Abstract
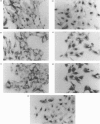
Creatine kinase (CK) plays an important role in buffering ATP and ADP levels in tissues which have intermittently high and fluctuating energy demands, such as skeletal muscle. This buffering function has a spatial, as well as a temporal aspect, which is dependent on the localization of different enzyme isoforms within the cell. We show here, by in situ hybridization, that the mRNAs for the cytoplasmic isoforms of CK are differentially localized in a mouse myoblast cell line (C2C12). The mRNA for the M form is localized at the cell periphery, while that for the B form is localized in the perinuclear region. Deletion of segments of the 3' untranslated regions of these mRNAs or swapping of these segments between the mRNAs for the two isoforms demonstrated that localization signals lie within these regions. Localization appears to be tissue-specific, since both the M and B mRNAs were distributed uniformly over the cytoplasm in a non-muscle cell line. These results, in conjunction with other studies which have shown that mRNA localization can lead to co-localization of the encoded protein, suggest that the localization of the mRNAs for the cytoplasmic isoforms of CK may be involved in the localization of the enzymes themselves.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassell G. J. High resolution distribution of mRNA within the cytoskeleton. J Cell Biochem. 1993 Jun;52(2):127–133. doi: 10.1002/jcb.240520203. [DOI] [PubMed] [Google Scholar]
- Benfield P. A., Graf D., Korolkoff P. N., Hobson G., Pearson M. L. Isolation of four rat creatine kinase genes and identification of multiple potential promoter sequences within the rat brain creatine kinase promoter region. Gene. 1988 Mar 31;63(2):227–243. doi: 10.1016/0378-1119(88)90527-6. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Carpenter C. L. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Billadello J. J., Kelly D. P., Roman D. G., Strauss A. W. The complete nucleotide sequence of canine brain B creatine kinase mRNA: homology in the coding and 3' noncoding regions among species. Biochem Biophys Res Commun. 1986 Jul 16;138(1):392–398. doi: 10.1016/0006-291x(86)90294-9. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T., Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle K., Braddock P., Fulton S. 31P NMR measurements of the ADP concentration in yeast cells genetically modified to express creatine kinase. Biochemistry. 1990 Apr 3;29(13):3295–3302. doi: 10.1021/bi00465a021. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Jaynes J. B., Chamberlain J. S., Hauschka S. D. The mouse muscle creatine kinase cDNA and deduced amino acid sequences: comparison to evolutionarily related enzymes. J Mol Evol. 1985;22(4):334–341. doi: 10.1007/BF02115689. [DOI] [PubMed] [Google Scholar]
- Ch'ng J. L., Ibrahim B. Transcriptional and posttranscriptional mechanisms modulate creatine kinase expression during differentiation of osteoblastic cells. J Biol Chem. 1994 Jan 21;269(3):2336–2341. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe L., Morris E., Fulton A. B. Vimentin mRNA location changes during muscle development. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2724–2728. doi: 10.1073/pnas.90.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby B., Glover D. M. Discrete sequence elements control posterior pole accumulation and translational repression of maternal cyclin B RNA in Drosophila. EMBO J. 1993 Mar;12(3):1219–1227. doi: 10.1002/j.1460-2075.1993.tb05763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Lipshitz H. D. Localized RNAs and their functions. Bioessays. 1993 Oct;15(10):651–658. doi: 10.1002/bies.950151004. [DOI] [PubMed] [Google Scholar]
- Dix D. J., Eisenberg B. R. Myosin mRNA accumulation and myofibrillogenesis at the myotendinous junction of stretched muscle fibers. J Cell Biol. 1990 Nov;111(5 Pt 1):1885–1894. doi: 10.1083/jcb.111.5.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking M. K., Hofstetter H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene. 1986;45(1):101–105. doi: 10.1016/0378-1119(86)90137-x. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B. Spatial organization of the synthesis of cytoskeletal proteins. J Cell Biochem. 1993 Jun;52(2):148–152. doi: 10.1002/jcb.240520206. [DOI] [PubMed] [Google Scholar]
- Garner C. C., Tucker R. P., Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988 Dec 15;336(6200):674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Hill M. A., Gunning P. Beta and gamma actin mRNAs are differentially located within myoblasts. J Cell Biol. 1993 Aug;122(4):825–832. doi: 10.1083/jcb.122.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Wold B. J., Hauschka S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989 Aug;9(8):3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keown W. A., Campbell C. R., Kucherlapati R. S. Methods for introducing DNA into mammalian cells. Methods Enzymol. 1990;185:527–537. doi: 10.1016/0076-6879(90)85043-n. [DOI] [PubMed] [Google Scholar]
- Kislauskis E. H., Li Z., Singer R. H., Taneja K. L. Isoform-specific 3'-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J Cell Biol. 1993 Oct;123(1):165–172. doi: 10.1083/jcb.123.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis E. H., Singer R. H. Determinants of mRNA localization. Curr Opin Cell Biol. 1992 Dec;4(6):975–978. doi: 10.1016/0955-0674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Lupker J. H., Roskam W. G., Miloux B., Liauzun P., Yaniv M., Jouannau J. Abundant excretion of human growth hormone by recombinant-plasmid-transformed monkey kidney cells. Gene. 1983 Oct;24(2-3):281–287. doi: 10.1016/0378-1119(83)90088-4. [DOI] [PubMed] [Google Scholar]
- Morris E. J., Fulton A. B. Rearrangement of mRNAs for costamere proteins during costamere development in cultured skeletal muscle from chicken. J Cell Sci. 1994 Mar;107(Pt 3):377–386. doi: 10.1242/jcs.107.3.377. [DOI] [PubMed] [Google Scholar]
- Okita T. W., Li X., Roberts M. W. Targeting of mRNAs to domains of the endoplasmic reticulum. Trends Cell Biol. 1994 Mar;4(3):91–96. doi: 10.1016/0962-8924(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Papenbrock T., Wille W. The 3' non-coding region of the mouse brain B creatine kinase mRNA: a sequence with exceptional homology among species. Nucleic Acids Res. 1986 Nov 11;14(21):8690–8690. doi: 10.1093/nar/14.21.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S., Herlihy W., Royal N., Pang H., Aposhian H. V., Pickering L., Belagaje R., Biemann K., Page D., Kuby S. Rabbit muscle creatine phosphokinase. CDNA cloning, primary structure and detection of human homologues. J Biol Chem. 1984 Dec 10;259(23):14317–14320. [PubMed] [Google Scholar]
- Ritchie M. E., Trask R. V., Fontanet H. L., Billadello J. J. Multiple positive and negative elements regulate human brain creatine kinase gene expression. Nucleic Acids Res. 1991 Nov 25;19(22):6231–6240. doi: 10.1093/nar/19.22.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer B. W., Perriard J. C. Intracellular targeting of isoproteins in muscle cytoarchitecture. J Cell Biol. 1988 Apr;106(4):1161–1170. doi: 10.1083/jcb.106.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H. RNA zipcodes for cytoplasmic addresses. Curr Biol. 1993 Oct 1;3(10):719–721. doi: 10.1016/0960-9822(93)90079-4. [DOI] [PubMed] [Google Scholar]
- Sowden M., Harrison S., Ashfield R., Kingsman A. J., Kingsman S. M. Multiple cooperative interactions constrain BPV-1 E2 dependent activation of transcription. Nucleic Acids Res. 1989 Apr 25;17(8):2959–2972. doi: 10.1093/nar/17.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Banker G. A. Getting the message from the gene to the synapse: sorting and intracellular transport of RNA in neurons. Trends Neurosci. 1992 May;15(5):180–186. doi: 10.1016/0166-2236(92)90170-d. [DOI] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Requirement of microfilaments in sorting of actin messenger RNA. Science. 1991 Sep 13;253(5025):1275–1277. doi: 10.1126/science.1891715. [DOI] [PubMed] [Google Scholar]
- Trask R. V., Billadello J. J. Tissue-specific distribution and developmental regulation of M and B creatine kinase mRNAs. Biochim Biophys Acta. 1990 Jun 21;1049(2):182–188. doi: 10.1016/0167-4781(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Trask R. V., Koster J. C., Ritchie M. E., Billadello J. J. The human M creatine kinase gene enhancer contains multiple functional interacting domains. Nucleic Acids Res. 1992 May 11;20(9):2313–2320. doi: 10.1093/nar/20.9.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T., Eppenberger H. M. The subcellular compartmentation of creatine kinase isozymes as a precondition for a proposed phosphoryl-creatine circuit. Prog Clin Biol Res. 1990;344:877–889. [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H. M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992 Jan 1;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. E., Vale R. D. RNA on the move: the mRNA localization pathway. J Cell Biol. 1993 Oct;123(2):269–274. doi: 10.1083/jcb.123.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisraeli J. K., Sokol S., Melton D. A. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990 Feb;108(2):289–298. doi: 10.1242/dev.108.2.289. [DOI] [PubMed] [Google Scholar]