Abstract
The community adherence support group (CASG) was one of the first differentiated service delivery (DSD) models introduced in Mozambique. This study assessed the impact of this model on retention in care, loss to follow-up (LTFU), and viral suppression among antiretroviral therapy (ART)-treated adults in Mozambique. A retrospective cohort study included CASG-eligible adults enrolled between April 2012 and October 2017 at 123 health facilities in Zambézia Province. Propensity score matching (1:1 ratio) was used to allocate CASG members and those who never enrolled in a CASG. Logistic regressions were performed to estimate the impact of CASG membership on 6- and 12-month retention and viral load (VL) suppression. Cox proportional regression was used to model differences in LTFU. Data from 26,858 patients were included. The median age at CASG eligibility was 32 years and 75% were female, with 84% residing in rural areas. A total of 93% and 90% of CASG members were retained in care at 6 and 12 months, respectively, while 77% and 66% non-CASG members were retained during the same periods. The odds of being retained in care at 6 and 12 months were significantly higher among patients receiving ART through CASG support (adjusted odds ratio [aOR] = 4.19 [95% confidence interval; CI: 3.79–4.63], p < .001, and aOR = 4.43 [95% CI: 4.01–4.90], p < .001, respectively). Among 7,674 patients with available VL measurements, the odds of being virally suppressed were higher among CASG members (aOR = 1.14 [95% CI: 1.02–1.28], p < .001). Non-CASG members had a significantly higher likelihood of being LTFU (adjusted hazard ratio = 3.45 [95% CI: 3.20–3.73], p < .001). While Mozambique rapidly scales up multi-month drug dispensation as the preferred DSD model, this study emphasizes the continued importance of CASG as an efficacious DSD alternative, especially among patients residing in rural areas, where CASG acceptability is higher.
Keywords: HIV viral suppression, retention in HIV care, community adherence support group, differentiated service delivery, sub-Saharan Africa, Mozambique
Introduction
Mozambique is challenged by a high HIV burden, with 2.1 million people currently living with HIV (2020).1 The UNAIDS 95–95-95 goals include 95% of persons living with HIV (PLWH) knowing their serostatus, 95% of people who know their positive status receiving anti-retroviral therapy (ART), and 95% of ART-treated persons being virally suppressed, allowing for the control of the epidemic by 2030.2 Strategies to improve retention to care include the use of differentiated service delivery (DSD) models.
The Community Adherence Support Group (CASG) (“CAAG—Grupos de Apoio de Adesão Comunitária,” in Portuguese) was one of the first DSD models introduced in Mozambique.3 The CASG DSD is based on a community- and group-based platform of sharing knowledge, providing psychosocial support among group members and responsibility for ART pickups, while creating shared patient responsibility and involvement to promote adherence.
The national strategy recommends that CASG members (3–6 members/group) (1) are at least 15 years of age; (2) are not pregnant and/or lactating women (women already enrolled in the CASG who become pregnant are only temporarily withdrawn and may rejoin postnatally); (3) know their HIV-positive status; (4) have been on ART for at least 6 months; (5) are clinically stable with evidence of viral suppression [viral load (VL) <1,000 copies/mL] or have a CD4 cell count of ≥200 cells/mm3; and (6) do not have any active World Health Organization (WHO) clinical stage 3 or 4 conditions.
Patients who were lost from care, but re-engaged, can also be included in a group if deemed to be otherwise stable.4,5 In 2016, Mozambique adopted the Test-and-Treat strategy6 where VL monitoring became part of routine care for all patients receiving ART for more than 6 months (and for PLWH who had been on ART for ≥3 months).
The CASG model has been shown to improve retention in care rates among ART-treated adults in Tete Province, where it was initially introduced in 2011, and later expanded countrywide in Mozambique.3,7–11 Since then, DSD models have been developed and implemented to improve patient adherence and retention in care, to improve male uptake of services, and to decongest already overcrowded health facilities. By the end of 2020, nationally in Mozambique, 40% of PLWH on ART were enrolled in one of the DSD models.12
We evaluated the effect of CASG participation on retention in care, loss to follow-up (LTFU), and viral suppression using a matched cohort study in Zambézia Province, a rural and populous province where an evaluation of the impact of CASGs among large numbers of ART-treated PLWH had not yet been performed.
Materials and Methods
Study setting
For this study, data were used from patients receiving care in Zambézia Province, a rural region of Mozambique with a population of ~5.5 million.13 It is one of the poorest provinces of the country and has one of the highest HIV prevalence rates in the country [15.1% among reproductive-aged adults (15–59 years of age)].14 Friends in Global Health (FGH), a wholly owned subsidiary of the Vanderbilt University Medical Center (VUMC), has been providing technical assistance and support for HIV prevention, care, and treatment, including prevention of mother-to-child transmission services, in Zambézia since 2006.
With funding from the U.S. Centers for Disease Control and Prevention (CDC) and the President’s Emergency Plan for AIDS Relief (PEPFAR), VUMC/FGH currently supports 147 health facilities in Zambézia.
Study design and study population
A matched, retrospective cohort study was performed among adult (≥15 years of age) PLWH enrolled in ART services in 123 health facilities within nine districts in Zambézia Province. Data from April 1, 2012, to September 30, 2018, were evaluated. Inclusion criteria were as follows: patients ≥15 years of age who enrolled in ART services between April 1, 2012 and September 30, 2017, and who were eligible for CASG.
As a proxy criterion for CASG eligibility, we used a minimum of four ART pickups over a period of six consecutive months (i.e., proxy for retention in care). Individuals who were registered in any given CASG with only one member were considered as non-CASG members, as the Mozambique Ministry of Health (MOH) recommends group sizes of 3–6 members.
Outcomes, data sources, and definitions
Routinely collected patient-level data were obtained from the electronic patient tracking system database, OpenMRS™, where individual patient-level data are securely stored. These data are entered into the system from paper-based patient medical records and ART pickup forms. Data for analysis were extracted and transferred to a secure deidentified database.
Our primary outcomes were retention in care at 6 and 12 months, viral suppression, and LTFU.
Loss to follow-up was defined as being more than 2 months overdue for the most recent clinical appointment or scheduled ART refill/pickup, as per the current MOH definition.15
Retention among PLWH was defined relative to time from eligibility to CASG. Patients were considered retained in care if they were not LTFU 6 or 12 months after CASG eligibility for the 6- and 12-month retention evaluations, respectively.
Viral suppression was defined as having a VL of <1,000 copies/mL. VL tests performed after August 2016 (when VL testing became part of routine monitoring for all PLWH on ART) were included in the viral suppression dataset, using the first available VL test >6 months following CASG eligibility.
Statistical analyses
Multiple imputation was used to impute missing values. We assumed that the data were missing at random and used 20 imputations.16 The imputation model included CASG membership, sex, district, education level (24% missing), marital status (27% missing), CD4 cell count value at first visit (35% missing), age at eligibility (none missing), and tuberculosis (TB) infection status at baseline (1% missing). A propensity score was calculated as the probability of being enrolled in a CASG, adjusting for age at eligibility, sex, education level, marital status, year of CASG eligibility, CD4 cell count at first visit, TB infection status at baseline, and district, for each imputed dataset.
We used logistic regression, with continuous variables (e.g., age, program year, and CD4 cell count) modeled via restricted cubic splines. The estimated propensity score was obtained as the average of all 20 imputed datasets. The averaged propensity score was then used to match CASG to non-CASG members. We used the R package, MatchIt,17 for matching, with some modifications to match patients with similar follow-up times, using 1:1 nearest-neighbor caliper matching, with the caliper set to 0.20 standard deviations (SD) of the estimated propensity scores.
To reduce selection bias, a patient who was enrolled in a CASG n days after being eligible could only be matched to a non-CASG member who was active for at least the same n days after his/her CASG eligibility date. Matches were also required to be from the same district.
An exploratory analysis was performed using descriptive statistics, with frequency tables for categorical variables and means (SD) and/or medians [interquartile ranges (IQR)] for continuous variables. Univariate comparisons were performed using the Wilcoxon–Mann–Whitney test for continuous variables, and categorical variables were compared using the Chi-square test. A Cox regression model was then used to estimate the impact of CASGs on LTFU, controlling for sex, age at CASG eligibility, education level, marital status, CD4 cell count at first clinical visit, TB infection status at baseline, and district, for each imputed dataset.
We used a sandwich estimator for the variance, with clusters defined as CASG identifiers for CASG members and patient IDs for non-CASG members. Time to LTFU was defined as time from CASG eligibility to LTFU. Patients were censored at LTFU date or at the database closure date. CASG members were considered “ever” in the CASG, although some had left his/her group, but continued in care. We also estimated the association between CASG and 6- and 12-month retention through logistic regression, adjusting for the same covariates as above, again for each imputed dataset. CASG members and their respective controls who were not followed for a minimum of 6 or 12 months by the end of database closure date were excluded from the 6- and 12-month retention analysis, respectively.
Data stratified by district type (urban or rural) were analyzed to evaluate whether CASG membership had a different effect on patients enrolled in CASGs in different settings. Retention was calculated 6 or 12 months after CASG enrollment for CASG members and 6 or 12 months after CASG eligibility for non-CASG members. The impact of CASGs on viral suppression was similarly assessed. More explicitly, the matching procedure for all patients with VL measurements was re-run, thus creating a new matched population.
The first VL result obtained after CASG eligibility for analysis was used, adjusting for the time between CASG eligibility and VL measurement in the regression analysis, in addition to all other variables cited above. Finally, a sensitivity analysis was performed to assess whether the retention results were subject to selection bias. That is, although VL could have been used to assign CASG membership, it was not included in the propensity score model due to significant missingness, especially in the earlier periods of evaluation before VL measurements were obtained as part of routine clinical care.
Therefore, for the sensitivity analyses, the dataset was restricted to patients having available VL test results and included VL suppression (yes/no) in the propensity score model. The procedures above were re-run to assess the impact of CASGs on 6- and 12-month retention as well as on the risk of being LTFU. All statistical analyses were conducted using R statistical software.18 Final estimates after multiple imputation were combined using Rubin’s rule.19
Ethical considerations
This data use and evaluation plan was approved by the VUMC Institutional Review Board (#201887) and the Institutional Research Ethics Committee for Health of Zambézia Province (Comité Institucional de Bioética para Saúde—Zambézia; 16-CIBS-Z-18). This project was reviewed in accordance with the U.S. CDC human research protection procedures and was determined to be research.
Individual informed consent was not required for this evaluation since only routinely collected deidentified data were used, and a waiver of informed consent was approved.
Results
Data were obtained from 131,089 patients who had initiated ART for HIV between April 1, 2012 and September 30, 2017, of whom 90,008 (68.7%) were eligible for CASGs. Of those eligible, 17,018 (18.9%) did ever enroll in a CASG. The matching algorithm was unable to find controls for all patients ever enrolled in a CASG, resulting in a matched population of 26,858 patients. Among 64,838 patients eligible for VL measurements, 39,196 had VL results available.
The matched algorithm was able to find a corresponding non-CASG member for 3,837 CASG members, leading to a study population of 7,674 patients. Supplementary Figures S1 and S2 show the distribution of propensity scores.
Baseline characteristics
Baseline characteristics of the study’s matched population are displayed in Table 1. The median age at CASG eligibility was 31.9 years [IQR 25.9–40.0]; 74.5% (n = 20,020) of the patients were female; and patients had a median baseline CD4 cell count of 375 cells/mm3 [IQR 231–555]. Sixteen percent (n = 4,317) of all patients were receiving care in the urban provincial capital and surrounding area (Quelimane district), with the remainder (84%) receiving care in rural health facilities.
Table 1.
Baseline Characteristics of the Matched Population (Comparing Those Ever Enrolling in Community Adherence Support Groups with Those Never Enrolling in a Community Adherence Support Group) (n = 26,858)
| All (n = 26,858) | Non-CASG members (n = 13,429) | CASG members (n = 13,429) | |
|---|---|---|---|
|
| |||
| Sex, n (%) | |||
| Female | 20,020 (74.5) | 10,051 (74.8) | 9,969 (74.2) |
| Male | 6,838 (25.5) | 3,378 (25.2) | 3,460 (25.8) |
| Age at CASG eligibility (years), median [IQR] | 31.9 [25.9–40.0] | 31.5 [25.6–39.5] | 32.5 [26.4–40.4] |
| Age at CASG eligibility (years), n (%) | |||
| 15–19 | 1,223 (4.55) | 731 (5.4) | 492 (3.7) |
| 20–24 | 4,469 (16.6) | 2,346 (17.5) | 2,123 (15.8) |
| 25–49 | 18,908 (70.4) | 9,231 (68.7) | 9,67, (72.1) |
| 50+ | 2,258 (8.4) | 1,121 (8.4) | 1,137 (8.5) |
| Marital status, n (%) | |||
| Cohabitating with partner | 9,415 (35.1) | 4,773 (35.5) | 4,642 (34.6) |
| Married | 3,516 (13.1) | 1,770 (13.2) | 1,746 (13.0) |
| Separated/divorced/widowed/single | 5,974 (22.2) | 2,900 (21.6) | 3,074 (22.9) |
| Missing | 7,953 (29.6) | 3,986 (29.7) | 3,967 (29.5) |
| Highest educational level attained, n (%) | |||
| None | 5,397 (20.1) | 2,593 (19.3) | 2,804 (20.9) |
| Primary school | 12,290 (45.8) | 6,142 (45.7) | 6,148 (45.8) |
| Post-secondary | 2,937 (10.9) | 1,569 (11.7) | 1,368 (10.2) |
| Missing | 6,234 (23.2) | 3,125 (23.3) | 3,109 (23.2) |
| CD4 cell count (cells/mm3), median [IQR] | 375 [231–555] | 376 [232–556] | 373 [230–554] |
| CD4 cell count (cells/mm3), n (%) | |||
| <200 | 3,666 (13.6) | 1,779 (13.2) | 1,887 (14.1) |
| 200–349 | 4,715 (17.6) | 2,277 (17.0) | 2,438 (18.2) |
| 350–499 | 4,156 (15.5) | 1,994 (14.8) | 2,162 (16.1) |
| ≥500 | 5,712 (21.3) | 2,804 (20.9) | 2,908 (21.7) |
| Missing | 8,609 (32.1) | 4,575 (34.1) | 4,034 (30) |
| TB infection status at enrollment, n (%) | |||
| No | 23,232 (86.5) | 11,502 (85.7) | 11,730 (87.3) |
| Yes | 3,440 (12.8) | 1,799 (13.4) | 1,641 (12.2) |
| Missing | 186 (0.7) | 128 (1.0) | 58 (0.4) |
| Health facility location—district, n (%) | |||
| Gilé | 1,920 (7.2) | 960 (7.2) | 960 (7.2) |
| Ile | 1,102 (4.1) | 551 (4.1) | 551 (4.1) |
| Inhassunge | 2,240 (8.3) | 1,120 (8.34) | 1,120 (8.3) |
| Maganja da Costa | 2,964 (11) | 1482 (11) | 1,482 (11) |
| Mocubela | 2,806 (10.4) | 1,403 (10.4) | 1,403 (10.4) |
| Alto Molócué | 1,244 (4.6) | 622 (4.6) | 622 (4.6) |
| Namacurra | 4,344 (16.2) | 2,172 (16.2) | 2,172 (16.2) |
| Pebane | 5,936 (22.1) | 2,968 (22.1) | 2,968 (22.1) |
| Quelimane | 4,302 (16) | 2,151 (16) | 2,151 (16) |
| Health facility location, n (%) | |||
| Periurban Quelimanea | 1,055 (3.9) | 534 (4) | 521 (3.9) |
| Semiurban Quelimaneb | 3,262 (12.1) | 1,623 (12.1) | 1,639 (12.2) |
| Rural districts | 22,541 (83.9) | 11,272 (83.9) | 11,269 (83.9) |
| Time to first VL (days), median [IQR]c | 351 [165–645] | 358 [163–687] | 344 [169–606] |
| Time to CASG eligibility (days), median [IQR] | 276 [188–640] | 273 [184–644] | 279 [194–636] |
| Year of CASG eligibility, n (%) | |||
| ≤2013 | 2,678 (10) | 1,543 (11.5) | 1,135 (8.45) |
| 2014 | 4,733 (17.6) | 2,583 (19.2) | 2,150 (16) |
| 2015 | 6,133 (22.8) | 3,209 (23.9) | 2,924 (21.8) |
| 2016 | 6,570 (24.5) | 3,184 (23.7) | 3,386 (25.2) |
| ≥2017 | 6,744 (25.1) | 2,910 (21.7) | 3,834 (28.6) |
Periurban: urban health facilities in Quelimane District.
Semiurban: peripheral health facilities in Quelimane District.
Time to VL is derived from VL measurements obtained from 4,748 patients, which were used in the viral suppression analysis.
CASG, community adherence support group; CI, confidence interval; IQR, interquartile range; OR, odds ratio; TB, tuberculosis; VL, viral load.
The median time from enrollment into ART services to CASG eligibility was 276 days [IQR 188–640]; specifically, 279 days [IQR 194–636] for CASG members and 273 days [IQR 184–644] for non-CASG members. The median duration of CASG membership was 520 days [IQR 302–775], computed as the time from CASG enrollment date to either the CASG end date, for patients who left his/her CASG, or to the end of study date, for patients currently on CASG.
Eighty-five percent of patients who enrolled in a CASG at any time of the study were still active CASG members at the end of the study follow-up period.
Retention in care at 6 and 12 months
Of the 13,424 CASG members who were matched to a control, 2,089 and 5,627 patients had less than 6 and 12 months of follow-up at the time of database closure (end of study period), respectively, and were excluded from the retention analyses. The final populations for 6- and 12-month retention analysis included 22,680 and 15,604 patients, respectively, of which half were CASG members (1:1 matching). Retention in care among CASG members was, on average, 93% and 90% at 6 and 12 months, respectively, while only 77% and 66% for non-CASG members (Supplementary Tables S1 and S2).
Results for the multivariable logistic regression analysis are displayed in Table 2. The odds of a person who was ever enrolled in a CASG being retained at 6 months were ~4.2-fold higher than a person who was never enrolled in a CASG (adjusted odds ratio [aOR] = 4.19 [95% confidence interval; CI: 3.79–4.64]), with all other covariates being held constant. At 12 months, a person who was ever in a CASG had, as well, more than fourfold higher odds of being retained (aOR = 4.43 [95% CI: 4.01–4.90]).
Table 2.
Results from Multivariable Logistic Regression Models Applied to the Matched Data
| 6-Month retention (n = 22,680), aOP [95% CI] | 12-Month retention (n = 15,604), aOR [95% CI] | Viral suppression (n = 7,674), aOR [95% CI] | |
|---|---|---|---|
|
| |||
| CASG member | |||
| No | Ref. | Ref. | Ref. |
| Yes | 4.19 [3.79–4.64] | 4.43 [4.01–4.90] | 1.14 [1.02–128] |
| Sex | |||
| Female | Ref. | Ref. | Ref. |
| Male | 0.90 [0.82–0.99] | 0.80 [0.73–0.88] | 0.72 [0.62–0.82] |
| Age at CASG eligibility, yearsa | |||
| 20 | 0.82 [0.70–0.95] | 0.72 [0.60–0.87] | 0.85 [0.77–0.93] |
| 35 | Ref. | Ref. | Ref. |
| 50 | 1.23 [0.95–1.59] | 1.08 [0.82–1.43] | 1.27 [1.10–1.46] |
| Highest educational level attained | |||
| None | 1.03 [0.92–1.14] | 0.97 [0.88–1.08] | 1.06 [0.91–1.23] |
| Primary | Ref. | Ref. | Ref. |
| Post-secondary | 1.06 [0.95–1.19] | 1.06 [0.93–1.22] | 1.34 [1.14–1.59] |
| Marital status | |||
| Cohabitating with partner | Ref. | Ref. | Ref. |
| Married | 0.99 [0.88–1.11] | 0.98 [0.87–1.11] | 1.14 [0.94–1.38] |
| Separated/divorced/widowed/single | 1.06 [0.96–1.17] | 0.98 [0.89–1.09] | 1.07 [0.94–1.21] |
| CD4 cell count (cell/mm3)a | |||
| 150 | 0.97 [0.66–1.10] | 0.95 [0.82–1.10] | 0.82 [0.75–0.90] |
| 300 | Ref. | Ref. | Ref. |
| 500 | 1.02 [0.95–1.09] | 1.00 [0.93–1.09] | 1.18 [1.12–1.24] |
| Health facility location—district | |||
| Quelimane | Ref. | Ref. | Ref. |
| Gilé | 0.54 [0.45–0.65] | 0.50 [0.42–0.61] | 0.43 [0.34–0.54] |
| Ile | 0.82 [0.65–1.04] | 0.69 [0.53–0.90] | 0.45 [0.35–0.60] |
| Inhassunge | 0.52 [0.44–0.62] | 0.47 [0.39–0.56] | 0.80 [0.62–1.01] |
| Maganja da Costa | 0.45 [0.38–0.53] | 0.31 [0.26–0.38] | 0.53 [0.42–0.65] |
| Mocubela | 0.50 [0.43–0.59] | 0.33 [0.28–0.39] | 0.87 [0.65–1.16] |
| Alto Molócué | 0.64 [0.51–0.80] | 0.61 [0.48–0.79] | 0.64 [0.48–0.87] |
| Namacurra | 0.60 [0.52–0.70] | 0.63 [0.54–0.74] | 0.63 [0.52–0.77] |
| Pebane | 0.74 [0.64–0.85] | 0.68 [0.59–0.80] | 0.87 [0.70–1.08] |
Age and CD4 cell count values were fitted using restricted cubic splines.
aOR, adjusted odds ratio.
Men were less likely to be retained in care (aOR = 0.90 [95% CI: 0.82–0.99]) at 6 months and at 12 months (aOR = 0.80 [95% CI: 0.73–0.88]). The odds of being retained increased with each year of age (aOR = 1.02 [95% CI: 1.01–1.02] at 6 months and 1.01 [95% CI: 1.01–1.01 at 12 months]) (Supplementary Tables S1 and S2).
The stratified analysis per area showed comparable results: patients who ever enrolled in a CASG had similar odds of being retained at 6 months (aOR = 4.58 [95% CI: 3.38–6.20] and aOR = 4.13 [95% CI: 3.70–4.60] for urban and rural settings, respectively) and at 12 months (aOR = 4.13 [95% CI: 3.08–5.54] and aOR = 4.37 [95% CI: 3.92–4.87] for urban and rural settings, respectively) after CASG eligibility (data not shown).
Loss to follow-up
Among the study population of 26,848 patients, 9% of patients who were ever enrolled in a CASG were LTFU versus 29% among those never enrolled in a CASG (Supplementary Table S3). Patients receiving ART through CASG support were less likely to be LTFU when compared with ART-treated patients not receiving ART through CASG support: the proportion of patients being LTFU was approximately three times higher for persons who never joined a CASG compared with those who joined a CASG (adjusted hazard ratio [aHR] = 3.45 [95% CI: 3.20–3.73]) (Fig. 1; Supplementary Table S5).
FIG. 1.
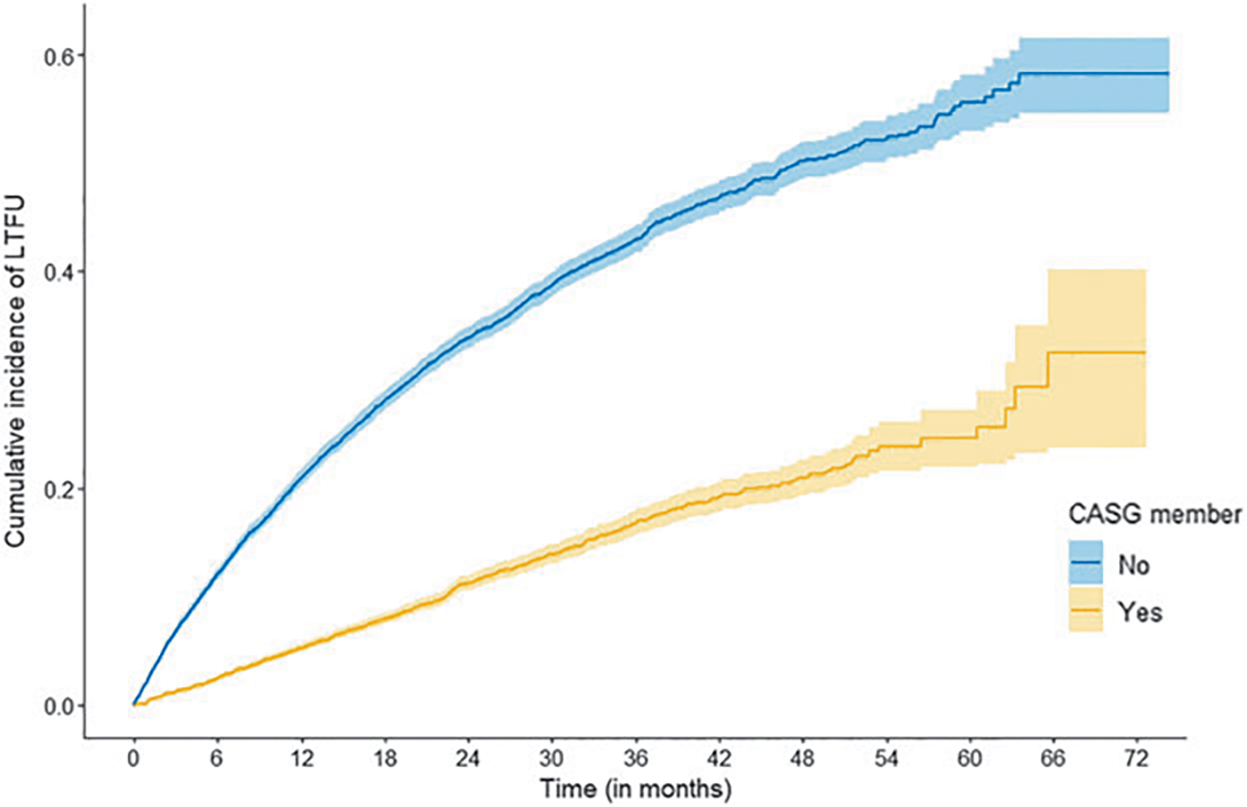
Cumulative incidence of LTFU among patients: adults receiving ART through CASG support (i.e., ever in a CASG) (yellow line) and adults receiving ART who were not ever in a CASG (i.e., eligible for, but never in a CASG) (blue line). ART, antiretroviral therapy; CASG, community adherence support group; LTFU, loss to follow-up.
Viral suppression
The median time from CASG eligibility to first VL result was slightly shorter for CASG members (408 days [IQR 207–717]) compared with non-CASG members (498 days [IQR 250–825]). The viral suppression rate overall was 80% among CASG members versus 77% among non-CASG members ( p = .002). Factors associated with viral suppression are shown in Supplementary Table S4. The adjusted regression showed that persons ever enrolled in CASGs had higher odds of being virally suppressed (aOR = 1.14 [95% CI: 1.02–1.28]) (Table 2).
Sensitivity analysis
Our sensitivity analysis used all 7,674 matched patients with VL measurements, but only 7,494 and 6,260 patients were followed for 6 and 12 months, respectively, thus composing the study population for the retention analysis. Among them, 95% and 94% CASG members were retained at 6 and 12 months, respectively, while 84% and 83% non-CASG members were retained at 6 and 12 months. By the end of the study period, 96% and 91% of CASG and non-CASG members, respectively, were still enrolled in care.
The impact of CASGs on overall retention remained the same, that is, results from our sensitivity analysis were very similar to the findings obtained without including VL in the propensity score. More specifically, CASG members were still more likely to be retained in care (aOR = 3.28 [95% CI: 2.73–3.93] for 6-month retention and aOR = 3.17 [95% CI: 2.63–3.82] for 12-month retention) and non-CASG members were more likely to be LTFU (aHR = 2.49 [95% CI: 2.00–3.11]) (data not shown).
Discussion
This 5-year, observational cohort study showed the impact of CASGs on retention in care and viral suppression rates among ART-treated adults in Zambézia Province, Mozambique. The study revealed that after adjustment for sex, baseline TB coinfection status, year of CASG eligibility, and age at ART initiation, the odds of being retained at 6 and 12 months were more than fourfold greater when an adult was receiving ART through CASG support versus not during the evaluation period.
A similar previously conducted study performed in Tete, another province within Mozambique, had also shown that patient-driven ART distribution through CASG support resulted in positive HIV continuum of care outcomes, including higher retention in care rates, among ART-treated adults.7,8,20
However, following a larger cohort for a longer follow-up period and comparing with matched patients who were eligible for, but not enrolled in, a CASG, the present study demonstrated that sustained favorable continuum of care outcomes were similarly achieved, thus adding to the published literature dating back a few years. This study was novel through its inclusion of viral suppression as an outcome following the inclusion of routine VL monitoring for all ART-treated patients, revealing a positive effect of CASGs on viral suppression.
DSD models of care, including CASGs, have shown beneficial continuum of care outcomes among ART-treated adults, especially among adults desiring to spend as little time in the health facility as possible.21 A review demonstrated favorable retention in care and viral suppression rates for a variety of DSD models that were evaluated, however, heterogeneity between the studies existed.22 Preferences for models differ as well and need to be considered when offering services.23
The CASG strategy, offering a combination of decreased number of health facility visits and community-based adherence support, has demonstrated benefits for patients. Factors such as time and financial benefits were seen to be important among patients entering a CASG.24 Additionally, health education benefits and psychosocial benefits (for members and their families) were also noted by Kun et al.25
Our study results do suggest that the CASG model should remain as a viable DSD model of care, with patients having the opportunity to opt into a CASG rather than adopting a new one. Being a predominantly rural country, CASG support might be a sustainable option in Mozambique as CASG uptake remains very high among persons residing in rural locales. This preferred location of ART pickup was also seen in Zambia among PLWH residing in rural settings and confirms the need for the contextualization of service delivery.23
Enrollment in one or other model could eventually follow an adaptive approach, responding to individual patient needs, thus placing them in the DSD model that best suits their individual needs for a particular period of time, realizing that life events are dynamic and, as such, individual patient preferences may change over time. A community strategy implemented in South Africa among postpartum women showed a high proportion shifting to other DSD models with encouraging results.26
Costing is an important aspect when optimizing care, where a study showed similar costs; however, 6-monthly drug dispensation appears to be less expensive.27 Health systems also benefit from the CASG model as individual clinic patient volumes diminish, a benefit clearly seen during the recent COVID-19 pandemic when additional mitigation measures were put into place and when the number of required clinic visits reduced due to scale up of other DSD models.
Our study had limitations. Routinely collected data are sensitive to data entry errors and/or missing data. Multiple imputation was done to address missing data. The suboptimal data quality did not allow us to measure the median number of members per group, which could possibly explain why the median time between clinic visits is relatively short. We used a proxy to determine CASG eligibility, being a minimum of four ART pickups within any 6 months after ART initiation.
Therefore, the outcomes of this analysis might be subject to a certain degree of selection bias. For women enrolled in a CASG, becoming pregnant resulted in temporary withdrawal from the CASG. The analysis was done comparing patients who ever participated in a CASG and those who did not (during the evaluation period). The WHO clinical staging was not reported and thus not included in our analysis, which could also lead to potential bias (being one of the CASG eligibility criteria). Coverage of VL testing was also low, which could have led to an underestimation of viral suppression rates.
Moreover, due to this high missingness, VL was not used in the propensity score matching algorithm. Sensitivity analysis with first VL measurement showed slightly smaller effect sizes obtained from the multivariable analyses, but still showed strong positive associations of CASG membership with better outcomes. The effect sizes obtained from all multivariable analyses were slightly smaller, but still showed strong positive associations of CASG membership with better outcomes.
Conclusions
Participation in community adherence groups significantly increased the likelihood of being retained in care. The higher viral suppression rates among those in CASGs further highlight the benefits of this DSD model. While Mozambique rapidly scales up multimonth drug dispensation as the preferred DSD model, this study emphasizes the continued importance of a CASG as an efficacious DSD alternative, especially among patients residing in rural areas, where CASG uptake is higher.
Supplementary Material
Acknowledgments
This evaluation was a collaborative partnership between VUMC/FGH investigators, the Provincial Health Directorate of Zambézia (DPS-Z), and the U.S. Centers for Disease Control and Prevention (CDC) Mozambique.
Funding Information
This evaluation has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreements #NU2GGH001943 and #NU2GGH002367 (PI/PD: C.W.W.).
Footnotes
Disclaimer
The findings, conclusions, and opinions expressed by authors contributing to this article are those of the author(s) and do not necessarily represent the official position of the funding agencies or the authors’ affiliated institutions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS. UNAIDS Country Factsheet Mozambique; 2020. Available from: https://www.unaids.org/en/regionscountries/countries/mozambique [Last accessed: September 15, 2022].
- 2.UNAIDS. Fast-Track—Ending the AIDS Epidemic by 2030. UNAIDS: Geneva; 2014. [Google Scholar]
- 3.Decroo T, Telfer B, Biot M, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic Syndr 2011;56(2):e39–e44; doi: 10.1097/QAI.0b013e3182055138 [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health (MOH). National Guidelines on Strategies of Community Support and Adherence Groups. Maputo; 2015. Available from: https://www.misau.gov.mz/index.php/hiv-sida-directrizes-nacionais [Last accessed: September 15, 2022].
- 5.Ministry of Health (MOH). National Guidelines on Differentiated Models of Care in Mozambique. 2018. Maputo. Available from: https://comitetarvmisau.co.mz/docs/orientacoes_nacionais/Guiao_Modelos_diferenciados_2018.pdf [Last accessed: September 15, 2022]. [Google Scholar]
- 6.Ministry of Health (MOH). National Guidelines on Test and Treat Approach 2018. Maputo; 2018. Available from: https://www.misau.gov.mz/index.php/guioes-de-prevencao-e-de-cuidados-e-tratamento [Last accessed: September 15, 2022].
- 7.Jobarteh K, Shiraishi RW, Malimane I, et al. Community ART support groups in Mozambique: The potential of patients as partners in care. PLoS One 2016;11(12):e0166444; doi: 10.1371/journal.pone.0166444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decroo T, Telfer B, Dores CD, et al. Effect of community ART groups on retention-in-care among patients on ART in Tete Province, Mozambique: A cohort study. BMJ Open 2017;7(8):e016800; doi: 10.1136/bmjopen-2017-016800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auld AF, Shiraishi RW, Couto A, et al. A decade of anti-retroviral therapy scale-up in Mozambique: Evaluation of outcome trends and new models of service delivery among more than 300,000 patients enrolled during 2004–2013. J Acquir Immune Defic Syndr 2016;73(2):e11–e22; doi: 10.1097/QAI.0000000000001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decroo T, Koole O, Remartinez D, et al. Four-year retention and risk factors for attrition among members of community ART groups in Tete, Mozambique. Trop Med Int Health 2014;19(5):514–521; doi: 10.1111/tmi.12278 [DOI] [PubMed] [Google Scholar]
- 11.Decroo T, Lara J, Rasschaert F, et al. Scaling up community ART groups in Mozambique. Int STD Res Rev 2013;1(2):49–58. [Google Scholar]
- 12.Ministry of Health (MOH). Annual Report—Activities of HIV/STI Program 2021. Maputo; 2021. Available from: https://www.misau.gov.mz/index.php/relatorios-anuais [Last accessed: September 15, 2022].
- 13.National Institute for Statistics (INE). IV Population Census; 2017. Available from: http://www.ine.gov.mz/iv-rgph-2017/mocambique/07-educacao/quadro-18-populacao-de-5-anos-e-mais-por-condicao-de-alfabetizacaoe-sexo-segundo-area-de-residencia-e-idade-mocambique-2017-p4xp18-p3.xlsx/view [Last accessed: September 15, 2022].
- 14.Ministry of Health (MOH). National Institute for Statistics (INE), and ICF. INS Survey of Indicators on Immunization, Malaria and HIV/AIDS in Mozambique 2015. INS: Maputo, Mozambique, and Rockville, Maryland, USA; 2015. [Google Scholar]
- 15.Ministry of Health (MOH). National Guidelines on Anti-retroviral Therapy and Opportunistic Infections in Adults, Adolescents, Pregnant Women and Children; 2014. Available from: https://www.misau.gov.mz/index.php/hiv-sidadirectrizes-nacionais [Last accessed: September 15, 2022].
- 16.Little R, Rubin D. Statistical Analysis with Missing Data, 2nd Ed. Wiley-Interscience: Hoboken, N.J., USA; 2002. [Google Scholar]
- 17.Ho DE, Imai K, King G, et al. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42(8):1–28. [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013. Available from: http://www.R-project.org/ [Last accessed: September 15, 2022].
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons Inc.: New York; 1987. Available from: 10.1002/9780470316696 [DOI] [Google Scholar]
- 20.Decroo T, Rasschaert F, Telfer B, et al. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: A systematic review. Int Health 2013;5(3):169–179; doi: 10.1093/inthealth/iht016 [DOI] [PubMed] [Google Scholar]
- 21.Pascoe SJS, Scott NA, Fong RM, et al. “Patients are not the same, so we cannot treat them the same”—A qualitative content analysis of provider, patient and implementer perspectives on differentiated service delivery models for HIV treatment in South Africa. J Int AIDS Soc 2020;23(6):e25544; doi: 10.1002/jia2.25544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long L, Kuchukhidze S, Pascoe S, et al. Retention in care and viral suppression in differentiated service delivery models for HIV treatment delivery in sub-Saharan Africa: A rapid systematic review. J Int AIDS Soc 2020;23(11):e25640; doi: 10.1002/jia2.25640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshun-Wilson I, Mukumbwa-Mwenechanya M, Kim HY, et al. Differentiated care preferences of stable patients on antiretroviral therapy in Zambia: A discrete choice experiment. J Acquir Immune Defic Syndr 2019;81(5):540–546; doi: 10.1097/QAI.0000000000002070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasschaert F, Telfer B, Lessitala F, et al. A qualitative assessment of a community antiretroviral therapy group model in Tete, Mozambique. PLoS One 2014;9(3):e91544; doi: 10.1371/journal.pone.0091544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kun KE, Couto A, Jobarteh K, et al. Mozambique’s Community Antiretroviral Therapy Support Group Program: The role of social relationships in facilitating HIV/AIDS treatment retention. AIDS Behav 2019;23(9):2477–2485; doi: 10.1007/s10461-019-02419-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zerbe A, Brittain K, Phillips TK, et al. Community-based adherence clubs for postpartum women on antiretroviral therapy (ART) in Cape Town, South Africa: A pilot study. BMC Health Serv Res 2020;20(1):621; doi: 10.1186/s12913-020-05470-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen S, Nichols B, Guthrie T, et al. Do differentiated service delivery models for HIV treatment in sub-Saharan Africa save money? Synthesis of evidence from field studies conducted in sub-Saharan Africa in 2017–2019. Gates Open Res 2021;5:177; doi: 10.12688/gatesopenres.13458.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


