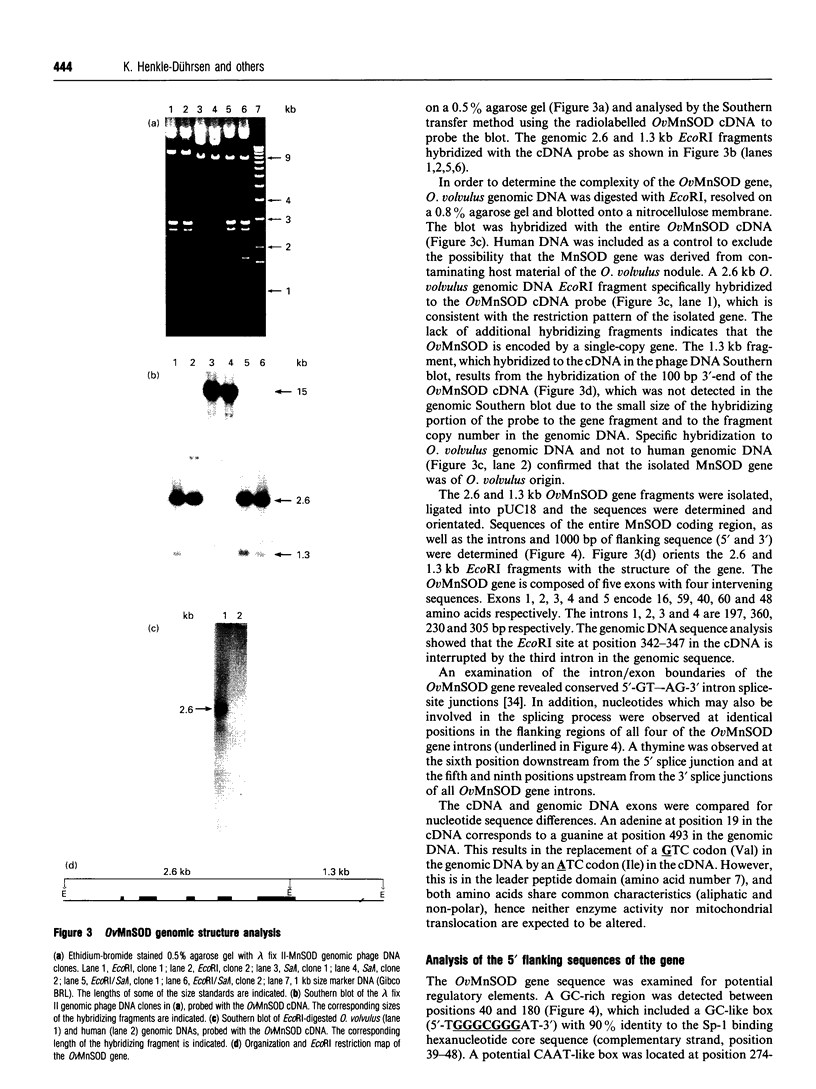
- Henkle K. J., Liebau E., Walter R. D. Characterization of the Onchocerca volvulus superoxide dismutase gene and mRNA processing. Mol Biochem Parasitol. 1993 Mar;58(1):173–176. doi: 10.1016/0166-6851(93)90103-5. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. Isolation and characterization of complementary DNAs encoding human manganese-containing superoxide dismutase. FEBS Lett. 1988 Mar 14;229(2):256–260. doi: 10.1016/0014-5793(88)81136-0. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Howard A. J., Crapo J. D. Molecular structure of a functional rat gene for manganese-containing superoxide dismutase. Am J Respir Cell Mol Biol. 1991 Mar;4(3):278–286. doi: 10.1165/ajrcmb/4.3.278. [DOI] [PubMed] [Google Scholar]
- James E. R., McLean D. C., Jr, Perler F. Molecular cloning of an Onchocerca volvulus extracellular Cu-Zn superoxide dismutase. Infect Immun. 1994 Feb;62(2):713–716. doi: 10.1128/iai.62.2.713-716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid R. W., Suquet C. M. A superoxide dismutase of metacestodes of Taenia taeniaeformis. Mol Biochem Parasitol. 1986 Mar;18(3):301–311. doi: 10.1016/0166-6851(86)90087-3. [DOI] [PubMed] [Google Scholar]
- Liebau E., Walter R. D., Henkle-Dührsen K. Isolation, sequence and expression of an Onchocerca volvulus glutathione S-transferase cDNA. Mol Biochem Parasitol. 1994 Feb;63(2):305–309. doi: 10.1016/0166-6851(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Liebau E., Walter R. D., Henkle-Dührsen K. Onchocerca volvulus: isolation and sequence of a second glutathione S-transferase cDNA. Exp Parasitol. 1994 Aug;79(1):68–71. doi: 10.1006/expr.1994.1062. [DOI] [PubMed] [Google Scholar]
- Liebau E., Wildenburg G., Walter R. D., Henkle-Dührsen K. A novel type of glutathione S-transferase in Onchocerca volvulus. Infect Immun. 1994 Nov;62(11):4762–4767. doi: 10.1128/iai.62.11.4762-4767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marres C. A., Van Loon A. P., Oudshoorn P., Van Steeg H., Grivell L. A., Slater E. C. Nucleotide sequence analysis of the nuclear gene coding for manganese superoxide dismutase of yeast mitochondria, a gene previously assumed to code for the Rieske iron-sulphur protein. Eur J Biochem. 1985 Feb 15;147(1):153–161. doi: 10.1111/j.1432-1033.1985.tb08731.x. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Magnuson M. A. Identification and functional characterization of the bovine manganous superoxide dismutase promoter. Am J Respir Cell Mol Biol. 1994 Jan;10(1):113–121. doi: 10.1165/ajrcmb.10.1.8292376. [DOI] [PubMed] [Google Scholar]
- Miao Z., Gaynor J. J. Molecular cloning, characterization and expression of Mn-superoxide dismutase from the rubber tree (Hevea brasiliensis). Plant Mol Biol. 1993 Oct;23(2):267–277. doi: 10.1007/BF00029003. [DOI] [PubMed] [Google Scholar]
- Reveillaud I., Niedzwiecki A., Bensch K. G., Fleming J. E. Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol Cell Biol. 1991 Feb;11(2):632–640. doi: 10.1128/mcb.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Schatz G. Mitochondrial presequences. J Biol Chem. 1988 Apr 5;263(10):4509–4511. [PubMed] [Google Scholar]
- Rushmore T. H., Morton M. R., Pickett C. B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991 Jun 25;266(18):11632–11639. [PubMed] [Google Scholar]
- Sanchez-Moreno M., Leon P., Garcia-Ruiz M. A., Monteoliva M. Superoxide dismutase activity in nematodes. J Helminthol. 1987 Sep;61(3):229–232. doi: 10.1017/s0022149x00010063. [DOI] [PubMed] [Google Scholar]
- Sato K., Aoki T., Nakano M. Dirofilaria immitis: a large-scale purification method and partial characteristics of a superoxide dismutase from adult worms. Exp Parasitol. 1994 Mar;78(2):210–216. doi: 10.1006/expr.1994.1021. [DOI] [PubMed] [Google Scholar]
- Schulz-Key H., Albiez E. J., Büttner D. W. Isolation of living adult Onchocerca volvulus from nodules. Tropenmed Parasitol. 1977 Dec;28(4):428–430. [PubMed] [Google Scholar]
- Shull S., Heintz N. H., Periasamy M., Manohar M., Janssen Y. M., Marsh J. P., Mossman B. T. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991 Dec 25;266(36):24398–24403. [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Strålin P., Marklund S. L. Effects of oxidative stress on expression of extracellular superoxide dismutase, CuZn-superoxide dismutase and Mn-superoxide dismutase in human dermal fibroblasts. Biochem J. 1994 Mar 1;298(Pt 2):347–352. doi: 10.1042/bj2980347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Hegamyer G., Colburn N. H. Sequence of manganese superoxide dismutase-encoding cDNAs from multiple mouse organs. Gene. 1993 Sep 15;131(2):301–302. doi: 10.1016/0378-1119(93)90311-p. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Ou X., Henkle-Dührsen K., Selkirk M. E. Extracellular and cytoplasmic CuZn superoxide dismutases from Brugia lymphatic filarial nematode parasites. Infect Immun. 1994 Mar;62(3):961–967. doi: 10.1128/iai.62.3.961-967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Molecular genetics of superoxide dismutases. Free Radic Biol Med. 1988;5(5-6):393–402. doi: 10.1016/0891-5849(88)90113-x. [DOI] [PubMed] [Google Scholar]
- White J. A., Scandalios J. G. Isolation and characterization of a cDNA for mitochondrial manganese superoxide dismutase (SOD-3) of maize and its relation to other manganese superoxide dismutases. Biochim Biophys Acta. 1988 Nov 10;951(1):61–70. doi: 10.1016/0167-4781(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Zeng W. L., Alarcon C. M., Donelson J. E. Many transcribed regions of the Onchocerca volvulus genome contain the spliced leader sequence of Caenorhabditis elegans. Mol Cell Biol. 1990 Jun;10(6):2765–2773. doi: 10.1128/mcb.10.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]