Abstract
Over recent years, the scientific community has acknowledged the crucial role of certain microbial strains inhabiting the intestinal ecosystem in promoting human health, and participating in various beneficial functions for the host. These microorganisms are now referred to as next-generation probiotics and are currently considered as biotherapeutic products and food or nutraceutical supplements. However, the majority of next-generation probiotic candidates pose nutritional demands and exhibit high sensitivity towards aerobic conditions, leading to numerous technological hurdles in large-scale production. This underscores the need for the development of suitable delivery systems capable of enhancing the viability and functionality of these probiotic strains. Currently, potential candidates for next generation probiotics (NGP) are being sought among gut bacteria linked to health, which include strains from the genera Bacteroids, Faecalibacterium, Akkermansia and Clostridium. In contrast to Lactobacillus spp. and Bifidobacterium spp., NGP, particularly Bacteroids spp. and Clostridium spp., appear to exhibit greater ambiguity regarding their potential to induce infectious diseases. The present review provides a comprehensive overview of NGPs in terms of their health beneficial effects, regulation framework and risk assessment targeting relevant criteria for commercialization in food and pharmaceutical markets.
Keywords: Clinical trials, Gut microbiota, Synbiotics, Live biotherapeutics, Next generation probiotics
1. Introduction
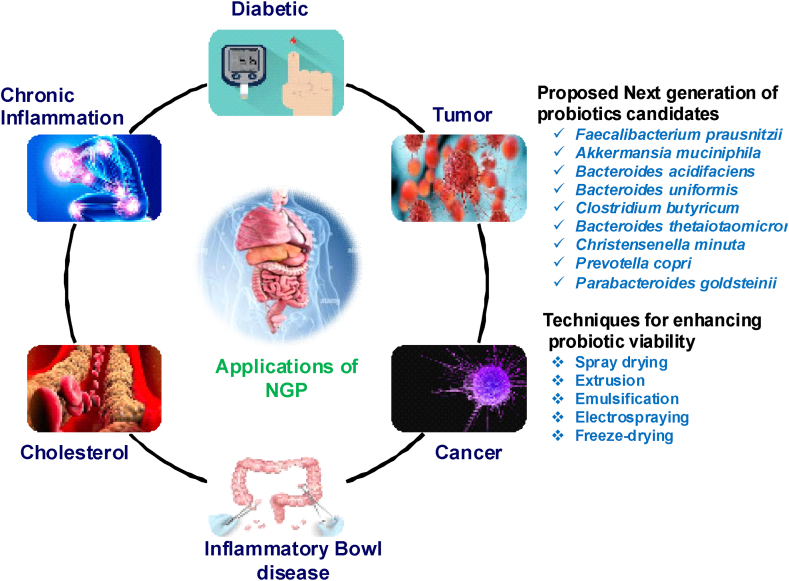
The conventional probiotics initially sprang from Elie Metchnikoff's report in 1907. The prevailing consensus held that the consumption of fermented dairy products, like yogurt, kefir, sauerkraut, tempeh and kombucha which are rich in lactic acid bacteria (LAB), provides health benefits. Probiotics are viable microorganisms which administered in sufficient quantities, contribute a health advantage to the host. These benefits can include improving digestion, supporting immune function, and contributing to overall well being. The Food and Agriculture Organization (FAO) and World Health Organization (WHO) collaborative group established criteria that probiotics must meet, which is the strain should be deemed safe for consumption [1]. Probiotics are formulations containing living microorganisms, such as bacteria, yeasts, or a combination of all, that commonly present in the natural gut microbiota [2]. The use of appropriate probiotic strains at sufficient doses is the first prerequisite for creating a probiotic food product. The primary criteria for choosing appropriate probiotic bacterial strains are their capacity to remain viable under food processing and storage conditions, their ability to survive during intestinal transit, and their potential to improve consumer health [3]. Additionally, recent clinical trials show significantly enhanced reporting of adverse events [4]. Recent advancements in methods such as metabolomics, gnotobiotics and next-generation sequencing have facilitated a more comprehensive understanding of topics like colonization resistance, susceptibility to external microorganisms, biogeographical diversity, variation in individual microbiome structures, and their potential impact on treatment outcomes [5]. The term NGPs was introduced by O'Toole PW (2017) that NGPs are live bacteria identified based on comparative microbiota analyses that when administered in right amounts, confer a health advantage on human health. On the other hand, live microorganisms in biological products are applicable to the prevention of disease in humans, and are not vaccines [6]. Over the past few years, the scientific community has been accumulating a growing body of knowledge about the mechanisms involved in metabolic and inflammatory disorders. These rapidly escalating conditions are reaching epidemic levels, posing fresh challenges for both clinicians and researchers. Gaining a comprehensive understanding of the exact roles and controlling characteristics of beneficial or probiotic bacteria in the gut ecosystem seems to be essential in the effort to prevent inflammatory and diet-related disorders. Akkermansia muciniphila, and Eubacterium hallii and Faecalibacterium prausnitzii, have been pinpointed as potential NGPs that hold great promise in preventing and treating diseases associated with dysbiosis [7] (Fig. 1).
Fig. 1.
Next generation probiotics for prevention of disease.
Nevertheless, these microorganisms pose difficulties in handling because of their high susceptibility to oxygen exposure and frequently to the gastric conditions they encounter post-ingestion [8]. One characteristic of certain NGPs is their ability to generate short-chain fatty acids, among which butyrate, in particular, is recognized for its role in promoting immune balance and human intestinal well-being [9]. The emerging knowledge is providing a basis for the selection of NGPs. The proposed candidates for NGP include Akkermansia muciniphila, Bacteroides thetaiotaomicron, Bacteroides acidifaciens, Bacteroides uniformis, Clostridium butyricum, Christensenella minuta, Faecalibacterium prausnitzii, Prevotella copri, and Parabacteroides goldsteinii. These selections are primarily based on their potential to prevent and alleviate conditions such as colitis, obesity, liver disease and diabetes [10]. Conversely, the use of conventional probiotics does not target particular diseases. In light of these circumstances, there is an urgent requirement for the discovery and profiling of new NGPs that are tailored to specific diseases [11]. To attain the objectives of pinpointing NGPs, the initial step involves uncovering whether there exist noteworthy correlations between the microbiota and the host across the various study groups, including the healthy, diseased, and experimental cohorts. These connections can be gleaned from either animal or clinical investigations [12]. The Human Genome Project, has led to a rise in next-generation sequencing technology that has enhanced the depth and velocity of phylogenetic analysis [13].
Due to the favorable results in manipulating gut microbiota for the management and prevention of numerous conditions, food nutraceutical firms and pharmaceutical companies may express keen interest in potential probiotic strains. Nonetheless, for the introduction of these advanced probiotics, also known as live biotherapeutics products [6], in the market a full assessment of safety parameters is mandatory. NGPs are microorganism species that align with the conventional probiotic criteria but have yet to establish a history of use in promoting health. NGPs also closely adhere to the description of live biotherapeutic products (LBPs) as outlined by the US Food and Drug Administration, which defines them as biological products that: “comprises living organisms, including microbes; are pertinent to preventing, treating, or alleviating human diseases or conditions; and are not a form of vaccination” [14]. This paves the way for the exploration of NGPs, given their suitability for pharmaceutical applications rather than being mere dietary supplements [15]. The present review deals with overall perspective of NGP candidates in terms of their health beneficial effects, related regulatory framework, and risk assessment criteria relevant for commercialization in food and pharmaceutical markets.
2. Microbial strains for next generation probiotics
Human are recognized as “Superorganisms”, and closely associated with microbiomes [16]. The human gastrointestinal tract (GIT), is home to 100 trillion microorganisms (bacteria, fungus, viruses, and protozoa), as revealed by research carried out by the human microbiome study [17]. Due to the dense population the bacteria residing in the gastrointestinal tract and the absence of prior expertise regarding their nutritional requirements and proper culture conditions, it can be challenging to grow every bacterium. The development of bioinformatics tools and nucleic acid sequencing techniques has allowed researchers to overcome this shortage by enabling them to detect and measure the numerous constituents of the gut microbiota [18]. Approximately 90 % phylogenetic analysis indicates that the gut bacteria are categorized within the Firmicutes and Bacteroidetes phyla [19]. The remaining bacteria include members of the Proteobacteria, Actinobacteria, Verrucomicrobia, and Fusobacteriota phyla. During infancy, the composition of the gut microbiota is relatively clear and includes a notable number of microorganisms such as Veillonella, Clostridium botulinum, C. coccoides, Bacteroides, and Akkermansia muciniphila [20].
The evidence of benefit of NGPs on human health is available from various clinical trials conducted on humans and mice over past few decades. Several clinical trials have been conducted on human and mice for treatment of many diseases. In a report by Gérard et al. [21], isolated Bacteroides sp. from human faces and the report concluded that it was the first cholesterol reducing bacterium. Similarly, Bacteroides fragilis was sorted out from a healthy breast fed infant. This bacterial strain showing the ability to enhances phagocytosis, polarises M1 macrophages and improve immune imbalance, inflammatory disease and mental disorders [22]. In another report, Clostridium butyricum was isolated and evaluated in patients with ulcerative colitis. The result during clinical trial showed prevention of pouchitis and alteration of the microbiota profile in patients [23]. In another clinical study of Li et al. [24] revealed that the strain inhibited Vibrio parahaemolyticus infection in mice. Zhang et al. [25] reported that Bacteroides fragilis isolated from human feces protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Another study concluded that bacterial strain Lactococcus lactis induced Parkinsonism by inhibiting ferroptosis, redressing dysbiosis and oxidative stress in human [26] (Table 1).
Table 1.
Microbial strains for next generation probiotics.
| S.No | Strain | Host | Benefit | Clinal Trails | References |
|---|---|---|---|---|---|
| 1. | Bacteroides fragilis | Human feces | Enhances phagocytosis and polarises M1 macrophages | LoVo cells | [22] |
| 2. | Bacteroides dorei | Human feces | Cholesterol reducing | Human | [21] |
| 3. | Bacteroides ovatus | Human Gut | Reduces Intestinal Inflammation | Mice | [27] |
| 4. | Bacteroides ovatus | – | Reduces Intestinal Inflammation | Mice | [28] |
| 5. | Lactococcus lactis | GMO(Food) | Reduces inflammatory bowel diseases | Mice | [29] |
| 6. | Faecalibacterium prausnitzii | Human feces | Mainly IBD, but also asthma, eczema and Type II diabete |
Human | [[30], [31], [32]] |
| 7. | Bacteroides ovatus | Human feces | Reduces the risk of certain types of cancer | – | [33] |
| 8. | Clostridium butyricm | Human | Prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis | Human | [23] |
| 9. | Bacteroides acidifaciens | Mouse feces | Clearance of infectious agents | – | [34] |
| 10. | Oscillospira sp. | Human Gut | Improved diabetes, obesity and reduced systematic chronic inflammation | – | [35] |
| 11. | Akkermansia muciniphila | Human Gut | Improves key components of metabolic syndrome, such as reducing fat mass, plasma glucose, gut permeability and metabolic inflammation | Mice | [36] |
| 12. | Bacteroides fragilis | Human feces | Antibiotic-associated diarrhea | Rat | [25] |
| 13. | Bacteroides fragilis | Human Gut | Oxazolone-induced experimental colitis | Mice | [37] |
| 14. | Bacteroides fragilis | Human feces | Vibrio parahaemolyticus infection | Mice | [24] |
| 15. | Bacteroides uniformis | Human feces | Overweight-associated disorders | Mice | [38] |
| 16. | Akkermansia muciniphila | Human Gut | Develop live biotherapeutic product | – | [39] |
| 17. | Ruminococcus bromii | Human Gut | Develop live biotherapeutic product | – | [39] |
| 18. | Faecalibacterium prausnitzii | Human Gut | Develop live biotherapeutic product | – | [39] |
| 19. | Anaerobutyricum hallii | Human Gut | Develop live biotherapeutic product | – | [39] |
| 20. | Roseburia intestinalis | Human Gut | Develop live biotherapeutic product | – | [39] |
| 21. | Faecalibacterium prausnitzii | Human gut | Reduced in patients with hyperlipidaemia, prediabetes and type 2 diabetes, non-alcoholic fatty liver diseaseand inflammatory bowel disease | Mice and Human | [40] |
| 22. | Lactococcus lactis | Fecal | Induced Parkinsonism are mediated by modulating oxidative stress, inhibiting ferroptosis, and redressing dysbiosis | Human | [26] |
| 23. | Bifidobacterium sp. | Promotes antitumor immunity and facilitates anti–PD-L1 efficacy | Mice | [41] | |
| 24. | Bifidobacterium longum | Human Blood | Robust CD8+ T cell response and better prognosis in HBV-related hepatocellular carcinoma | Human | [42] |
| 25. | Enterococcus hirae | Human Blood | Robust CD8+ T cell response and better prognosis in HBV-related hepatocellular carcinoma | Human | [42] |
| 26. | Lactobacillus rhamnosus | Human blood | Improve immune system | Human | [43] |
| 27. | Bifidobacterium lactis | Human blood | Improve immune system | Human | [43] |
| 28. | Akkermansia muciniphila | – | Combat cancer disease | Mice | [44] |
| 29. | Faecalibacterium prausnitzii | Human | Anti-tumor response | Mice | [45] |
| 30. | Akkermansia muciniphila | – | Reducing systematic inflammation and potentially lowering cancer risk | Human | [46] |
| 31. | Clostridium butyricum | Stool samples | CBM reduced the changes in the intestinal flora and decreased the incidence of gastrointestinal side effects | – | [47] |
| 32. | Clostridium butyricum | – | Showed Antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8 and novel intravesical therapy for bladder cancer | Human | [48] |
| 33. | Clostridium butyricum | – | Reduces the incidence of diarrhea in digestive diseases, including inflammatory bowel disease | Human | [49] |
| 34. | Eubacterium limosum | Increases mucosal integrity and shows anti-inflammatory action modulation of mucosal defense system via TLR4 | Mice | [50] | |
| 35. | Eubacterium hallii | – | Improves insulin sensitivity and increases energy metabolism in severely obese and diabetic | Mice | [51] |
| 36. | Akkermansia muciniphila | – | Enhance the efficacy of cancer immunotherapy's | Mice | [52] |
| 37. | Enterococcus hirae | – | Th1 Cell Immune Responses in Chemotherapy-Treated Cancer | Mice | [53] |
| 38. | Barnesiellaintestinihominis | – | Th1 Cell Immune Responses in Chemotherapy-Treated Cancer |
Mice | [53] |
| 39. | Bacteroides fragilis | Feces of a healthy breast-fed infant | Enhances the phagocytic functions of macrophages, polarising them to an M1 phenotype | [22] |
In a finding, the clinical study evaluated the effect of Clostridium butyricum on human, showing that it reduced the incidence of diarrhea in digestive diseases, including inflammatory bowel disease [49]. In a different finding five bacterial strains Roseburia intestinalis, Anaerobutyricum hallii, Faecalibacterium prausnitzii, Ruminococcus bromii, and Akkermansia muciniphila were isolated from human gut and developed as live biotherapeutic product [39]. In a study, Faecalibacterium prausnitzii was isolated from the human gut, and a clinical trial was conducted on mice and humans. The study revealed that patients treated with this strain showed a reduction in non-alcoholic fatty liver disease, hyperlipidaemia, prediabetes, inflammatory bowel disease and type 2 diabetes, demonstrating its potential as an efficient NGP strain [40]. In summary, NGP is still in its early stages as a medical concept, but it shows great promise and will require much investigation before it can be used in preventive care.
3. Characteristics of next generation probiotics
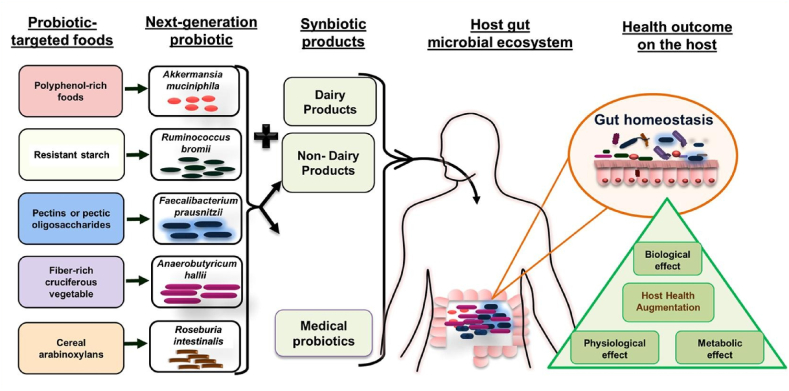
NGPs' functioning mechanisms in the gut are more complex than those of traditional probiotics, and they are far more susceptible to the harsh conditions found in the human gastrointestinal tract. It has been established that NGPs' are essential for enhancing the efficacy and survivability of beneficial gut bacteria. Probiotics, which are defined as substrates that the host microbes specifically use to provide a health benefit, are also crucial for NGPs [54]. Probiotics have the potential to be beneficial to health in part because they aid in the growth and activity of beneficial gut microbes through fermentation [55]. Additionally, encapsulating materials for probiotics it has been shown that giving inulin (a fructan) orally can greatly increase the proliferation of Faecalibacteriumprausnitzii and Bifidobacterium adolescentis in the human gut microbiota [39]. Remarkably, a different study showed that adding riboflavin, cysteine, and inulin increased the survival rate of F. Prausnitzii in the air by 70 %. F. prausnitzii is capable of surviving in partially oxygenized environments such as the gut mucosa through the transfer of electrons to oxygen. For this purpose, the bacterium utilizes extracellular antioxidants, including cysteine and riboflavin that are abundant in the gut. These antioxidants can maintain the viability of F. Prausnitzii in ambient air [56]. Galacto-oligosaccharides are frequently added to meals and are thought of as prebiotics. Probiotic development in the complex gut environment may be stimulated by galact-oligosaccharides [57].
Probiotics have garnered ongoing interest due to their ability to ferment in the colon, promoting bacterial activity that may positively impact on colon health, even though they are not widely recognized as prebiotics [58]. When it comes to gut microecology, vitamin D has been extensively studied and is one of the well-researched vitamins. Studies have shown that vitamin D deprivation exerts a noteworthy influence on the diversity and makeup of microbiome. For instance, vitamin D3 treatment was observed to increase the proportional prevalence of Bacteroidetes, while decreasing certain of Proteobacteria in an interventional, open-label pilot investigation [59]. The human body often obtains vitamin K via food supplements. The gene necessary for vitamin K synthesis is also present in the gut microbiota [60], like Bacillus subtilis, Eubacterium rectal, and various species of Bacteroides have the ability to reshape dietary vitamin K into menaquinones, which can control the gut microbiota [54]. The small intestine absorbs just 5–6% of the polyphenols, and very few are eliminated with feces. The remaining polyphenols are then available to support probiotics by the gut microbiota in the colon or large intestine [61].
Plant polyphenols also have an attractive antioxidant impact that may increase the viability rates NGPs sensitive to oxygen within the gastrointestinal tract. Polyphenol-rich extracts, such as those derived from berries, grapes, and caffeic acid, could enhance the growth of Akkermansia muciniphila within the microbiome [62]. In animal models, this has been demonstrated to improve colonic inflammation and metabolic disorders, and other diseases [63]. A common plant polyphenol among vegetables and fruits, resveratrol has shown promising effects when used to enhance the activity of NGPs. Research findings indicate that resveratrol modifies the physicochemical attributes of the microbial surface, and promotes Lactobacillus paracasei ATCC334 adherence and biofilm formation [64]. Consuming anthocyanins has been associated with an increase in Bifidobacterium spp. abundance in the gut microbiota [65]. While anthocyanins have been demonstrated to exhibit antioxidant and anti-inflammatory properties in mice, their impact on NGPs has not yet been assessed. While the small intestine is the primary site of mineral absorption, the gut microbiota's composition in the colon can alter the mineral bioavailability [66].
During food processing, storage, and gastrointestinal transit, the ratio of live probiotics is typically significantly decreased. NGPs have demonstrated effectiveness in treating a variety of disorders associated with the gut microbiota; however, this positive outcome is only likely to occur when a sufficient number of live bacteria enter the intestinal tract and effectively establish residence in the gastrointestinal tract [67]. Tragically, bile salts, gastric acids, and oxygen can all cause severe sensitivity in NGPs such A. muciniphila and F. Prausnitzii [54]. Combining different probiotics with probiotics during therapy has been shown to increase probiotic colonization and survival [54].
4. Mechanisms of action of next generation probiotics
The vital roles of the human gut microbiota encompass the management of systemic immunity, assisting in nutrient absorption by the host, safeguarding the intestinal barrier to facilitate the establishment of indigenous flora, and competing with external pathogenic bacteria [68]. The substantial influence of the gut microbiome on human well-being is widely recognized [69]. The human digestive system hosts a plethora of microorganisms that have forged intricate connections with the host. Significant attention is dedicated to understanding the composition and function of the gut microbiota in both states of health and disease [70]. The human gastrointestinal microbiota, commonly referred to as the "overlooked organ" of the human body, remains inadequately characterized, even with regard to its composition [71]. Certainly, changes in the composition of the gut microbiome, known as dysbiosis, have been associated with various intestinal and systemic disorders, such as obesity, inflammatory bowel disease, diabetes, allergies, immune disorders, metabolic syndrome, cardiovascular diseases and Crohn's disease [72,73]. Apart from their immune-regulating properties, the gut microbiota also enhance the host's well-being by providing a protective barrier against pathogens, assisting in the process of digestion by breaking down indigestible food components and generating vital metabolites [74]. Furthermore, these metabolic and inflammatory conditions have been associated with substantial changes in both the abundance and diversity of the human gut microbiota [75]. The microbiota assist in the process of digestion, contributes to nutrition, and plays a role in shaping our immune system [76]. It is acknowledged that diet is a fundamental factor that influences the makeup of the gut microbiome. Over a prolonged duration, a consistent dietary pattern shapes both the structure and operations of the gut microbiome [77].
Faecalibacterium prausnitzii, Eubacterium hallii and Akkermansia muciniphila, are frequently found in the human microorganisms identified for their potential as probiotic candidates [78]. Akkermansia muciniphila can degrade components of intestinal mucin, resulting in a competitive inhibitory effect on other pathogens that also degrade mucin [79]. It is a common resident of the human gastrointestinal tract, representing roughly 1–3% of the total gut microbiota [80]. Recent discoveries have revealed that A. muciniphila can act as a marker for a favorable metabolic profile in a host. Indeed, a decrease in the presence of A. muciniphila in the gut has been linked to a range of metabolic and inflammatory conditions, including obesity, type-2 diabetes, and inflammatory bowel disease [81]. Faecalibacterium prausnitzii serves as the primary source of butyrate, with the capacity to influence the expression of tight junction proteins and holds the potential to improve the integrity of the intestinal barrier in people dealing with IBD [82]. This bacterial species ranks among the most prevalent microorganisms in the human gut, comprising approximately 5–20 % of the complete microbiota found in the feces of healthy individuals [83]. Furthermore, it has been documented that F. prausnitzii can metabolize the degradation byproducts of complex carbohydrates, including glucose, maltose, and N-acetylglucosamine, a constituent of glycoprotein's present in the gut mucosa [84]. Eubacterium hallii engages in nutrient exchange within the gut microbiota by producing lactate and acetate [85]. Recently, various strains from the Bacteroides genera were isolated from the fecal samples of healthy individuals using high-throughput in vitro screening assays. These strains exhibited anti-inflammatory characteristics and were regarded as promising NGPs [86]. Most Clostridium species are commensal bacteria with the ability to activate intestinal epithelial cells, fortify the intestinal barrier's integrity, and produce short-chain fatty acids [87].
Propionibacterium plays a crucial part in immunomodulation through the expression of dihydrolipoamide acetyltransferase, resulting in the proliferation of Th17 cells and ultimately decreasing the mortality linked to necrotizing enterocolitis [88]. F. prausnitzii, E. hallii and A. muciniphila are standout contenders among the identified keystone species, and they hold great promise for making a significant contribution to combating diseases stemming from the dysbiosis of inflammatory and metabolic origins. One of the most effective methods to induce beneficial alterations in the gut microbiome is through dietary modification, achieved by avoiding processed, high-carbohydrate, or high-fat foods, as well as artificial sweeteners and sugar, and by increasing the intake of vegetables, fermented foods and fibers that encourage the growth of beneficial bacteria [89]. Although positive outcomes have been observed with probiotics across various circumstances [90], the means by which they provide these benefits to humans remain poorly understood. Furthermore, it is acknowledged that the impact of probiotics depends on the precise strain and dosage, which may, to some extent, explain the varying results observed when using different probiotic strains, even if they belong to the same genus or species. Moreover, multiple factors could potentially impact the outcomes observed in clinical trials involving probiotics, such as the utilization of different probiotic strains, either on their own or in combination with other therapies: The host's initial health status, the capacity of the host's microbiota to permanently accommodate new microorganisms, and the ecological niche created (and controlled) by the host's immune system during the early stages of life [91] (Fig. 2).
Fig. 2.
Mechanism of actions of next generation probiotics. Adapted with permission from Ref. [39].
5. Techniques for enhance viability and stability of next generation probiotics
The use of appropriate probiotic strains at sufficient doses is the first prerequisite for creating a probiotic food product. The primary criteria for choosing appropriate probiotic bacterial strains are their capacity to remain viable under food processing and storage conditions, their ability to survive intestinal transit, and their potential to improve consumer health. Strain-specificity governs the ability of bacteria in the food matrix to survive against various extreme conditions during product production, processing, and storage [3]. Since prolonged storage is frequently required, commercial probiotic products must have an extended shelf life. Lowering the water activity (aw) to about 0.1 and the intracellular moisture level below 4 % inhibits cellular functions and maintains the cells in an inert condition [92]. Microencapsulation is among the most effective methods for enhancing the survival and durability of probiotic strains under industrial processing conditions, while also providing protection from the gastrointestinal environment [93]. Probiotics are microencapsulated to protect certain compounds or biological cells from external factors that could destroy their essential components. During the formulation development process, it enhances the flow characteristics and shields the bacteria from heat, oxygen, and moisture [94]. It has been demonstrated that probiotic encapsulation technology can improve the biological activity and survival of the microorganisms when utilised as a carrier or targeted delivery system [95]. In general, a minimum level of more than 106 live probiotic bacteria per millilitre or gram of food product is approved, while the exact number of cells needed to generate therapeutic effects is unknown and may vary depending on the strain and the desired health effect [96]. As viability is usually regarded as a requirement for probiotics' efficacy in relation to their health-promoting qualities for consumers, it represents an industrial hurdle. Numerous studies have shown the impact of live cells on the functional attributes of probiotics [97]. An effective microencapsulation strategy must protect the probiotics from the harsh conditions of upper gastrointestinal tract, release them within the colon, maintain their stability during storage, and ultimately improve their ability to adhere to mucosal surfaces for colonization [98].
5.1. Spray drying
Spray drying (SD) is a commonly used method for microencapsulation. The fundamental idea underlying this is the exchange of mass and heat between the air and the atomized droplets, and vice versa [99]. This special drying method allows for the continuous creation of probiotic powdered particles, which is followed by the internal spraying of liquid stock culture within the drying chamber [100]. According to the numerous authors, enveloping lactic acid and diverse probiotic cultures using a range of carrier substances through the spray drying process is long-term, cost effective, safe and energy-efficient preservation technique [101]. Spray drying involves three stages: atomization, which creates droplets; mixing, which evaporates water between droplets and hot air; and separation, which gathers the dried powders from the cyclone separator [102]. Spray drying provides a number of benefits over other techniques. These consist of low cost, short drying time, and continuous operation capability; together, they allow drying huge quantities of suspension within a relatively brief timeframe. It is also possible to alter the characteristics of the powder, and the scaling-up process is relatively straightforward [103]. The benefits of spray drying, including its elevated efficiency, cost-effectiveness, and good powder characteristics, have made it a potential method for producing probiotic powders. However, the adverse environmental conditions from digestion and drying can substantially lower cell viability, resulting in poor bioaccessibility and bioavailability of living cells. Thus, in order to preserve bacteria and their physiological processes in the targeted regions, efficient targeted delivery systems utilising spray drying must be developed [104]. The main disadvantage of this method is that it uses high temperatures, which might negatively impact encapsulation efficiency [105]. There is a need to concentrate on more practical desiccation techniques like freeze-drying because spray-drying requires high temperatures and oxygen, which makes it unsuitable for next-generation probiotics. The protective effects of antioxidants, osmoadaptation and stress therapy during the freeze drying of next-generation probiotics should be studied in order to optimise this process [8].
5.2. Extrusion
Extrusion is the predominant technique for encapsulating probiotic microorganisms within hydrocolloid gel matrices. This method, also known as sprinkle, is easy to use, inexpensive and produces probiotics with a high rate of encapsulation retention due to its mild environmental conditions [106]. Extrusion offers good cell viability and is a simple method to execute. Encapsulated gel beads are created during the extrusion process by dripping or spraying the feed solution via a nozzle at high pressure. The pulsation or vibration technology used in this method is described as a prilling or vibrational jet that may produce capsules in a standardised manner without compromising the viability of the microflora [107]. Despite the previously noted favorable conditions, its drawbacks include slowness, which hinders its large-scale application; inefficiency in producing microspheres smaller than 500 μm and the requirement for low to moderate viscosity hydrocolloid solutions [108].
5.3. Emulsification
Another low-cost approach to the probiotic microencapsulation is emulsification, which is more readily scaled up than extrusion. Probiotic cells and coated polymer are combined in both combining aqueous and oil phases to generate a substance resembling a "solution," comprising minute droplets. Upon the introduction of cross-linking agents, the water-soluble polymer transitions into an insoluble state, leading to the creation of gel particles within the oil phase. Subsequently, microcapsules can be retrieved through the filtration process [109]. Although this encapsulating method is typically employed in lab settings, there are certain drawbacks for probiotic cell and food sector applications [110]. L. casei and Lactiplantibacillus plantarum can be effectively utilised when RS-4 (phosphorylated starch type 4) is employed; encapsulation is achieved through the emulsion technique. These probiotics are effectively shielded from gastrointestinal conditions, exhibiting robust survival capacity even under harsh heat treatments and prolonged storage [111].
5.4. Electrospraying
Electrospraying is a cutting-edge drying technique employed in nutraceutical and food industries. It relies on the electrohydrodynamic process, involving the application of a high-voltage electrical field. The electrical interactions between charged particles in a fluid medium are the foundation of electrohydrodynamic approach. Research investigations have recently shown a great deal of interest in food drying using this method. This method provides inexpensive, quick drying times, efficient encapsulation, and great product quality [112,113]. This approach has a number of benefits, including high adaptability, simplicity, and ease of scaling up. The encapsulated probiotic cells in this technology suffer the least amount of thermal damage because heat is not involved [114]. This approach is simple, easy to regulate, and free of serious hazards, making it a potentially useful substitute for encasing delicate substances. This method can be utilised to successfully encapsulate medicinal molecules in micro- and nanoscale forms by adjusting the process variables, solvent type, and process parameters [115]. Due to its basic design, electrospraying may produce particles with a monodisperse distribution and is easy to operate in moderate weather situations [116]. The biological macromolecule encapsulation of food bioactive components by the electrospraying approach has garnered significant attention in the field of food science [117]. Due to its lack of necessity for elevated temperatures, pressures, or harsh chemical environments, research is currently underway to produce drug and probiotic-laden particles using electrospraying [118].
5.5. Freeze-drying
This procedure was initially commercialized during World War II to maintain the stability of penicillin and blood plasma. Afterwards, freeze-drying technology was employed by French virologist Charles Merieux to preserve vaccines. In 1938, Max Mortgenthaler, a scientist, pioneered freeze-dried coffee, paving the way for the development of powdered food item. The low operating temperature of the freeze-drying process reduces product denaturation, which is typically a problem with other drying techniques [119]. Lyophilisation, also known as freeze drying, is a popular technique for dehydrating probiotic bacterial cells so that their storage durability is guaranteed. Three steps make up the freeze-drying process: freezing the cell culture, sublimation, and final drying. Typically, the initial stage is carried out beyond the drying apparatus. In the subsequent phase of this method, sublimation under reduced pressure is employed to eliminate the frozen water, while the ultimate stage involves desorption to remove the non-frozen water, resulting in the desired final water content [120,121]. Foods, medicines, and biological materials that are heat-sensitive are frequently dried via freeze drying method [122]. Since freeze drying doesn't require any freezing temperatures while the product is being distributed, it's the most practical method for protecting probiotic bacteria. However, in comparison to other drying techniques, the freeze drying procedure is more costly (4–7 times) and time-consuming [123]. The two main disadvantages of the freeze-drying method are its increased drying time and power usage [124]. When probiotics are freeze-dried, their encapsulated structure becomes more porous, negatively impacting their barrier strength [94]. According to reports, probiotics with a significant air-solid interface area during storage die off more quickly [125] (Table 2).
Table 2.
Techniques of next generation probiotics.
| S.No | Food | Techniques | Probiotic Strains | References |
|---|---|---|---|---|
| 1. | Apple juice | Spray drying | Lactobacillus rhamnosus GG | [126] |
| 2. | Ice cream | Emulsion | Lactobacillus casei Lc-01 and Bifidobacterium lactis Bb-12 | [127] |
| 3. | kefir | Extrusion | Bifidobacterium animals | [128] |
| 4. | Yogurt | Ionic gelation and complexation | Lactobacillus acidophilus LA-5 | [129] |
| 5. | Yogurt | Extrusion | Bifidobacterium bifidum F-35 | [130] |
| 6. | Carrot juice | Extrusion | Lactobacillus acidophilus | [131] |
| 7. | Fruit juices | Freeze drying | Bifidobacterium longum, Bifidobacterium breve | [132] |
| 8. | Mango juice | Gelation | Lactobacillus plantarum | [133] |
6. Regulatory framework of next generation probiotics
Probiotic regulations vary from nation to nation; no single framework is accepted worldwide. Within the European Union, food supplements and probiotics are subject to the regulations outlined in the Food Products Directive and Regulation (2000/13/EU; Regulation 178/2002/EC). The EFSA must approve every health claim related to probiotics. The qualified presumption of safety for a number of microbial cultures has been published by the EFSA [134]. All of the probiotics' filed health claims have been rejected by EFSA so far. As a result, while product claims are subject to strict inspection, the manufacturing process is not heavily regulated, and there is hardly any post-marketing regulatory follow-up [135]. After reviewing over 400 probiotic applications, the EFSA panel on dietetic products, nutrition, and allergens was unable to find any evidence supporting any health claim. “In reality, an adjustment regarding the use of general descriptors has essentially rendered the use of the term ‘probiotic’ unlawful” [136]. It remains uncertain whether any NGPs would be subject to further regulatory oversight [6]. Government bodies such as the Food Safety and Standards Authority of India (FSSAI), the U.S. Food and Drug Administration (FDA) and the Dietary Supplement Health and Education Act (DSHEA) in the United States, The European Food Safety Authority (EFSA) in Europe, the Joint Health Claims Initiative (JHCI) in the UK, the State Food and Drug Administration (SFDA) in China, the Canadian Food and Drugs Act Under Natural Health Products Regulations in Canada, and the Food Safety and Quality Division (FSQD), Food for Specified Health Use (FOSHU) in Japan and the National Health Surveillance Agency in Brazil have gained international recognition to describe the regulatory pathways for probiotics marketed as medicinal products including clinical trial data or as components in functional foods [137]. Regulatory challenges affecting the commercialization of NGP's include their classification which can vary across regions (e.g., as drugs or dietary supplements), resulting in diverse market pathways and compliance requirements. Standards for safely and efficacy also vary, complicating and increasing the cost of meeting regulatory expectations globally. Restrictions on health claims concerning probiotics add complexity to market positioning and consumer comprehension. Navigating labelling requirements is crucial to ensure compliance and uphold consumer trust in product benefits. These challenges highlight the importance of harmonized regulatory approaches to promote the global acceptance and market success of NGP's.
7. Safety and effectiveness considerations for next generation probiotics
The idea of NGPs was first formally proposed by Nature Microbiology in 2017. They believe that NGPs are distinct from conventional probiotics and meet the criteria for "active biological agents" as per the US FDA guidelines [138]. The NGPs should undergo clinical trials and need to be approved by relevant regulatory authorities prior to being released onto the market [139]. Potential NGPs are currently required to satisfy the following criteria: safety, individualised treatment, and internal interaction within the flora [140]. In addition to the functional examination of individual bacterial strains, Lactobacillus rhamnosus and Bifidobacterium lactis have been extensively studied in clinical trials to assess their safety profile, demonstrating high gastrointestinal tolerance and minimal risk of adverse effects in infants and elderly individuals. However safety considerations may vary greatly among different strains, even within the same species. Therefore safety appears to be the most crucial factor in the development of NGPs. These NGP candidates still need to be advanced clinically for the purpose of treating chronic inflammation-related disorders [141]. The safety evolution of NGP varies in different countries. New food products, such as NGP, must be safety evaluated by the European Food Safety Authority (EFSA). Key elements of the assessment of microbes include clear species-level taxonomic classification, whole-genome sequencing analysis to fully characterise strains, antibiotic resistances and their potential horizontal transfer, and other potentially harmful metabolic properties like those associated with obesity, diabetes, and metabolic syndrome etc. [142]. In addition to safety concern, the NGP's attributes must include a comprehensive understanding of the diseases it targets, as well as the genetic characteristics and physiological attributes of the bacteria, such as growth dynamics and antibiotic sensitivity patterns. Furthermore, it is necessary to define the underlying molecular ameliorative pathways. The next step in achieving this goal is to perform strict functional validation of the novel probiotics using state-of-the-art NGS (next generation sequencing) and bioinformatics methodologies to screen and isolate the NGPs [11]. Testing genetic stability and conducting human clinical trials underscore the importance of rigorous assessment methods to ensure the safety and efficacy of NGP's. These measures help establish their reliability and effectiveness across various health applications.
Significant studies on NGPs utilise a variety of methods to evaluate effectiveness and safety. These include clinical trials, such as randomized controlled trials and longitudinal studies, which employee validated outcome measures like symptom severity scores, biomarkers, and assessment of quality of life. Animal models are also employed to explore mechanisms of action and establish initial safety profiles before proceeding to human trials. Participant selection criteria usually encompass age, health status, and specific conditions being studied, while interventions involve the controlled administration of probiotic strains or formulations.
8. Potential health benefits and applications of next generation probiotics
The human gut microbiota is essential for regulating systemic immunity, promoting nutrient uptake by the host, preserving the integrity of the gut barrier, allowing for the colonization of native flora, and posing a threat to external pathogenic bacteria [143]. New developments high-throughput DNA sequencing, culturing methods, and molecular analysis technologies have enabled the collection of sufficient data to distinguish functionally distinct bacterial species. The past decade has seen a rapid development of microbiota related analysis platforms, leading to a quick unravelling of the composition, structure, and roles of gut microbial communities [144]. Microbiota based strategies are employed to improve intestinal barrier integrity, reduce inflammation, and ameliorate various diseases [145]. Among them, the fast developing practise of fecal microbiota transplantation (FMT) involves transferring the microbiota from a healthy donor to a recipient who suffers from a medical condition associated with a dysbiotic, diseased gut microbiome. A review of recent developments show that FMT has the most effective results when treating Clostridium difficile infections associated with recurrent antibiotic use [146].
There is growing interest in the human gut microbiota as an aspect of the environment that might impact either wellness or disease [147]. The creation of NGP represents a practical method for modifying the gut microbiota and improving human health [39]. Researchers like Neef, Sanz [148] have paved the way for the discovery of NGPs following the pharmaceutical regulatory guidelines outlined by both the food and drug administration and the European pharmacopoeia, NGPs have been identified as live biotherapeutic products (LBPs) since they are more appropriate for pharmaceutical use rather than as dietary supplements. Developing NGP strains is closely associated with addressing specific diseases such as bowel diseases, neuropsychiatric diseases, chronic inflammation, metabolic syndromes, inflammatory asthma, dysbiosis, cardiovascular disorders, and cancers [11].
However, a number of important prospective NGPs are strictly anaerobic and might need to grow in concert with other bacteria to perform at their most effectively [6]. In addition to standard probiotics, the functions of newly discovered, unconventional, indigenous gut microbiota bacteria for the promotion of health and potential medicinal applications have quickly generated increased excitement. Currently, strains of gut bacteria associated with health, such as those from the genera such as Akkermansia, Faecalibacterium, Bacteroides, Clostridium, Eubacterium, Propionibacterium and Roseburias, as well as genetically modified (GM) strains are being looked at as potential next-generation probiotics (NGPs) [39]. Appropriate accumulation of comprehensive physiological and molecular data is enabling the separation, recognition, and functional analysis of these different bacteria. These potentially advantageous bacteria are gradually being categorized to create the NGPs [54]. Several NGP bacterial strains belong to different family such as Actinobacteria, Bacteroidetes, Firmicutes and Verrucomicrobia [141]. Significantly, NGPs might be subject to drug-like regulations; consequently, the criteria for categorizing NGPs are considerably more rigorous compared to conventional probiotics [149]. Extensive interpretation of effectiveness in the improvement of disease, physiological, safety, and metabolomics characteristics, as well as the drug vulnerability pattern, drug resistance genes, and potential severity, is required [7]. Addressing the interaction between NPGs and the host to uphold intestinal immunity and host stability is a topic that needs attention. While most NGP products are not yet commercially available, research and development in this field continue to advance [39].
The high sensitivity of nearly all of these putative NGPs to oxygen will significantly impact their growth in pure culture and make large-scale production more challenging [150]. The development of NGPs for additional disease areas is rapidly expanding, in contrast to standard probiotics that target a general population and emphasise gut health [151]. NGPs for gastrointestinal issues, allergies and eczema in children, prevention of skin infections and allergies, and pregnancy and nursing are just a few of the constantly expanding categories in which these products are being developed [152]. NGPs targeted at various life phases are also expected to see significant development as more and more scientific information rapidly accumulates. Next generation probiotics offer innovative approaches to improving health beyond traditional benefits examples include precision targeting probiotics designed to address specific conditions or symptoms such as gastrointestinal disorders, immune modulation probiotics that enhance immune function by supporting the gut microbiota, reducing the risk of infections and autoimmune diseases, mental health support contributing to alleviating symptoms of anxiety, depression or stress and nutrient synthesis etc.
NGP's such as Lactobacillus, Bifidobacterium, Akkermansia muciniphila, and Faecalibacterium prausnitzii, demonstrate promising health benefits in scientific studies [153]. Lactobacillus and Bifidobacterium are recognized for enhancing gut health and immune function, with certain strains proving effective in conditions such as IBS and IBD. Akkermansia muciniphila promotes gut barrier function and metabolic health, aiding individuals dealing with obesity and metabolic disorders. Faecalibacterium prausnitzii, known for its anti-inflammatory properties, holds promise for managing inflammatory bowel diseases.
9. Conclusions
In recent times, there has been a notable surge in research interest regarding the influence of commensal gut bacteria on enhancing health, surpassing the focus traditionally placed on disease-related studies. These non-traditional germs that promote health are commonly known as NGPs, and if they are utilised for pharmaceutical purposes, they are subject to FDA live biotherapeutic products demands. The main obstacle lies in the identification of NGPs, as the approach is heavily reliant on assumptions and predominantly built upon correlation studies that compare the relative abundance of microbes in healthy individuals and those with diseases. After being identified, the organism needs to undergo testing using suitable in vitro, and in vivo models for a variety of functional, safety, and technological criteria. To produce LBP on a large scale, the unusual growth requirements must be considered, and the composition must guarantee the culture's viability and bioactivity until it is consumed. The produced product needs to clear regulatory approval requirements and undergo phase 1–3 clinical studies to confirm dose, assess safety, and evaluate efficacy. Although there is a wealth of research, further work is necessary to progress beyond the established correlation between gut microbiota in a healthy state and one in a diseased condition. The main reason could be that a harmonious intestinal microflora plays a role in establishing a balanced host microbiome environment that is linked to good health, which could be shown by the abundance of these health-associated bacteria. Another possible cause for the anomalous "immune set point" could be that a smaller number of these NGP candidates have been responsible. Nonetheless, further investigation is required to ascertain whether a solitary bacterial strain can offer such beneficial effects or if a consortium is necessary to achieve the intended outcomes of live bacterial biotherapeutics. Further research is needed to determine whether to use NGPs as preventive medicine, and this will involve the use of preclinical models and clinical trials to distinguish between all of the possibilities.
Funding statement
The authors are grateful to the Eternal University, Baru Sahib for for providing the facilities and financial support, to undertake the investigations
Data availability statement
No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Tawseefa Jan: Writing – original draft. Rajeshwari Negi: Writing – original draft. Babita Sharma: Writing – original draft. Sanjeev Kumar: Writing – review & editing. Sangram Singh: Writing – review & editing. Ashutosh Kumar Rai: Writing – review & editing. Sheikh Shreaz: Writing – review & editing. Sarvesh Rustagi: Writing – review & editing. Nisha Chaudhary: Writing – review & editing. Tanvir Kaur: Writing – review & editing. Divjot Kour: Writing – review & editing. Mohd Aaqib Sheikh:Writing – review & editing. Krishan Kumar: Writing – review & editing. Ajar Nath Yadav: Conceptualization. Naseer Ahmed: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financialinterestsor personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ajar Nath Yadav, Email: ajarbiotech@gmail.com.
Naseer Ahmed, Email: naseerfst@gmail.com.
References
- 1.Akuzawa R., Miura T., Surono I. Asian fermented milks. Encyclopedia of Dairy Science. 2011;2:507–511. [Google Scholar]
- 2.Éliás A.J., Barna V., Patoni C., Demeter D., Veres D.S., Bunduc S., Erőss B., Hegyi P., Földvári-Nagy L., Lenti K. Probiotic supplementation during antibiotic treatment is unjustified in maintaining the gut microbiome diversity: a systematic review and meta-analysis. BMC Med. 2023;21:262. doi: 10.1186/s12916-023-02961-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tripathi M.K., Giri S.K. Probiotic functional foods: survival of probiotics during processing and storage. J. Funct.Foods. 2014;9:225–241. [Google Scholar]
- 4.Žuntar I., Petric Z., Bursać Kovačević D., Putnik P. Safety of probiotics: functional fruit beverages and nutraceuticals. Foods. 2020;9:947. doi: 10.3390/foods9070947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zmora N., Zilberman-Schapira G., Suez J., Mor U., Dori-Bachash M., Bashiardes S., Kotler E., Zur M., Regev-Lehavi D., Brik R.B.-Z. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174:1388–1405. e1321. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 6.O'Toole P.W., Marchesi J.R., Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:1–6. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 7.Almeida D., Machado D., Andrade J.C., Mendo S., Gomes A.M., Freitas A.C. Evolving trends in next-generation probiotics: a 5W1H perspective. Crit. Rev. Food Sci. Nutr. 2020;60:1783–1796. doi: 10.1080/10408398.2019.1599812. [DOI] [PubMed] [Google Scholar]
- 8.Torp A.M., Bahl M.I., Boisen A., Licht T.R. Optimizing oral delivery of next generation probiotics. Trends in Food Sci Techno. 2022;119:101–109. [Google Scholar]
- 9.Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai Q., Feng S., Arjan N., Chen W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019;59:3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 11.Chang C.-J., Lin T.-L., Tsai Y.-L., Wu T.-R., Lai W.-F., Lu C.-C., Lai H.-C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019;27:615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilen M., Dufour J.-C., Lagier J.-C., Cadoret F., Daoud Z., Dubourg G., Raoult D. The contribution of culturomics to the repertoire of isolated human bacterial and archaeal species. Microbiome. 2018;6:1–11. doi: 10.1186/s40168-018-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronaghi M., Uhlén M., Nyrén P. A sequencing method based on real-time pyrophosphate. Science. 1998;281:363–365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 14.Yahfoufi N., Alsadi N., Jambi M., Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10:1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouanet A., Bolca S., Bru A., Claes I., Cvejic H., Girgis H., Harper A., Lavergne S.N., Mathys S., Pane M. Live biotherapeutic products, a road map for safety assessment. Front. Med. 2020:237. doi: 10.3389/fmed.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvucci E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019;70:781–795. doi: 10.1080/09637486.2019.1580682. [DOI] [PubMed] [Google Scholar]
- 17.Anwar H., Irfan S., Hussain G., Faisal M.N., Muzaffar H., Mustafa I., Mukhtar I., Malik S., Ullah M.I. Gut microbiome: a new organ system in body. Parasitol Microbiol Res. 2019;1:17–21. [Google Scholar]
- 18.Feng X.-w., Ding W.-p., Xiong L.-y., Guo L., Sun J.-m., Xiao P. Recent advancements in intestinal microbiota analyses: a review for non-microbiologists. Curr Med Sci. 2018;38:949–961. doi: 10.1007/s11596-018-1969-z. [DOI] [PubMed] [Google Scholar]
- 19.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh T.P., Natraj B.H. Next-generation probiotics: a promising approach towards designing personalized medicine. Crit. Rev. Microbiol. 2021;47:479–498. doi: 10.1080/1040841X.2021.1902940. [DOI] [PubMed] [Google Scholar]
- 21.Gérard P., Lepercq P., Leclerc M., Gavini F., Raibaud P., Juste C. Bacteroides sp. strain D8, the first cholesterol-reducing bacterium isolated from human feces. Appl. Environ. Microbiol. 2007;73:5742–5749. doi: 10.1128/AEM.02806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng H., Li Z., Tan Y., Guo Z., Liu Y., Wang Y., Yuan Y., Yang R., Bi Y., Bai Y. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci. Rep. 2016;6 doi: 10.1038/srep29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasueda A., Mizushima T., Nezu R., Sumi R., Tanaka M., Nishimura J., Kai Y., Hirota M., Osawa H., Nakajima K. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg. Today. 2016;46:939–949. doi: 10.1007/s00595-015-1261-9. [DOI] [PubMed] [Google Scholar]
- 24.Li Z., Deng H., Zhou Y., Tan Y., Wang X., Han Y., Liu Y., Wang Y., Yang R., Bi Y. Bioluminescence imaging to track Bacteroides fragilis inhibition of Vibrio parahaemolyticus infection in mice, Fron. Cell Infect Microbiol. 2017;7:170. doi: 10.3389/fcimb.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., Zhu B., Xu J., Liu Y., Qiu E., Li Z., Li Z., He Y., Zhou H., Bai Y. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018;9:1040. doi: 10.3389/fimmu.2018.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boesmans L., Valles-Colomer M., Wang J., Eeckhaut V., Falony G., Ducatelle R., Van Immerseel F., Raes J., Verbeke K. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of Butyricicoccus pullicaecorum to healthy volunteers. mSystems. 2018;3:10.1128. doi: 10.1128/mSystems.00094-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamady Z.Z., Scott N., Farrar M.D., Lodge J.P.A., Holland K.T., Whitehead T., Carding S.R. Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut. 2010;59:461–469. doi: 10.1136/gut.2008.176131. [DOI] [PubMed] [Google Scholar]
- 28.Hamady Z.Z., Scott N., Farrar M.D., Wadhwa M., Dilger P., Whitehead T.R., Thorpe R., Holland K.T., Lodge P.J., Carding S.R. Treatment of colitis with a commensal gut bacterium engineered to secrete human tgf-β1 under the control of dietary xylan. Inflamm. Bowel Dis. 2011;17:1925–1935. doi: 10.1002/ibd.21565. [DOI] [PubMed] [Google Scholar]
- 29.Motta J.-P., Bermúdez-Humarán L.G., Deraison C., Martin L., Rolland C., Rousset P., Boue J., Dietrich G., Chapman K., Kharrat P. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci. Transl. Med. 2012;4:158ra144. doi: 10.1126/scitranslmed.3004212. [DOI] [PubMed] [Google Scholar]
- 30.Sjödin K.S., Vidman L., Rydén P., West C.E. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Curr. Opin. Allergy Clin. Immunol. 2016;16:390–395. doi: 10.1097/ACI.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 31.Song H., Yoo Y., Hwang J., Na Y.-C., Kim H.S. Faecalibacterium prausnitzii subspecies–level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016;137:852–860. doi: 10.1016/j.jaci.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Rossi O., Van Berkel L.A., Chain F., Tanweer Khan M., Taverne N., Sokol H., Duncan S.H., Flint H.J., Harmsen H.J., Langella P. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 2016;6 doi: 10.1038/srep18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulsemer P., Toutounian K., Kressel G., Goletz C., Schmidt J., Karsten U., Hahn A., Goletz S. Impact of oral consumption of heat-treated Bacteroides xylanisolvens DSM 23964 on the level of natural TFα-specific antibodies in human adults. Benef. Microbes. 2016;7:485–500. doi: 10.3920/BM2015.0143. [DOI] [PubMed] [Google Scholar]
- 34.Yanagibashi T., Hosono A., Oyama A., Tsuda M., Suzuki A., Hachimura S., Takahashi Y., Momose Y., Itoh K., Hirayama K. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA+ B cells. Immunobiology. 2013;218:645–651. doi: 10.1016/j.imbio.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 35.Yang J., Li Y., Wen Z., Liu W., Meng L., Huang H. Oscillospira-a candidate for the next-generation probiotics. Gut Microb. 2021;13 doi: 10.1080/19490976.2021.1987783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cani P.D., Van Hul M. Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr. Opin. Biotechnol. 2015;32:21–27. doi: 10.1016/j.copbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 37.An D., Oh S.F., Olszak T., Neves J.F., Avci F.Y., Erturk-Hasdemir D., Lu X., Zeissig S., Blumberg R.S., Kasper D.L. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauffin Cano P., Santacruz A., Moya Á., Sanz Y. 2012. Bacteroides Uniformis CECT 7771 Ameliorates Metabolic and Immunological Dysfunction in Mice with High-Fat-Diet Induced Obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumari M., Singh P., Nataraj B.H., Kokkiligadda A., Naithani H., Ali S.A., Behare P.V., Nagpal R. Fostering next-generation probiotics in human gut by targeted dietary modulation: an emerging perspective. Food Res Int150. 2021 doi: 10.1016/j.foodres.2021.110716. [DOI] [PubMed] [Google Scholar]
- 40.Khan M.T., Dwibedi C., Sundh D., Pradhan M., Kraft J.D., Caesar R., Tremaroli V., Lorentzon M., Bäckhed F. Synergy and oxygen adaptation for development of next-generation probiotics. Nature. 2023;620:381–385. doi: 10.1038/s41586-023-06378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Man Lei Y., Jabri B., Alegre M.-L. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rong Y., Dong Z., Hong Z., Jin Y., Zhang W., Zhang B., Mao W., Kong H., Wang C., Yang B. Reactivity toward Bifidobacterium longum and Enterococcus hirae demonstrate robust CD8+ T cell response and better prognosis in HBV-related hepatocellular carcinoma. Exp. Cell Res. 2017;358:352–359. doi: 10.1016/j.yexcr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Roller M., Clune Y., Collins K., Rechkemmer G., Watzl B. Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. Br. J. Nutr. 2007;97:676–684. doi: 10.1017/S0007114507450292. [DOI] [PubMed] [Google Scholar]
- 44.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.-L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaput N., Lepage P., Coutzac C., Soularue E., Le Roux K., Monot C., Boselli L., Routier E., Cassard L., Collins M. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017;28:1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 46.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M., Karpinets T., Prieto P., Vicente D., Hoffman K., Wei S.C. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimbo I., Yamaguchi T., Odaka T., Nakajima K., Koide A., Koyama H., Saisho H. Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J. Gastroenterol. 2005;11:7520. doi: 10.3748/wjg.v11.i47.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinnoh M., Horinaka M., Yasuda T., Yoshikawa S., Morita M., Yamada T., Miki T., Sakai T. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int. J. Oncol. 2013;42:903–911. doi: 10.3892/ijo.2013.1790. [DOI] [PubMed] [Google Scholar]
- 49.Tian Y., Li M., Song W., Jiang R., Li Y.Q. Effects of probiotics on chemotherapy in patients with lung cancer. Oncol. Lett. 2019;17:2836–2848. doi: 10.3892/ol.2019.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanauchi O., Fukuda M., Matsumoto Y., Ishii S., Ozawa T., Shimizu M., Mitsuyama K., Andoh A. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J. Gastroenterol.: WJG. 2006;12:1071. doi: 10.3748/wjg.v12.i7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Udayappan S., Manneras-Holm L., Chaplin-Scott A., Belzer C., Herrema H., Dallinga-Thie G.M., Duncan S.H., Stroes E.S., Groen A.K., Flint H.J. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. Npj Biofilms Microbi. 2016;2:1–10. doi: 10.1038/npjbiofilms.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Routy B., Le Chatelier E., Derosa L., Duong C.P., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 53.Daillère R., Vétizou M., Waldschmitt N., Yamazaki T., Isnard C., Poirier-Colame V., Duong C.P., Flament C., Lepage P., Roberti M.P. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Fei Y., Chen Z., Han S., Zhang S., Zhang T., Lu Y., Berglund B., Xiao H., Li L., Yao M. Role of prebiotics in enhancing the function of next-generation probiotics in gut microbiota. Crit Rev Food Sci. 2023;63:1037–1054. doi: 10.1080/10408398.2021.1958744. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg E., Rosenberg E. 2021. Microbiomes in Medicine and Agriculture, Microbiomes: Current Knowledge and Unanswered Questions; pp. 353–412. [Google Scholar]
- 56.Khan M.T., van Dijl J.M., Harmsen H.J. Antioxidants keep the potentially probiotic but highly oxygen-sensitive human gut bacterium Faecalibacterium prausnitzii alive at ambient air. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maathuis A.J., van den Heuvel E.G., Schoterman M.H., Venema K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a 13C-labeling technique. J. Nutr. 2012;142:1205–1212. doi: 10.3945/jn.111.157420. [DOI] [PubMed] [Google Scholar]
- 58.Yao C., Muir J., Gibson P. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016;43:181–196. doi: 10.1111/apt.13456. [DOI] [PubMed] [Google Scholar]
- 59.Bashir M., Prietl B., Tauschmann M., Mautner S.I., Kump P.K., Treiber G., Wurm P., Gorkiewicz G., Högenauer C., Pieber T.R. Effects of high doses of vitamin D 3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eu J Nutr. 2016;55:1479–1489. doi: 10.1007/s00394-015-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai L., Mafra D., Shiels P.G., Hackeng T.M., Stenvinkel P., Schurgers L.J. Vitamin K and hallmarks of ageing: focus on diet and gut microbiome. Nutrients. 2023;15:2727. doi: 10.3390/nu15122727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aravind S.M., Wichienchot S., Tsao R., Ramakrishnan S., Chakkaravarthi S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021;142 doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- 62.Chakkaravarthi S., Aravind S. In: Gomes da Cruz A., Ranadheera C.S., Nazzaro F., Mortazavian A., editors. Vol. 62. Academic Press; 2021. pp. 219–232. (Probiotics and Prebiotics in Foods). [Google Scholar]
- 63.Tomás‐Barberán F.A., González‐Sarrías A., García‐Villalba R., Núñez‐Sánchez M.A., Selma M.V., García‐Conesa M.T., Espín J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201500901. [DOI] [PubMed] [Google Scholar]
- 64.Al Azzaz J., Al Tarraf A., Heumann A., Da Silva Barreira D., Laurent J., Assifaoui A., Rieu A., Guzzo J., Lapaquette P. Resveratrol favors adhesion and biofilm formation of Lacticaseibacillus paracasei subsp. paracasei Strain ATCC334. Int. J. Mol. Sci. 2020;21:5423. doi: 10.3390/ijms21155423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Q., Liang Q., Balakrishnan B., Belobrajdic D.P., Feng Q.-J., Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12:381. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bielik V., Kolisek M. Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome. Int. J. Mol. Sci. 2021;22:6803. doi: 10.3390/ijms22136803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ducarmon Q., Zwittink R., Hornung B., Van Schaik W., Young V., Kuijper E. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 2019;83:10. doi: 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 69.Manor O., Dai C.L., Kornilov S.A., Smith B., Price N.D., Lovejoy J.C., Gibbons S.M., Magis A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020;11:5206. doi: 10.1038/s41467-020-18871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajilić‐Stojanović M., Smidt H., De Vos W.M. Diversity of the human gastrointestinal tract microbiota revisited. Environ. Microbiol. 2007;9:2125–2136. doi: 10.1111/j.1462-2920.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- 71.O'Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 73.De Filippis F., Vitaglione P., Cuomo R., Berni Canani R., Ercolini D. Dietary interventions to modulate the gut microbiome—how far away are we from precision medicine. Inflamm. Bowel Dis. 2018;24:2142–2154. doi: 10.1093/ibd/izy080. [DOI] [PubMed] [Google Scholar]
- 74.Ottman N., Smidt H., De Vos W.M., Belzer C. The function of our microbiota: who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012:104. doi: 10.3389/fcimb.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hartstra A.V., Bouter K.E., Bäckhed F., Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 76.Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ecklu-Mensah G., Gilbert J., Devkota S. Dietary selection pressures and their impact on the gut microbiome. Cell. Mol. Gastroenterol. Hepatol. 2022;13:7–18. doi: 10.1016/j.jcmgh.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Hage R., Hernandez-Sanabria E., Van de Wiele T. Emerging trends in “smart probiotics”: functional consideration for the development of novel health and industrial applications. Front. Microbiol. 2017;8:1889. doi: 10.3389/fmicb.2017.01889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belzer C., De Vos W.M. Microbes inside—from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Derrien M., Belzer C., de Vos W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Schneeberger M., Everard A., Gómez-Valadés A.G., Matamoros S., Ramírez S., Delzenne N.M., Gomis R., Claret M., Cani P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015;5 doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plöger S., Stumpff F., Penner G.B., Schulzke J.D., Gäbel G., Martens H., Shen Z., Günzel D., Aschenbach J.R. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann. NY Acad. Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 83.Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., Ze X., Brown D., Stares M.D., Scott P., Bergerat A. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopez-Siles M., Khan T.M., Duncan S.H., Harmsen H.J., Garcia-Gil L.J., Flint H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012;78:420–428. doi: 10.1128/AEM.06858-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duncan S.H., Louis P., Flint H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hiippala K., Kainulainen V., Suutarinen M., Heini T., Bowers J.R., Jasso-Selles D., Lemmer D., Valentine M., Barnes R., Engelthaler D.M. Isolation of anti-inflammatory and epithelium reinforcing Bacteroides and Parabacteroides spp. from a healthy fecal donor. Nutrients. 2020;12:935. doi: 10.3390/nu12040935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo P., Zhang K., Ma X., He P. Clostridium species as probiotics: potentials and challenges. J. Anim. Sci. Biotechnol. 2020;11:1–10. doi: 10.1186/s40104-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colliou N., Ge Y., Sahay B., Gong M., Zadeh M., Owen J.L., Neu J., Farmerie W.G., Alonzo F., Liu K. Commensal Propionibacterium strain UF1 mitigates intestinal inflammation via Th17 cell regulation. J. Clin. Invest. 2017;127:3970–3986. doi: 10.1172/JCI95376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O'Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 90.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 91.Celiberto L.S., Graef F.A., Healey G.R., Bosman E.S., Jacobson K., Sly L.M., Vallance B.A. Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology. 2018;155:36–52. doi: 10.1111/imm.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Broeckx G., Vandenheuvel D., Claes I.J., Lebeer S., Kiekens F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm. 2016;505:303–318. doi: 10.1016/j.ijpharm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Rajam R., Subramanian P. Encapsulation of probiotics: past, present and future. Beni-Suef Univ. J. Basic Appl. Sci. 2022;11:1–18. [Google Scholar]
- 94.Zuidam N.J., Shimoni E. In: Encapsulation Technologies for Active Food Ingredients and Food Processing. Zuidam N., Nedovic V., editors. 2010. Overview of microencapsulates for use in food products or processes and methods to make them; pp. 3–29. [Google Scholar]
- 95.Abbas M.S., Saeed F., Afzaal M., Jianfeng L., Hussain M., Ikram A., Jabeen A. Recent trends in encapsulation of probiotics in dairy and beverage: a review. J. Food Process. Preserv. 2022;46 [Google Scholar]
- 96.Ouwehand A.C., Salminen S.J. The health effects of cultured milk products with viable and non-viable bacteria. Int. Dairy J. 1998;8:749–758. [Google Scholar]
- 97.Lahtinen S.J., Gueimonde M., Ouwehand A.C., Reinikainen J.P., Salminen S.J. Probiotic bacteria may become dormant during storage. Appl. Environ. Microbiol. 2005;71:1662–1663. doi: 10.1128/AEM.71.3.1662-1663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rokka S., Rantamäki P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur. Food Res. Technol. 2010;231:1–12. [Google Scholar]
- 99.Arslan S., Erbas M., Tontul I., Topuz A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT-Food Sci. Technol. 2015;63:685–690. [Google Scholar]
- 100.Peighambardoust S., Tafti A.G., Hesari J. Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food. Sci. Tech. 2011;22:215–224. [Google Scholar]
- 101.Riveros B., Ferrer J., Borquez R. Spray drying of a vaginal probiotic strain of Lactobacillus acidophilus. Dry. Technol. 2009;27:123–132. [Google Scholar]
- 102.Anandharamakrishnan C., Gimbun J., Stapley A., Rielly C.D. Application of computational fluid dynamics (CFD) simulations to spray-freezing operations. Dry. Technol. 2009;28:94–102. [Google Scholar]
- 103.Huang S., Vignolles M.-L., Chen X.D., Le Loir Y., Jan G., Schuck P., Jeantet R. Spray drying of probiotics and other food-grade bacteria: a review. Trends Food Sci. 2017;63:1–17. [Google Scholar]
- 104.Xu Y., Dong M., Xiao H., Young Quek S., Ogawa Y., Ma G., Zhang C. Advances in spray-dried probiotic microcapsules for targeted delivery: a review. Crit. Rev. Food Sci. Nutr. 2023:1–17. doi: 10.1080/10408398.2023.2235424. [DOI] [PubMed] [Google Scholar]
- 105.Anandharamakrishnan C., Rielly C.D., Stapley A.G. Loss of solubility of α-lactalbumin and β-lactoglobulin during the spray drying of whey proteins. LWT-Food Sci. Technol. 2008;41:270–277. [Google Scholar]
- 106.Heinzen C. Workshop. vol. 53. 2002. Microencapsulation by prilling and coextrusion; pp. 26–28. [Google Scholar]
- 107.Eckert C., Agnol W.D., Dallé D., Serpa V.G., Maciel M.J., Lehn D.N., de Souza C.F.V. Development of alginate-pectin microparticles with dairy whey using vibration technology: effects of matrix composition on the protection of Lactobacillus spp. from adverse conditions. Food Res. Int. 2018;113:65–73. doi: 10.1016/j.foodres.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 108.Reis C.P., Neufeld R.J., Vilela S., Ribeiro A.J., Veiga F. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J. Microencapsul. 2006;23:245–257. doi: 10.1080/02652040500286086. [DOI] [PubMed] [Google Scholar]
- 109.Krasaekoopt W., Bhandari B., Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003;13:3–13. [Google Scholar]
- 110.Burgain J., Gaiani C., Linder M., Scher J. Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J. Food Eng. 2011;104:467–483. [Google Scholar]
- 111.Ashwar B.A., Gani A., Gani A., Shah A., Masoodi F.A. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018;239:287–294. doi: 10.1016/j.foodchem.2017.06.110. [DOI] [PubMed] [Google Scholar]
- 112.Wu X., Wang C., Guo Y. Effects of the high-pulsed electric field pretreatment on the mechanical properties of fruits and vegetables. J. Food Eng. 2020;274 [Google Scholar]
- 113.Anukiruthika T., Moses J., Anandharamakrishnan C. Electrohydrodynamic drying of foods: principle, applications, and prospects. J. Food Eng. 2021;295 [Google Scholar]
- 114.Haffner F.B., Diab R., Pasc A. Encapsulation of probiotics: insights into academic and industrial approaches. AIMS Mater. Sci. 2016;3:114–136. [Google Scholar]
- 115.Tanhaei A., Mohammadi M., Hamishehkar H., Hamblin M.R. Electrospraying as a novel method of particle engineering for drug delivery vehicles. J. Control. Release. 2021;330:851–865. doi: 10.1016/j.jconrel.2020.10.059. [DOI] [PubMed] [Google Scholar]
- 116.Premjit Y., Mitra J. Optimization of electrospray-assisted microencapsulation of probiotics (Leuconostoc lactis) in soy protein isolate-oil particles using Box-Behnken experimental design. Food Bioprocess Technol. 2021;14:1712–1729. [Google Scholar]
- 117.Wang P., Ding M., Zhang T., Wu T., Qiao R., Zhang F., Wang X., Zhong J. Electrospraying technique and its recent application advances for biological macromolecule encapsulation of food bioactive substances. Food Rev. Int. 2022;38:566–588. [Google Scholar]
- 118.Zaeim D., Sarabi-Jamab M., Ghorani B., Kadkhodaee R., Tromp R.H. Electrospray-assisted drying of live probiotics in acacia gum microparticles matrix. Carbohydr. Polym. 2018;183:183–191. doi: 10.1016/j.carbpol.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 119.Geankoplis C. Prentice Hall Press; 2003. Transport Processes and Separation Process Principles (Includes Unit Operations) [Google Scholar]
- 120.Gaidhani K.A., Harwalkar M., Bhambere D., Nirgude P.S. Lyophilization/freeze drying–a review. World J. Pharm. Res. 2015;4:516–543. [Google Scholar]
- 121.Kiepś J., Dembczyński R. Current trends in the production of probiotic formulations. Foods. 2022;11:2330. doi: 10.3390/foods11152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marcial-Coba M.S., Cieplak T., Cahú T.B., Blennow A., Knøchel S., Nielsen D.S. Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct. 2018;9:5868–5879. doi: 10.1039/c8fo01331d. [DOI] [PubMed] [Google Scholar]
- 123.Chávez B., Ledeboer A. Drying of probiotics: optimization of formulation and process to enhance storage survival. Dry. Technol. 2007;25:1193–1201. [Google Scholar]
- 124.Ray S., Raychaudhuri U., Chakraborty R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016;13:76–83. [Google Scholar]
- 125.Krasaekoopt W., Bhandari B. In: Technologies and Delivery Systems for Food Ingredients and Nutraceuticals. Garti N., McClements D.J., editors. Elsevier; 2012. Properties and applications of different probiotic delivery systems; pp. 541–594. [Google Scholar]
- 126.Ying D., Schwander S., Weerakkody R., Sanguansri L., Gantenbein-Demarchi C., Augustin M.A. Microencapsulated Lactobacillus rhamnosus GG in whey protein and resistant starch matrices: probiotic survival in fruit juice. J. Funct.Foods. 2013;5:98–105. [Google Scholar]
- 127.Homayouni A., Azizi A., Ehsani M., Yarmand M., Razavi S. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008;111:50–55. [Google Scholar]
- 128.González‐Sánchez F., Azaola A., Gutiérrez‐López G.F., Hernández‐Sánchez H. Viability of microencapsulated Bifidobacterium animalis ssp. lactis BB12 in kefir during refrigerated storage. Int. J. Dairy Technol. 2010;63:431–436. [Google Scholar]
- 129.Ribeiro M.C.E., Chaves K.S., Gebara C., Infante F.N., Grosso C.R., Gigante M.L. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res. Int. 2014;66:424–431. [Google Scholar]
- 130.Mousa A., Liu X.m., Chen Y.q., Zhang H., Chen W. Evaluation of physiochemical, textural, microbiological and sensory characteristics in set yogurt reinforced by microencapsulated Bifidobacterium bifidum F‐35. Int J Food Sci. 2014;49:1673–1679. [Google Scholar]
- 131.Petreska-Ivanovska T., Petrushevska-Tozi L., Grozdanov A., Petkovska R., Hadjieva J., Popovski E., Stafilov T., Mladenovska K. From optimization of synbiotic microparticles prepared by spray-drying to development of new functional carrot juice. Chem. Ind. Chem. Eng. Q. 2014;20:549–564. [Google Scholar]
- 132.Bhat A., Irorere V., Bartlett T., Hill D., Kedia G., Charalampopoulos D., Nualkaekul S., Radecka I. Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int. J. Food Microbiol. 2015;196:24–31. doi: 10.1016/j.ijfoodmicro.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 133.Praepanitchai O.-A., Noomhorm A., Anal A.K. Survival and behavior of encapsulated probiotics (Lactobacillus plantarum) in calcium-alginate-soy protein isolate-based hydrogel beads in different processing conditions (pH and temperature) and in pasteurized mango juice. BioMed Res. Int. 2019;13(2019) doi: 10.1155/2019/9768152. 9768152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Simone C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019;17:809–817. doi: 10.1016/j.cgh.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 135.Kolacek S., Hojsak I., Berni Canani R., Guarino A., Indrio F., Orel R., Pot B., Shamir R., Szajewska H., Vandenplas Y., a f.P. Commercial probiotic products: a call for improved quality control. A position paper by the espghan working group for probiotics and prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017;65:117–124. doi: 10.1097/MPG.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 136.Parliament E. E Parliament. 2006. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods; p. 1924. 2006. [Google Scholar]
- 137.Arora M., Baldi A. Regulatory categories of probiotics across the globe: a review representing existing and recommended categorization. Indian J. Med. Microbiol. 2015;33:S2–S10. doi: 10.4103/0255-0857.150868. [DOI] [PubMed] [Google Scholar]
- 138.da Cruz A.G., Ranadheera C.S., Nazzaro F., Mortazavian A. Academic Press; 2021. Probiotics and prebiotics in foods: challenges, innovations, and advances; p. 23. 2021. [Google Scholar]
- 139.O’Toole P., Marchesi J., Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2(5) doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 140.Zhang H., Duan Y., Cai F., Cao D., Wang L., Qiao Z., Hong Q., Li N., Zheng Y., Su M. Next-generation probiotics: microflora intervention to human diseases. BioMed Res. Int. 2022;16:5633403. doi: 10.1155/2022/5633403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aravind S.M., Chakkaravarthi S. In: Vitro Functionality of Probiotics in Foods. Mortazavian A.M., Da Cruz A.G., Khorshidian N., editors. Nova Medicine and Health; 2021. Safety assessment of probiotics for use in food products; pp. 219–246. [Google Scholar]
- 142.Efsa Panel on Dietetic Products, Allergies N., Turck D., Bresson J.L., Burlingame B., Dean T., Fairweather‐Tait S., Heinonen M., Hirsch‐Ernst K.I., Mangelsdorf I., McArdle H. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016;14 [Google Scholar]
- 143.Bajinka O., Darboe A., Tan Y., Abdelhalim K.A., Cham L.B. Gut microbiota and the human gut physiological changes. Ann. Microbiol. 2020;70:1–9. [Google Scholar]
- 144.Shetty S.A., Hugenholtz F., Lahti L., Smidt H., de Vos W.M. Intestinal microbiome landscaping: insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol. Rev. 2017;41:182–199. doi: 10.1093/femsre/fuw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Plaza-Díaz J., Solís-Urra P., Rodríguez-Rodríguez F., Olivares-Arancibia J., Navarro-Oliveros M., Abadía-Molina F., Álvarez-Mercado A.I. The gut barrier, intestinal microbiota, and liver disease: molecular mechanisms and strategies to manage. Int. J. Mol. Sci. 2020;21:8351. doi: 10.3390/ijms21218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hui W., Li T., Liu W., Zhou C., Gao F. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: an updated randomized controlled trial meta-analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: a review. Anton. Leeuw. Int. J. G. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 148.Neef A., Sanz Y. Future for probiotic science in functional food and dietary supplement development. Curr. Opin. Clin. Nutr. 2013;16:679–687. doi: 10.1097/MCO.0b013e328365c258. [DOI] [PubMed] [Google Scholar]
- 149.Kaistha S.D., Deshpande N. Wound Healing Research: Cur Trends Future Dir; 2021. Traditional Probiotics, Next-Generation Probiotics and Engineered Live Biotherapeutic Products in Chronic Wound Healing; pp. 247–284. [Google Scholar]
- 150.Tezel B.U., Şanlıbaba P., Akçelik N., Akçelik M. Advances in Probiotics. Elsevier; 2021. Selection criteria for identifying putative probiont; pp. 23–35. [Google Scholar]
- 151.Bellais S., Nehlich M., Ania M., Duquenoy A., Mazier W., van den Engh G., Baijer J., Treichel N.S., Clavel T., Belotserkovsky I. Species-targeted sorting and cultivation of commensal bacteria from the gut microbiome using flow cytometry under anaerobic conditions. Microbiome. 2022;10:1–17. doi: 10.1186/s40168-021-01206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vassilopoulou E., Guibas G.V., Papadopoulos N.G. Mediterranean-type diets as a protective factor for asthma and atopy. Nutrients. 2022;14:1825. doi: 10.3390/nu14091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jan T., Negi R., Sharma B., Singh S., Kumar S., Rustagi S., Shreaz S., Rai A.K., Rai P.K., Sheikh M.A., et al. Probiotic formulations for human health: current research and future perspective. J. Appl. Biol. Biotechnol. 2024;12(2024):14–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.