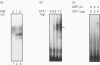Abstract
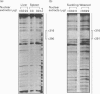
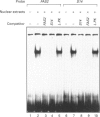
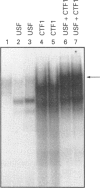
We have shown previously that fatty acid synthase (FAS) gene expression is positively regulated by glucose in rat adipose tissue and liver. In the present study, we have identified in the first intron of the gene a sequence closely related to known glucose-responsive elements such as in the L-pyruvate kinase and S14 genes, including a putative upstream stimulatory factor/major late transcription factor (USF/MLTF) binding site (E-box) (+ 292 nt to + 297 nt). Location of this sequence corresponds to a site of hypersensitivity to DNase I which is present in the liver but not in the spleen. Moreover, using this information from a preliminary report of the present work, others have shown that a + 283 nt to + 303 nt sequence of the FAS gene can confer glucose responsiveness to a heterologous promoter. The protein binding to this region has been investigated in vitro by a combination of DNase I footprinting and gel-retardation experiments with synthetic oligonucleotides and known nuclear proteins. DNase I footprinting experiments using a + 161 nt to + 405 nt fragment of the FAS gene demonstrate that a region from + 290 nt to + 316 nt is protected by nuclear extracts from liver and spleen. This region binds two ubiquitous nuclear factors, USF/MLTF and the CAAT-binding transcription factor/nuclear factor 1 (CTF/NF1). Binding of these factors is similar in nuclear extracts from liver which does or does not express the FAS gene as observed for glucose-responsive elements in the L-pyruvate kinase and S14 genes. This suggests a posttranslational modification of a factor of the complex after glucose stimulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy C. M., Williams-Ahlf B., Naggert J., Smith S. Molecular cloning of the mammalian fatty acid synthase gene and identification of the promoter region. Biochem J. 1990 Nov 1;271(3):675–679. doi: 10.1042/bj2710675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentero M. T., Horwitz M., Mermod N. Targeting of DNA polymerase to the adenovirus origin of DNA replication by interaction with nuclear factor I. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11537–11541. doi: 10.1073/pnas.91.24.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K. F., Schreglmann R., Stathopulos I., Klein H., Hoch J., Schweizer M. The fatty acid synthase (FAS) gene and its promoter in Rattus norvegicus. DNA Seq. 1992;2(6):359–386. doi: 10.3109/10425179209020817. [DOI] [PubMed] [Google Scholar]
- Bergot M. O., Diaz-Guerra M. J., Puzenat N., Raymondjean M., Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992 Apr 25;20(8):1871–1877. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun T., Roche E., Kim K. H., Prentki M. Glucose regulates acetyl-CoA carboxylase gene expression in a pancreatic beta-cell line (INS-1). J Biol Chem. 1993 Sep 5;268(25):18905–18911. [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foufelle F., Gouhot B., Perdereau D., Girard J., Ferre P. Regulation of lipogenic enzyme and phosphoenolpyruvate carboxykinase gene expression in cultured white adipose tissue. Glucose and insulin effects are antagonized by cAMP. Eur J Biochem. 1994 Aug 1;223(3):893–900. doi: 10.1111/j.1432-1033.1994.tb19066.x. [DOI] [PubMed] [Google Scholar]
- Foufelle F., Gouhot B., Pégorier J. P., Perdereau D., Girard J., Ferré P. Glucose stimulation of lipogenic enzyme gene expression in cultured white adipose tissue. A role for glucose 6-phosphate. J Biol Chem. 1992 Oct 15;267(29):20543–20546. [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Liu Z., Thompson K. S., Towle H. C. Carbohydrate regulation of the rat L-type pyruvate kinase gene requires two nuclear factors: LF-A1 and a member of the c-myc family. J Biol Chem. 1993 Jun 15;268(17):12787–12795. [PubMed] [Google Scholar]
- MacDougald O. A., Jump D. B. Identification of functional cis-acting elements within the rat liver S14 promoter. Biochem J. 1991 Dec 15;280(Pt 3):761–767. doi: 10.1042/bj2800761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie S., Diaz-Guerra M. J., Miquerol L., Kahn A., Iynedjian P. B. The pyruvate kinase gene as a model for studies of glucose-dependent regulation of gene expression in the endocrine pancreatic beta-cell type. J Biol Chem. 1993 Nov 15;268(32):23881–23890. [PubMed] [Google Scholar]
- Moustaïd N., Sakamoto K., Clarke S., Beyer R. S., Sul H. S. Regulation of fatty acid synthase gene transcription. Sequences that confer a positive insulin effect and differentiation-dependent expression in 3T3-L1 preadipocytes are present in the 332 bp promoter. Biochem J. 1993 Jun 15;292(Pt 3):767–772. doi: 10.1042/bj2920767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder K., Klein H., Kranz H., Beck K. F., Schweizer M. The tripartite DNA element responsible for diet-induced rat fatty acid synthase (FAS) regulation. Gene. 1994 Jul 8;144(2):189–195. doi: 10.1016/0378-1119(94)90377-8. [DOI] [PubMed] [Google Scholar]
- Shih H. M., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem. 1992 Jul 5;267(19):13222–13228. [PubMed] [Google Scholar]
- Shih H., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Context of the CACGTG motif determines the specificity of carbohydrate regulation. J Biol Chem. 1994 Mar 25;269(12):9380–9387. [PubMed] [Google Scholar]
- Sudo Y., Mariash C. N. Two glucose-signaling pathways in S14 gene transcription in primary hepatocytes: a common role of protein phosphorylation. Endocrinology. 1994 Jun;134(6):2532–2540. doi: 10.1210/endo.134.6.8194479. [DOI] [PubMed] [Google Scholar]
- Vaulont S., Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994 Jan;8(1):28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- Vaulont S., Puzenat N., Levrat F., Cognet M., Kahn A., Raymondjean M. Proteins binding to the liver-specific pyruvate kinase gene promoter. A unique combination of known factors. J Mol Biol. 1989 Sep 20;209(2):205–219. doi: 10.1016/0022-2836(89)90273-8. [DOI] [PubMed] [Google Scholar]