SUMMARY
In 2011, over 35,000 ostriches were slaughtered in the Oudtshoorn district of the Western Cape province of South Africa following the diagnosis of highly pathogenic avian influenza virus H5N2. We describe the pathology and virus distribution via immunohistochemistry in juvenile birds that died rapidly in this outbreak after showing signs of depression and weakness. Associated sialic acid (SA) receptor distribution in uninfected birds is also described. At necropsy, enlarged spleens, swollen livers, and generalized congestion were noted. Birds not succumbing to acute influenza infection often became cachectic with serous atrophy of fat, airsacculitis, and secondary infections. Necrotizing hepatitis, splenitis, and airsacculitis were prominent histopathologic findings. Virus was detected via immunohistochemistry in abundance in the liver and spleen but also in the air sac and gastrointestinal tract. Infected cells included epithelium, endothelium, macrophages, circulating leukocytes, and smooth muscle of a variety of organs and vessel walls. Analysis of SA receptor distribution in uninfected juvenile ostriches via lectin binding showed abundant expression of SAα2,3Gal (avian type) and little or no expression of SAα2,6Gal (human type) in the gastrointestinal and respiratory tracts, as well as leukocytes in the spleen and endothelial cells in all organs, which correlated with H5N2 antigen distribution in these tissues.
Keywords: highly pathogenic avian influenza virus, H5N2, immunohistochemistry, ostrich, sialic acid receptors, South Africa
RESUMEN
Nota de Investigación—Patobiología de la infección por el virus de la influenza aviar altamente patógeno, subtipo H5N2 en avestruces juveniles de Sudáfrica.
En el año 2011, más de 35,000 avestruces fueron sacrificadas en el distrito de Oudtshoorn en la provincia del Cabo Occidental en Sudáfrica como resultado del diagnóstico del virus de la influenza aviar altamente patógena H5N2. Se describe la distribución de la patología y del virus a través de técnicas de inmunohistoquímica en aves jóvenes que murieron rápidamente en este brote después de mostrar signos de depresión y debilidad. También se describe la distribución de receptores asociados con el ácido siálico en las aves infectadas. En la necropsia se observó agrandamiento del bazo, hígados inflamados, y congestión generalizada. Las aves que no murieron por la infección por la influenza aguda a menudo mostraron caquexia con atrofia serosa de la grasa, aerosaculitis, e infecciones secundarias. La hepatitis necrotizante, esplenitis y aerosaculitis fueron los hallazgos histopatológicos importantes. El virus se detectó en abundancia a través de técnicas de inmunohistoquímica en el hígado y en el bazo, pero también se detectó en los sacos aéreos y en el tracto gastrointestinal. Las células infectadas incluyeron células epiteliales, endoteliales, macrófagos, leucocitos circulantes, y el músculo liso de una variedad de órganos y paredes de los vasos. El análisis de la distribución de los receptores de ácido siálico en avestruces jóvenes no infectadas a través de la unión de la lectina mostró abundante expresión del receptor tipo SAα2, 3Gal (tipo aviar) y poca o ninguna expresión del tipo SAα2,6Gal (tipo humano) en los tractos gastrointestinal y respiratorio, así como en los leucocitos en el bazo y en las células endoteliales en todos los órganos, lo que se correlacionó con la distribución del antígeno H5N2 en estos tejidos.
Preemptive slaughter of more than 35,000 ostriches followed the diagnosis of highly pathogenic avian influenza virus (HPAIV) H5N2 in the Oudtshoorn district of the Western Cape Province of South Africa in April 2011. We describe the microscopic pathology and virus distribution via immunohistochemistry in juvenile birds from two farms affected during this outbreak. One farm, the index case, had 130 deaths out of 400 birds that were 4–14 mo of age, and the second farm had 150 deaths out of 550 birds that were 7 wk to 4 mo of age; in each case deaths followed inclement weather. Although clinical cases were rare, the virus was isolated from juvenile birds that died rapidly after showing signs of depression and weakness. Green urine, thought to signify liver damage, was observed in both acute and subacutely affected groups and has been previously reported in ostriches infected with HPAIV H7N1 (1). Gross necropsy findings in 37 birds included hepatomegaly, splenomegaly, and generalized congestion. Cachexia, serous atrophy of fat, airsacculitis, and secondary infections were observed in presumably recovered birds that died. In addition to reporting the microscopic pathology in these birds, we examined sialic acid (SA) receptor distribution in uninfected birds to possibly explain pathogenesis and susceptibility of ostriches to avian influenza viruses.
MATERIALS AND METHODS
Of 37 postmortem examinations performed on birds that died on two farms, selected tissues from seven birds (3 of 13 necropsied on index farm and 4 of 24 necropsied on the second) were fixed in 10% buffered formalin and paraffin embedded for histopathology. Tissues included liver (n = 5); spleen (n = 7); trachea (n = 4); lung (n = 3); air sac (n = 3); kidney (n = 2); pancreas (n = 4); ventriculus, small intestine, and large intestine (n = 2); and brain (n = 7). Birds ranged from 7 to 16 wk old and were positive for HPAIV H5N2 by virus isolation and/or reverse transcription PCR and sequencing. Paraffin sections were stained with hematoxylin and eosin (HE) for histopathology, and selected sections were stained with Lilly-Twort gram stain for evaluation of secondary bacterial infections. Sections were also stained for influenza A nucleoprotein via immunohistochemistry as previously described (3).
Formalin fixed and paraffin embedded tissues (trachea, lung, proventriculus, ventriculus, small and large intestines, pancreas, liver, spleen, kidney, and brain) from uninfected ostriches 6–9 mo old or older were stained with Maackia amurensis (MAA) lectins (both MAA1 and MAA2) for detection of avian-type α2,3-linked SA receptors (avian type) or Sambucus nigra lectin for detection of α2,6-linked SA receptors (human type) as previously described with modifications (4).
RESULTS AND DISCUSSION
Lesions were severe in liver, spleen, gastrointestinal tract (ventriculus, small and large intestine), and air sac but were also present in trachea and airways of the lung. Lesions were not present in lung parenchyma, kidney, or brain. Multifocal fibrinonecrotic splenitis and hepatitis with fibrinoid necrosis of vessels were characteristic findings in spleen and liver, respectively (Fig. 1A,B). In the respiratory tract, tracheitis with mucosal damage and regeneration and severe caseous to fibrinonecrotic airsacculitis with epithelial hyperplasia (Fig. 1C) were observed. Hemorrhagic and/or necrotic mucosal lesions were present in ventriculus and small and large intestine with secondary infection by gram-positive and gram-negative bacilli. Septicemia due to gram-negative bacilli was present in one bird. Changes were similar to previously reported natural HPAIV H7N1; however, birds infected with HPAIV H7N1 had prominent brain changes not observed in these birds (1). Lesions were also similar to those seen in ostriches experimentally infected with an emu-derived HPAIV H5N2, particularly the airsacculitis (2).
Fig. 1.
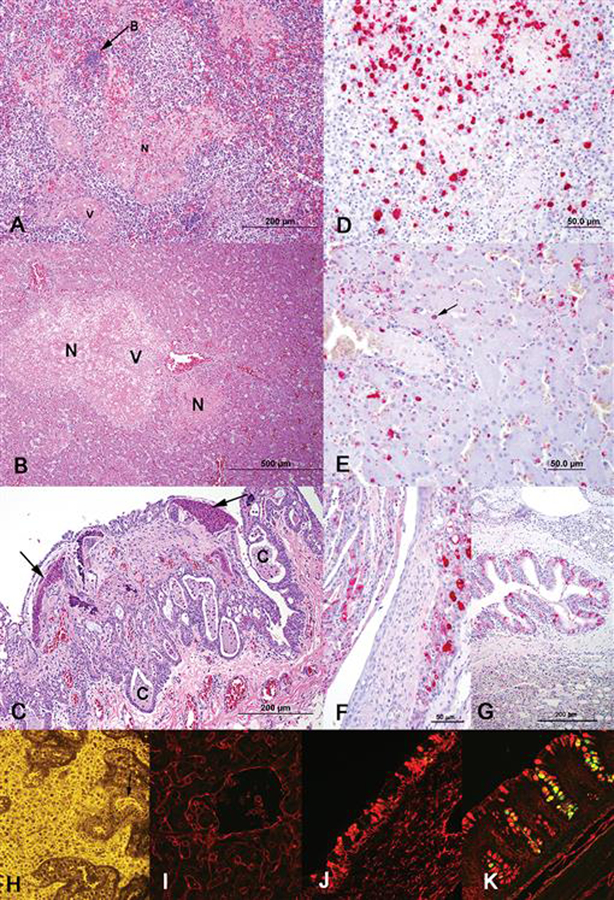
(A) Liver and (B) spleen were extensively involved with multifocal necrosis (N) and multifocal vascular necrosis (V). (A) Secondary infections, such as sepsis (bacterial colony at B, which were gram-negative rods on gram staining), also occurred. (C) Air sacs had severe ulceration, severe epithelial hyperplasia with cyst (C) formation and accumulation of caseous (arrows) to fibrinonecrotic exudate on the surface. (D) In spleen, viral antigen (red staining), both intranuclear and intracytoplasmic, was present in cells in areas of necrosis and also in smooth muscle of vessels and endothelium (not shown). (E) In liver, viral antigen (red staining), mostly intranuclear, was present in hepatocytes (arrow), endothelium, and Kupffer cells, and also in cells within necrotic areas (latter not shown). The respiratory tract had viral antigen in trachea, bronchi, and air sac, (F) but air sac had the most abundant viral antigen present (red staining). (G) Bronchial epithelium had abundant viral antigen (red staining) but few associated changes; parenchymal changes, e.g., pneumonia, were not evident. Lectin binding demonstrated abundant SAα2,3Gal (avian type) receptor expression (H, I, J, K). (H) Spleen single labeled for MAA2 showing binding of MAA2 (yellow fluorescence) in leukocytes and endothelium (arrow). (I) Liver, (J) trachea, and (K) colon were double labeled for MAA1 (green fluorescence) and MAA2 (red fluorescence; cells binding both MAA1 and MAA2 are yellow). Liver (sinusoidal lining cells) and trachea (mostly mucosal goblet cells) have only MAA2 staining. Colon has binding of both MAA1 and 2. (A–C) HE staining. (D–G) Immunohistochemistry using mouse monoclonal to influenza A nucleoprotein with fast red chromogen in an alkaline phosphatase technique. (H–K) Lectin binding was visualized with (H) an epifluorescent microscope or (I–K) confocal microscope.
Virus antigen, as detected by immunohistochemistry for influenza A nucleoprotein, was abundant in spleen (six of seven positive; Fig. 1D) and liver (four of five positive; Fig. 1E) but was also detected in trachea (two of four positive), air sac (three of three positive; Fig. 1F), bronchi (one of three positive; Fig. 1G), gastrointestinal tract (two of two positive), and pancreas (one of four positive); viral antigen was not detected in brain or kidney. Infected cells included epithelium, endothelium, macrophages, circulating leukocytes, and smooth muscle of the tunica muscularis of a variety of organs and tunica media of vessels, the later particularly in spleen, liver, and gastrointestinal tract; staining was intranuclear and/or intracytoplasmic. Trachea had segmental areas where glands were distorted and lined by regenerating hyperplastic epithelium with only small amounts of viral antigen, mostly in inflammatory cells in the lamina propria and vascular endothelium. In ventriculus and intestine, viral antigen, predominantly intranuclear, was present in epithelial cells and within large vessels with necrosis and inflammation. Pancreas had severe depletion of zymogen granules (atrophy) and ductal epithelial necrosis with viral antigen in ductal epithelium. Capua et al. (1) performed immunohistochemistry in ostriches infected with HPAIV H7N1 and described viral antigen in necrotic lesions, but specific distribution and cells types involved were not detailed (1). Multiorgan antigen distribution in the birds of this report was similar to the tissue distribution determined by virus isolation in ostriches experimentally infected with emu-origin HPAIV H5N2 (2), except ostriches infected with the emu-origin HPAIV H5N2 had virus isolated from brain and kidney, and virus was not detected in those organs by immunohistochemistry in these birds. Other than spleen and brain, similar tissues were not collected from all birds, so it is difficult to make comparisons between birds as to virus distribution and abundance. However, all seven birds had splenitis with abundant viral antigen in six of seven, while zero of seven birds had brain lesions and virus was not demonstrated in any of the brains.
Expression and distribution of sialic acid (SA) receptors in tissues, specifically those with a basic composition of SAα2,3Galβ1,3(4)Glc-Nacβ1 (7), may contribute to the susceptibility of different species of birds to avian influenza virus infections (6). Strong expression of α2,3-linked SA receptors is seen in the respiratory and intestinal tracts of birds highly susceptible to avian influenza viruses, such as chickens and Pekin ducks (4), but more resistant species, such as pigeons, have weak expression of α2,3-linked SA in most tissues (5). In this report, analysis of SA receptor distribution in uninfected ostriches via lectin binding showed abundant expression of SAα2,3Gal (avian type) predominantly in goblet cells of the gastrointestinal and respiratory tracts, as well as leukocytes in the spleen and endothelial cells in all organs (Figs. 1H–K), which might help explain susceptibility of ostriches to avian influenza viruses as well as the multiorgan distribution. Distribution of SA receptors correlated with HPAIV H5N2 antigen distribution in most tissues but failed to explain lack of virus in brain, where vascular endothelium expressed SA receptors, and kidney, where both tubular epithelium and vascular endothelium expressed SA receptors. There was little or no expression of SAα2,6Gal (human type) except in endothelial cells.
ACKNOWLEDGMENTS
Funding was provided by the National Institutes of Health (NIH) and Department of Health and Human Services under Contract No. HHSN266200700007C. Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Abbreviations:
- HE
hematoxylin and eosin
- HPAIV
highly pathogenic avian influenza virus
- MAA
Maackia amurensis
- SA
sialic acid
REFERENCES
- 1.Capua I, Mutinelli F, Bozza MA, Terregino C, and Cattoli G. Highly pathogenic avian influenza (H7N1) in ostriches (Struthio camelus). Avian Pathol 29:643–646. 2000. [DOI] [PubMed] [Google Scholar]
- 2.Clavijo A, Riva R, Copps J, Robinson Y, and Zhou E. Assessment of the pathogenicity of an emu-origin influenza A H5 virus in ostriches (Struthio camelus). Avian Pathol 30:83–89. 2001. [DOI] [PubMed] [Google Scholar]
- 3.Driskell EA, Jones CA, Stallknecht DE, Howerth EW, and Tompkins SM. Avian influenza virus isolates from wild birds replicate and cause disease in a mouse model of infection. Virology 399:280–289. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Kuchipudi SV, White GA, Bain M, Chang KC, and Dunham S. Differences in influenza virus receptors in chickens and ducks: implications for interspecies transmission. J. Mol. Genet. Med 3:143–151. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Han C, Wang X, Lin J, Ma M, Shu Y, Zhou J, Yang H, Liang Q, Guo C, Zhu J, Wei H, Zhao J, Ma Z, and Pan J. Influenza A virus receptors in the respiratory and intestinal tracts of pigeons. Avian Pathol 38:263–266. 2009. [DOI] [PubMed] [Google Scholar]
- 6.Suarez DL Influenza A virus. In: Avian influenza, 1st ed. Swayne DE, ed. Blackwell Publishing, Ames, IA. pp. 3–22. 2008. [Google Scholar]
- 7.Suzuki Y Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull 28:399–408. 2005. [DOI] [PubMed] [Google Scholar]


