Abstract
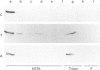
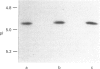
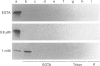
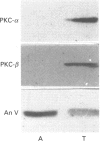
Annexins are a family of calcium-binding proteins that have been implicated in a wide range of intracellular processes. We have previously reported that stimulation of platelets with thrombin can induce the association of intracellular annexin V with membranes in two distinct ways. First, in such a way that it can be eluted from the membrane with EGTA and secondly in a manner such that it is tightly bound to the membrane and requires the non-ionic detergent Triton X-100 for its solubilization. We report that exposure of platelets to the calcium ionophore A23187 mimics the relocation induced by stimulation with thrombin. In separate experiments we demonstrate that a calcium ion concentration [Ca2+] of 0.8 microM is sufficient for maximum binding of the EGTA-resistant form to membranes. In contrast a higher [Ca2+] was required to induce maximal binding of the annexin V which could be extracted with EGTA. We demonstrate that following temperature-induced phase separation in Triton X-114, the membrane-associated annexin V partitions predominantly into the aqueous phase. We also show that the isoelectric point of annexin V does not change following membrane association. These observations suggest that a covalent modification, of annexin V itself, is not responsible for its association with the membrane. Millimolar [Ca2+] is required for maximal binding of purified annexin V to phospholipid vesicles. We show that binding to phospholipids can be reversed entirely by subsequent treatment with EGTA. This suggests that the EGTA-resistant form of annexin V is binding to a membrane component other than phosphatidylserine. Annexin V has previously been shown to bind to protein kinase C. Relocation of annexin V to membranes paralleled that of protein kinase C in thrombin-stimulated cells but not in cells treated with A23187, suggesting that these proteins are not functionally linked in platelet activation. Using bifunctional cross-linking reagents we have identified an 85 kDa complex containing annexin V. This may represent an association between annexin V and an annexin V-binding protein with a molecular mass of approximately 50 kDa.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Henderson P. A., Burns D., Loomis C., Fisher G. J. Translocation of protein kinase C isozymes in thrombin-stimulated human platelets. Correlation with 1,2-diacylglycerol levels. J Biol Chem. 1992 Aug 5;267(22):15585–15590. [PubMed] [Google Scholar]
- Bewley M. C., Boustead C. M., Walker J. H., Waller D. A., Huber R. Structure of chicken annexin V at 2.25-A resolution. Biochemistry. 1993 Apr 20;32(15):3923–3929. doi: 10.1021/bi00066a011. [DOI] [PubMed] [Google Scholar]
- Bianchi R., Garbuglia M., Verzini M., Giambanco I., Donato R. Calpactin I binds to the glial fibrillary acidic protein (GFAP) and cosediments with glial filaments in a Ca(2+)-dependent manner: implications for concerted regulatory effects of calpactin I and S100 protein on glial filaments. Biochim Biophys Acta. 1994 Sep 29;1223(3):361–367. doi: 10.1016/0167-4889(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Bianchi R., Giambanco I., Ceccarelli P., Pula G., Donato R. Membrane-bound annexin V isoforms (CaBP33 and CaBP37) and annexin VI in bovine tissues behave like integral membrane proteins. FEBS Lett. 1992 Jan 20;296(2):158–162. doi: 10.1016/0014-5793(92)80369-r. [DOI] [PubMed] [Google Scholar]
- Bianchi R., Pula G., Ceccarelli P., Giambanco I., Donato R. S-100 protein binds to annexin II and p11, the heavy and light chains of calpactin I. Biochim Biophys Acta. 1992 Nov 10;1160(1):67–75. doi: 10.1016/0167-4838(92)90039-g. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Boustead C. M., Brown R., Walker J. H. Isolation, characterization and localization of annexin V from chicken liver. Biochem J. 1993 Apr 15;291(Pt 2):601–608. doi: 10.1042/bj2910601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Clague M. J. Annexins in the endocytic pathway. Trends Biochem Sci. 1994 Jun;19(6):231–232. doi: 10.1016/0968-0004(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Crompton M. R., Moss S. E., Crumpton M. J. Diversity in the lipocortin/calpactin family. Cell. 1988 Oct 7;55(1):1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Eldering J. A., Kocher M., Clemetson J. M., Clemetson K. J., Frey F. J., Frey B. M. Presence of lipocortins I and IV, but not II and VI, in human platelets. FEBS Lett. 1993 Mar 8;318(3):231–234. doi: 10.1016/0014-5793(93)80518-y. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Feldman H., Rodbard D., Levine D. Mathematical theory of cross-reactive radioimmunoassay and ligand-binding systems of equilibrium. Anal Biochem. 1972 Feb;45(2):530–556. doi: 10.1016/0003-2697(72)90216-3. [DOI] [PubMed] [Google Scholar]
- Flaherty M. J., West S., Heimark R. L., Fujikawa K., Tait J. F. Placental anticoagulant protein-I: measurement in extracellular fluids and cells of the hemostatic system. J Lab Clin Med. 1990 Feb;115(2):174–181. [PubMed] [Google Scholar]
- Funakoshi T., Heimark R. L., Hendrickson L. E., McMullen B. A., Fujikawa K. Human placental anticoagulant protein: isolation and characterization. Biochemistry. 1987 Aug 25;26(17):5572–5578. doi: 10.1021/bi00391a053. [DOI] [PubMed] [Google Scholar]
- Giambanco I., Pula G., Bianchi R., Donato R. Interaction of two brain annexins, CaBP33 and CaBP37, with membrane-skeleton proteins. FEBS Lett. 1990 Jul 2;267(1):171–175. doi: 10.1016/0014-5793(90)80316-b. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Analytical isoelectric focusing using a high-voltage vertical slab polyacrylamide gel system. Anal Biochem. 1984 Nov 1;142(2):421–436. doi: 10.1016/0003-2697(84)90486-x. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Isacke C. M., Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Emans N. Annexins in membrane traffic. Trends Cell Biol. 1993 Jul;3(7):224–227. doi: 10.1016/0962-8924(93)90116-i. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
- Harder T., Gerke V. The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J Cell Biol. 1993 Dec;123(5):1119–1132. doi: 10.1083/jcb.123.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Berendes R., Burger A., Schneider M., Karshikov A., Luecke H., Römisch J., Paques E. Crystal and molecular structure of human annexin V after refinement. Implications for structure, membrane binding and ion channel formation of the annexin family of proteins. J Mol Biol. 1992 Feb 5;223(3):683–704. doi: 10.1016/0022-2836(92)90984-r. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Suda M., Nakao H., Nagoya T., Saino Y., Arai K., Mizoguchi T., Sato F., Yoshizaki H., Hirata M. Structure and expression of cDNA for an inhibitor of blood coagulation isolated from human placenta: a new lipocortin-like protein. J Biochem. 1987 Nov;102(5):1261–1273. doi: 10.1093/oxfordjournals.jbchem.a122165. [DOI] [PubMed] [Google Scholar]
- Kim K. M., Kim D. K., Park Y. M., Kim C. K., Na D. S. Annexin-I inhibits phospholipase A2 by specific interaction, not by substrate depletion. FEBS Lett. 1994 May 2;343(3):251–255. doi: 10.1016/0014-5793(94)80566-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees A. D., Wilson J., Orchard C. H., Orchard M. A. Ouabain enhances basal and stimulus-induced cytoplasmic calcium concentrations in platelets. Thromb Haemost. 1989 Nov 24;62(3):1000–1005. [PubMed] [Google Scholar]
- Lew D. P. Receptor signalling and intracellular calcium in neutrophil activation. Eur J Clin Invest. 1989 Aug;19(4):338–346. doi: 10.1111/j.1365-2362.1989.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Murphy C. T., Peers S. H., Forder R. A., Flower R. J., Carey F., Westwick J. Evidence for the presence and location of annexins in human platelets. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1739–1746. doi: 10.1016/0006-291x(92)90279-t. [DOI] [PubMed] [Google Scholar]
- Nakata T., Sobue K., Hirokawa N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J Cell Biol. 1990 Jan;110(1):13–25. doi: 10.1083/jcb.110.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Browning J. L., Mattaliano R. J., Smart J. E., Chow E. P., Falbel T., Ribolini A., Garwin J. L., Wallner B. P. Purification and partial sequence analysis of a 37-kDa protein that inhibits phospholipase A2 activity from rat peritoneal exudates. J Biol Chem. 1986 Mar 25;261(9):4239–4246. [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K. Epidermal growth factor-dependent phosphorylation of lipocortin. Nature. 1986 May 1;321(6065):81–84. doi: 10.1038/321081a0. [DOI] [PubMed] [Google Scholar]
- Pula G., Bianchi R., Ceccarelli P., Giambanco I., Donato R. Characterization of mammalian heart annexins with special reference to CaBP33 (annexin V). FEBS Lett. 1990 Dec 17;277(1-2):53–58. doi: 10.1016/0014-5793(90)80808-v. [DOI] [PubMed] [Google Scholar]
- Raynal P., Hullin F., Ragab-Thomas J. M., Fauvel J., Chap H. Annexin 5 as a potential regulator of annexin 1 phosphorylation by protein kinase C. In vitro inhibition compared with quantitative data on annexin distribution in human endothelial cells. Biochem J. 1993 Jun 15;292(Pt 3):759–765. doi: 10.1042/bj2920759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Römisch J., Schüler E., Bastian B., Bürger T., Dunkel F. G., Schwinn A., Hartmann A. A., Pâques E. P. Annexins I to VI: quantitative determination in different human cell types and in plasma after myocardial infarction. Blood Coagul Fibrinolysis. 1992 Feb;3(1):11–17. [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E. F., Morimoto Y. M., Matsuno T., Miyahara M., Utsumi K. Neutrophil specific 33 kDa protein: its Ca2+- and phospholipid-dependent intracellular translocation. FEBS Lett. 1987 Apr 6;214(1):181–186. doi: 10.1016/0014-5793(87)80038-8. [DOI] [PubMed] [Google Scholar]
- Sato E. F., Tanaka Y., Utsumi K. cDNA cloning and nucleotide sequence of lipocortin-like 33 kDa protein in guinea pig neutrophils. FEBS Lett. 1989 Feb 13;244(1):108–112. doi: 10.1016/0014-5793(89)81173-1. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. In vitro protein kinase C phosphorylation sites of placental lipocortin. Biochemistry. 1988 Jun 14;27(12):4253–4258. doi: 10.1021/bi00412a008. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Jones J., Haigler H. T. Inhibition of protein kinase C by annexin V. Biochemistry. 1992 Feb 18;31(6):1886–1891. doi: 10.1021/bi00121a043. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Mehlman T., Burgess W. H., Haigler H. T. Structural and functional characterization of endonexin II, a calcium- and phospholipid-binding protein. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6078–6082. doi: 10.1073/pnas.84.17.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton M. C. The platelet as a Ca(2+)-driven cell: mechanisms which may modulate Ca(2+)-driven responses. Adv Exp Med Biol. 1993;344:1–15. doi: 10.1007/978-1-4615-2994-1_1. [DOI] [PubMed] [Google Scholar]
- Smallwood M., Keen J. N., Bowles D. J. Purification and partial sequence analysis of plant annexins. Biochem J. 1990 Aug 15;270(1):157–161. doi: 10.1042/bj2700157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumitsu H., Mizutani A., Minami H., Kobayashi R., Hidaka H. A calcyclin-associated protein is a newly identified member of the Ca2+/phospholipid-binding proteins, annexin family. J Biol Chem. 1992 May 5;267(13):8919–8924. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter P. J., Orchard M. A., Walker J. H. Thrombin stimulates the intracellular relocation of annexin V in human platelets. Biochim Biophys Acta. 1994 Jun 30;1222(2):135–140. doi: 10.1016/0167-4889(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Valentine-Braun K. A., Hollenberg M. D., Fraser E., Northup J. K. Isolation of a major human placental substrate for the epidermal growth factor (urogastrone) receptor kinase: immunological cross-reactivity with transducin and sequence homology with lipocortin. Arch Biochem Biophys. 1987 Dec;259(2):262–282. doi: 10.1016/0003-9861(87)90494-2. [DOI] [PubMed] [Google Scholar]
- Walker J. H., Boustead C. M., Koster J. J., Bewley M., Waller D. A. Annexin V, a calcium-dependent phospholipid-binding protein. Biochem Soc Trans. 1992 Nov;20(4):828–833. doi: 10.1042/bst0200828. [DOI] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Johnsson N., Plessmann U., Van P. N., Söling H. D., Ampe C., Vandekerckhove J. The amino acid sequence of protein II and its phosphorylation site for protein kinase C; the domain structure Ca2+-modulated lipid binding proteins. EMBO J. 1987 Jun;6(6):1599–1604. doi: 10.1002/j.1460-2075.1987.tb02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F. Y., Gerke V., Gabius H. J. Identification of annexin II, annexin VI and glyceraldehyde-3-phosphate dehydrogenase as calcyclin-binding proteins in bovine heart. Int J Biochem. 1993 Jul;25(7):1019–1027. doi: 10.1016/0020-711x(93)90116-v. [DOI] [PubMed] [Google Scholar]