Abstract
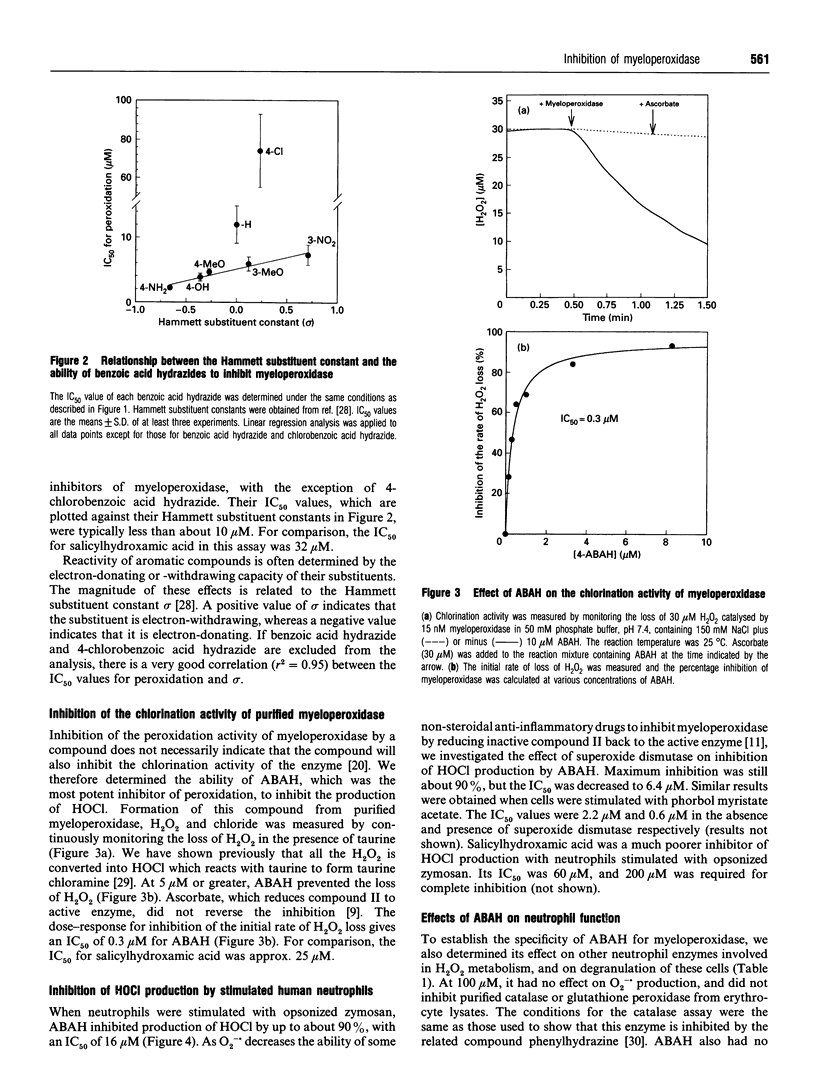
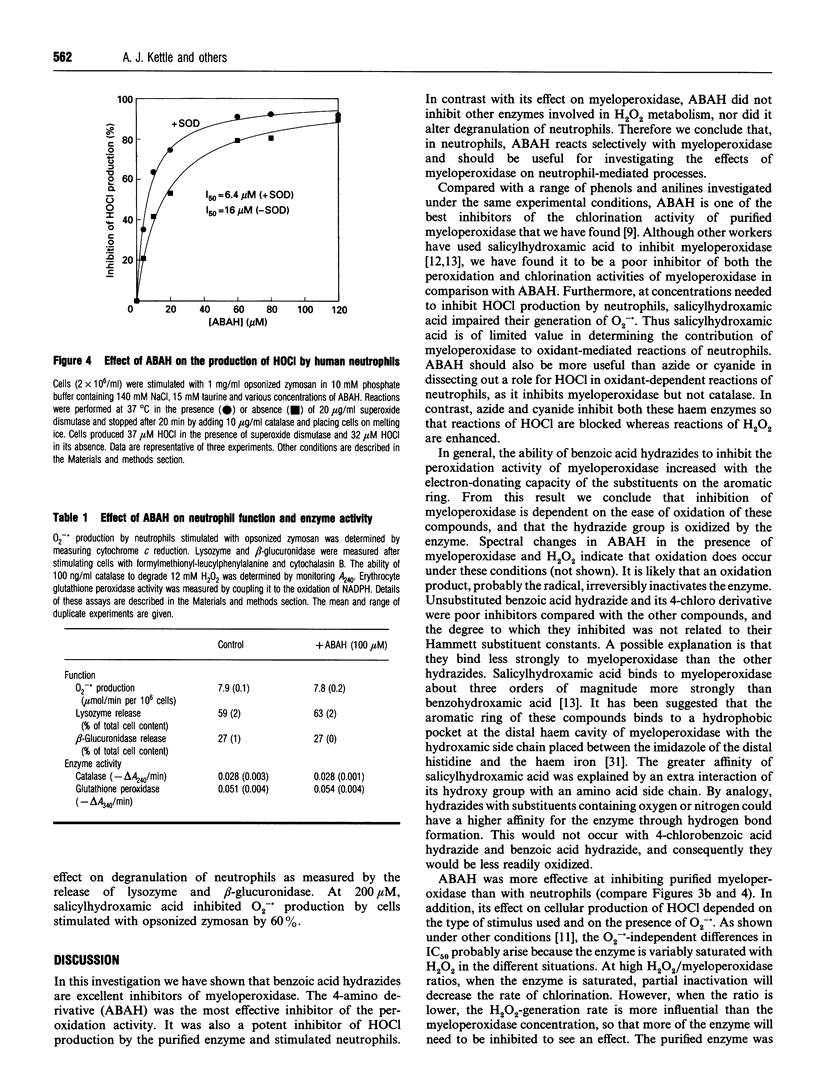
Myeloperoxidase is the most abundant protein in neutrophils and catalyses the conversion of H2O2 and chloride into HOCl. To help clarify the role of this enzyme in bacterial killing and inflammation, a specific and potent inhibitor needs to be identified. We have studied a series of benzoic acid hydrazides and found that in general they inhibit the peroxidation activity of myeloperoxidase with an IC50 value of less than 10 microM. The IC50 values of derivatives with substituents containing oxygen or nitrogen were related to their Hammett substituent constants. This indicates that myeloperoxidase oxidizes the hydrazide group of these compounds, and the degree to which they inhibit the enzyme is dependent on the ease of their oxidation. Unsubstituted benzoic acid hydrazide and its 4-chloro derivative were poor inhibitors of peroxidation. Thus it is likely that hydrogen-bonding of the enzyme to substituents containing oxygen or nitrogen increases the binding affinity of the hydrazides and enhances their oxidation by myeloperoxidase. 4-Aminobenzoic acid hydrazide (ABAH) was the most potent inhibitor of peroxidation. It irreversibly inhibited HOCl production by the purified enzyme, having an IC50 value of 0.3 microM. With neutrophils stimulated with opsonized zymosan or phorbol myristate acetate, ABAH inhibited HOCl production by up to 90% and the IC50 values were 16 microM and 2.2 microM respectively. In the presence of superoxide dismutase, these values decreased to 6.4 microM and 0.6 microM respectively. ABAH had no effect on superoxide radical (O2-.) production and degranulation by neutrophils, nor did it inhibit catalase or glutathione peroxidase. Thus ABAH is an effective and selective inhibitor that should be useful for determining the contribution of myeloperoxidase to oxidant-mediated reactions of neutrophils.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ator M. A., David S. K., Ortiz de Montellano P. R. Structure and catalytic mechanism of horseradish peroxidase. Regiospecific meso alkylation of the prosthetic heme group by alkylhydrazines. J Biol Chem. 1987 Nov 5;262(31):14954–14960. [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Davies B., Edwards S. W. Inhibition of myeloperoxidase by salicylhydroxamic acid. Biochem J. 1989 Mar 15;258(3):801–806. doi: 10.1042/bj2580801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Kettle A. J., Fatur D. J. Mechanism of the inhibition of catalase by ascorbate. Roles of active oxygen species, copper and semidehydroascorbate. J Biol Chem. 1986 Jan 25;261(3):1193–1200. [PubMed] [Google Scholar]
- Foote C. S., Goyne T. E., Lehrer R. I. Assessment of chlorination by human neutrophils. Nature. 1983 Feb 24;301(5902):715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Hori H., Fenna R. E., Kimura S., Ikeda-Saito M. Aromatic substrate molecules bind at the distal heme pocket of myeloperoxidase. J Biol Chem. 1994 Mar 18;269(11):8388–8392. [PubMed] [Google Scholar]
- Ikeda-Saito M., Shelley D. A., Lu L., Booth K. S., Caughey W. S., Kimura S. Salicylhydroxamic acid inhibits myeloperoxidase activity. J Biol Chem. 1991 Feb 25;266(6):3611–3616. [PubMed] [Google Scholar]
- Kettle A. J., Gedye C. A., Winterbourn C. C. Superoxide is an antagonist of antiinflammatory drugs that inhibit hypochlorous acid production by myeloperoxidase. Biochem Pharmacol. 1993 May 25;45(10):2003–2010. doi: 10.1016/0006-2952(93)90010-t. [DOI] [PubMed] [Google Scholar]
- Kettle A. J., Winterbourn C. C. Assays for the chlorination activity of myeloperoxidase. Methods Enzymol. 1994;233:502–512. doi: 10.1016/s0076-6879(94)33056-5. [DOI] [PubMed] [Google Scholar]
- Kettle A. J., Winterbourn C. C. Influence of superoxide on myeloperoxidase kinetics measured with a hydrogen peroxide electrode. Biochem J. 1989 Nov 1;263(3):823–828. doi: 10.1042/bj2630823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettle A. J., Winterbourn C. C. Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem Pharmacol. 1991 May 15;41(10):1485–1492. doi: 10.1016/0006-2952(91)90565-m. [DOI] [PubMed] [Google Scholar]
- Kettle A. J., Winterbourn C. C. Superoxide modulates the activity of myeloperoxidase and optimizes the production of hypochlorous acid. Biochem J. 1988 Jun 1;252(2):529–536. doi: 10.1042/bj2520529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettle A. J., Winterbourn C. C. Superoxide-dependent hydroxylation by myeloperoxidase. J Biol Chem. 1994 Jun 24;269(25):17146–17151. [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloperoxidase of the leukocyte of normal blood. I. Reaction of myeloperoxidase with hydrogen peroxide. Biochim Biophys Acta. 1970 Apr 22;206(1):71–77. doi: 10.1016/0005-2744(70)90083-5. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kerr D. E. Inactivation of catalase by phenylhydrazine. Formation of a stable aryl-iron heme complex. J Biol Chem. 1983 Sep 10;258(17):10558–10563. [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- Shacter E., Lopez R. L., Pati S. Inhibition of the myeloperoxidase-H2O2-Cl- system of neutrophils by indomethacin and other non-steroidal anti-inflammatory drugs. 1991 Mar 15-Apr 1Biochem Pharmacol. 41(6-7):975–984. doi: 10.1016/0006-2952(91)90204-i. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., van den Berg J. J., Roitman E., Kuypers F. A. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch Biochem Biophys. 1992 Aug 1;296(2):547–555. doi: 10.1016/0003-9861(92)90609-z. [DOI] [PubMed] [Google Scholar]
- van Zyl J. M., Basson K., Uebel R. A., van der Walt B. J. Isoniazid-mediated irreversible inhibition of the myeloperoxidase antimicrobial system of the human neutrophil and the effect of thyronines. Biochem Pharmacol. 1989 Jul 15;38(14):2363–2373. doi: 10.1016/0006-2952(89)90477-2. [DOI] [PubMed] [Google Scholar]


