Abstract
Background
Cutaneous neurofibromas (cNFs) are a major cause of disfigurement in patients with Neurofibromatosis Type 1 (NF1). However, clinical trials investigating cNF treatments lack standardised outcome measures to objectively evaluate changes in cNF size and appearance. 3D imaging has been proposed as an objective standardised outcome measure however various systems exist with different features that affect useability in clinical settings. The aim of this study was to compare the accuracy, precision, feasibility, reliability and accessibility of three imaging systems.
Materials and methods
We compared the Vectra‐H1, LifeViz‐Micro and Cherry‐Imaging systems. A total of 58 cNFs from 13 participants with NF1 were selected for imaging and analysis. The primary endpoint was accuracy as measured by comparison of measurements between imaging systems. Secondary endpoints included reliability between two operators, precision as measured with the average coefficient of variation, feasibility as determined by time to capture and analyse an image and accessibility as determined by cost.
Results
There was no significant difference in accuracy between the three devices for length or surface area measurements (p > 0.05), and reliability and precision were similar. Volume measurements demonstrated the most variability compared to other measurements; LifeViz‐Micro demonstrated the least measurement variability for surface area and image capture and analysis were fastest with LifeViz‐Micro. LifeViz‐Micro was better for imaging smaller number of cNFs (1–3), Vectra‐H1 better for larger areas and Cherry for uneven surfaces.
Conclusions
All systems demonstrated excellent reliability but possess distinct advantages and limitations. Surface area is the most consistent and reliable parameter for measuring cNF size in clinical trials.
Keywords: 3D imaging system, cutaneous neurofibroma, Neurofibromatosis Type 1, outcome measure
Abbreviations
- cNF
cutaneous neurofibromas
- CV
coefficient of variation
- HFUS
high‐frequency ultrasound
- MDD
minimal detectable difference
- NF1
Neurofibromatosis Type 1
1. INTRODUCTION
Neurofibromatosis Type 1 (NF1) is an autosomal dominant neurocutaneous condition affecting approximately 1 in 1900 to 1 in 3500 individuals worldwide. 1 , 2 A hallmark feature of the disease is the development of cutaneous neurofibromas (cNFs), which are benign peripheral nerve sheath tumours manifesting in over 99% of adult NF1 patients. 3 cNFs present as discrete skin‐coloured nodules that may appear on any area of the skin. Most cNFs begin to manifest after puberty and are known to increase with age to the order of up to tens to thousands in a single individual. 4 There is wide variability in cNF burden between each patient, with phenotypes ranging from barely visible flat nodules to large pedunculated masses. 5 There is minimal genotype‐phenotype correlation between NF1 variant and severity of cutaneous manifestations, with only four genotype‐phenotype correlations, affecting 10%–15% of NF1 population, reported in the literature. 6 Although not malignant, several studies have identified that most patients find cNFs to be the most burdensome aspect of NF1 due to cosmetic disfigurement, physical discomfort and psychosocial impacts reducing their quality of life.
The management of cNFs remains a clinical challenge, with current treatment limited to tumour removal by procedures such as surgical excision, CO2 laser, electrodesiccation, radiofrequency ablation and photocoagulation. 7 , 8 , 9 , 10 , 11 , 12 There is a need for better treatment options with recent clinical trials investigating new topical and systemic drug therapies to reduce tumour burden. 4 , 13 , 14 However, a major limitation of these studies is the lack of a standardised approach to objectively measure cNFs. To properly evaluate the efficacy of treatments for improving cNF appearance, a consistent set of measurement instruments and endpoints must be established. Previous studies have explored modalities such as digital callipers and high‐frequency ultrasound (HFUS). 15 , 16 However, these methods have notable drawbacks—callipers are time‐consuming to measure multiple tumours, and HFUS is expensive and requires specialist training. 3D imaging has been proposed as a potential tool for cNF evaluation. 15 A study comparing the Vectra H1 3D Imaging camera to digital callipers and high‐frequency ultrasound for the measurement of cNFs in clinical trials found excellent reliability and feasibility with 3D imaging. 17 A comparison of five different handheld 3D imaging systems to monitor small volume enhancement in face, vulva and hand found differences in accuracy, reliability, image quality, speed of image acquisition, ease of use and cost. 18 Hence, further research is needed to identify the optimal 3D imaging system for measurements of cNFs.
This study aimed to compare and assess the accuracy, reliability, precision, feasibility and accessibility of three different 3D imaging systems: Cherry Imaging, LifeViz Micro and the Vectra H1 to inform choice of outcome measures for cNF clinical trials. Preliminary data from this study was included in a recently published review. 19
2. MATERIALS AND METHODS
2.1. Study design
Thirteen participants were recruited for imaging from the NF1 skin clinic at Royal North Shore Hospital, Sydney, Australia, from January to April 2022. Inclusion criteria were a diagnosis of NF1, age over 18 years and the presence of at least one visible cNF. Participants were excluded if they had a history of epilepsy and severe migraines, as this is a contraindication for Cherry Imaging. We used convenience sampling to maximize recruitment within the study period.
Images of up to eight cNFs from each participant were captured and analysed using each 3D imaging system: Vectra H1 (Canfield Scientific Inc, Fairfield, NJ, USA), LifeViz Micro (QuantifiCare, Biot, France) and Cherry Imaging (Yokneam, Israel). The Vectra H1 and LifeViz Micro were chosen due to their previous use in past NF1 studies measuring cNFs. 14 , 17 Cherry Imaging was chosen as a novel scanner device of interest to the authors for potential use in NF1 studies and other dermatological research. 20 There was no participant dropout as all participants were consented and imaged at the same appointment.
The primary endpoint was accuracy as measured by comparing measurements between imaging systems. Secondary endpoints included the reliability, precision, feasibility and accessibility of the imaging systems. The times required to capture an image, generate a 3D model and analyse a cNF were recorded to assess feasibility. The financial cost of the hardware and software for each imaging system was used as a metric of accessibility.
2.2. Image acquisition
cNFs were selected from the head, torso or limbs depending on suitability for imaging. Prior to imaging, a 2D photograph of the cNF region was taken for baseline comparison and location tracking (Figure 1). Images were then taken with each 3D device following manufacturer instructions.
FIGURE 1.
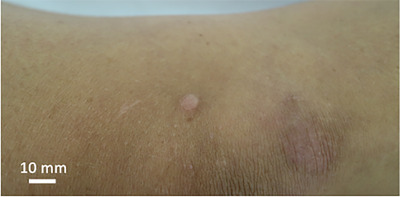
Cutaneous neurofibroma 2D image. Image was taken using a standard digital camera. Scale bar represents 10 mm.
To assess intra‐ and inter‐rater reliability, images of each cNF were taken three times with each device; twice by one investigator (J.L.) and once by a second investigator (M.G.). Repeat images were taken at least 3 min apart on the same day.
2.3. Image analysis
Images from each device were analysed by a single investigator (J.L.) on a dedicated laptop using the recommended software provided by each imaging system manufacturer. Example images are shown in Figure 2. Measurements for cNF length (maximum diameter), width (perpendicular maximum diameter), height, surface area and volume were performed on 3D models generated by all three imaging systems and recorded in millimetres. Vectra software was unable to provide tumour height measurements. Measurements performed using Cherry Imaging software were accessed via the debug console to obtain measurements in millimetres (default was centimetres).
FIGURE 2.
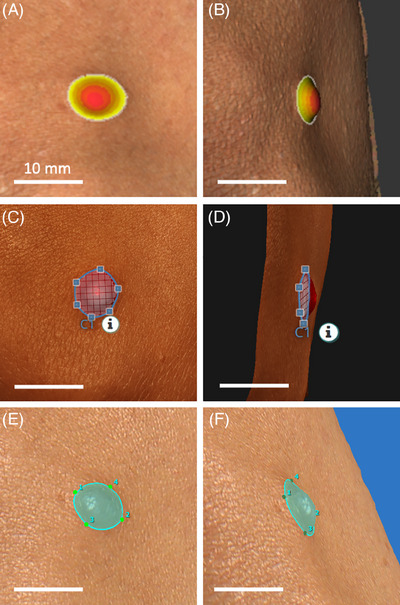
Cutaneous neurofibroma analysis on different 3D imaging systems. (A, B) 3D model analysis using Cherry Imaging Trace software. (C, D) 3D model analysis using LifeViz App software. (E, F) 3D model analysis using Vectra software. Scale bars represent 10 mm.
2.4. Statistical assessment
Statistical analyses were conducted using IBM SPSS Statistics (version 27.01). Friedman tests and Dunn–Bonferroni post hoc tests were performed to assess accuracy by comparing measurements of cNF length, width, surface area and volume between each imaging system. Height measurements between LifeViz Micro and Cherry Imaging were compared using a Wilcoxon signed‐rank test.
The intra‐ and inter‐rater reliability for image acquisition was assessed using intraclass correlation coefficients (ICC). To remain consistent with outcome measures by Thalheimer et al., ICCs ranging from 0 to 1 were considered: 0.9–1.0 ‘Excellent’, 0.75–0.89 ‘Good’, 0.5–0.74 ‘Moderate’ and < 0.5 ‘Poor’ reliability. 17
To assess precision, the average coefficient of variation (CV) was calculated for measurements from triplicate images of small (≤5 mm diameter) and large (> 5 mm diameter) cNFs. Tumours were categorised into small and large based on their median diameter as measured across all imaging systems, rounded to the nearest millimetre integer. The minimal detectable difference (MDD) for each imaging system was defined as at least twice the CV. 17
3. RESULTS
3.1. Study cohort
A total of 58 cNFs were imaged across thirteen participants with NF1 (Table 1). cNFs were located on the face, neck, chest, abdomen, back, arms and legs. There were 22 (38%) small cNFs (≤5 mm diameter) and 36 (62%) large cNFs (> 5 mm diameter). The median diameter was 6.80 mm (range 3.32–18.20 mm). Vectra H1 software was only able to render 3D models of 49 cNFs for measurement analysis, due to a technical failure. The remaining Vectra H1 images were not included in the analysis.
TABLE 1.
Participant demographics and tumour characteristics.
| Feature | Value |
|---|---|
| Participants, n | 13 |
| Age, years | 38 (24–66) |
| Sex (female) | 9 (69) |
| Fitzpatrick skin type | |
| I | 3 |
| II | 4 |
| III | 6 |
| Total cNFs imaged, n | 58 a |
| cNF locations | |
| Face | 4 (7) |
| Neck | 7 (12) |
| Chest | 9 (16) |
| Abdomen | 9 (16) |
| Back | 18 (31) |
| Arm | 9 (16) |
| Leg | 2 (3) |
| cNFs ≤ 5 mm | 22 (38) |
| cNFs > 5 mm | 36 (62) |
| Median diameter, mm | 6.80 (3.32–18.20) |
Note: Values are median (range) or n (%).
Vectra H1 software was only able to render 3D models of 49 cNFs for measurement analysis, due to a technical failure.
Abbreviation: cNF, cutaneous neurofibroma.
3.2. Accuracy
Whilst images of 58 cNFs were taken using all three devices, the Vectra H1 software was unable to render 3D models for 9 cNFs due to a software malfunction (Table 1). Hence only measurements of 49 cNFs could be used to compare the Vectra H1, LifeViz Micro and Cherry Imaging systems. Friedman tests found no significant differences between the three imaging systems for length (χ 2(2) = 3.617, p = 0.164) and surface area (χ 2(2) = 2.571, p = 0.276) measurements. Significant differences were detected for width (χ 2(2) = 15.600, p < 0.001) and volume (χ 2(2) = 31.633, p < 0.001). Compared to the Vectra H1, post hoc tests found significant differences with the LifeViz Micro for width (p < 0.001) and Cherry Imaging for volume (p < 0.001). Post hoc tests also showed differences between the LifeViz Micro and Cherry Imaging for both width (p = 0.003) and volume (p < 0.001) (Figure 3A–D).
FIGURE 3.
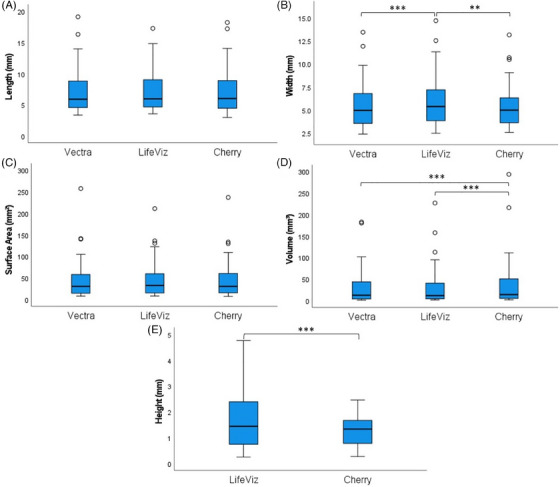
Comparison of cNF measurements between Vectra H1, LifeViz Micro and Cherry Imaging systems. Measurements were performed by a single rater. Statistical analyses for length (A), width (B), surface area (C) and volume (D) measurements were performed using the Friedman test and post hoc Dunn–Bonferroni tests (n = 49). Statistical analyses for height (E) were performed using the Wilcoxon signed‐rank test (n = 58). All analyses were conducted on IBM SPSS Statistics (ver. 27.01). Data is represented as boxplots displaying the median, IQR, minimum and maximum range of measurements. Circles represent outlier measurements. ** p < 0.01, *** p < 0.001. cNF, cutaneous neurofibromas.
For the 58 cNFs measured by the LifeViz Micro and Cherry Imaging systems, a Wilcoxon signed‐rank test showed that cNF height measurements were significantly higher by LifeViz Micro than Cherry Imaging (Z = −5.579, p < 0.001) (Figure 3E). The median (IQR) height was 1.43 (0.77–2.37) mm measured by LifeViz Micro and 1.33 (0.77–1.66) mm measured by Cherry Imaging.
3.3. Reliability
The intra‐ and inter‐rater ICC for image acquisition by each device is summarised in Table 2. All three imaging systems demonstrated “excellent” reliability (ICC ≥0.9).
TABLE 2.
Image acquisition reliability.
| Intra‐rater ICC | Inter‐rater ICC | |
|---|---|---|
| Vectra H1 | ||
| Length | 0.99 | 0.99 |
| Width | 0.99 | 0.99 |
| Surface Area | 0.99 | 0.99 |
| Volume | 0.99 | 0.99 |
| LifeViz Micro | ||
| Length | 0.99 | 0.99 |
| Width | 0.99 | 0.99 |
| Height | 0.97 | 0.99 |
| Surface Area | 0.99 | 0.99 |
| Volume | 0.97 | 0.99 |
| Cherry Imaging | ||
| Length | 0.99 | 0.99 |
| Width | 0.99 | 0.99 |
| Height | 0.99 | 0.99 |
| Surface Area | 0.99 | 0.99 |
| Volume | 0.99 | 0.99 |
| ICC | Reliability | |
|---|---|---|
| <0.5 | Poor | |
| 0.5–0.75 | Moderate | |
| 0.75–0.9 | Good | |
| 0.9–1.0 | Excellent |
Note: The inter‐rater and intra‐rater reliability for image acquisition by each 3D imaging system was assessed via ICC.
Abbreviations: cNF, cutaneous neurofibroma; ICC, intraclass correlation coefficients.
3.4. Measurement variability and MDD
The average CV and MDD for cNF measurements are summarised in Table 3. Measurements of small cNFs generally had a higher CV than for large cNFs. Overall, volume measurements displayed the most measurement variability, followed by height measurements. For surface area, the LifeViz Micro demonstrated the least measurement variability compared to the other two imaging systems.
TABLE 3.
Measurement variability and proposed treatment response thresholds.
| CV | MDD | |||
|---|---|---|---|---|
| Small cNF CV | Large cNF CV | Threshold to detect change in size of small cNF | Threshold to detect change in size of large cNF | |
| Vectra H1 | ||||
| Length | 3.8% | 3.4% | 10% | 10% |
| Width | 5.5% | 3.2% | 15% | 10% |
| Surface Area | 5.7% | 3.9% | 15% | 10% |
| Volume | 8.8% | 7.1% | 20% | 20% |
| LifeViz Micro | ||||
| Length | 3.2% | 3.2% | 10% | 10% |
| Width | 4.8% | 4.2% | 10% | 10% |
| Height | 8.0% | 6.3% | 20% | 15% |
| Surface Area | 4.8% | 3.5% | 10% | 10% |
| Volume | 11.0% | 6.6% | 25% | 15% |
| Cherry Imaging | ||||
| Length | 5.5% | 4.3% | 15% | 10% |
| Width | 4.3% | 3.9% | 10% | 10% |
| Height | 7.6% | 5.7% | 20% | 15% |
| Surface Area | 7.3% | 6.6% | 15% | 15% |
| Volume | 8.8% | 9.8% | 20% | 20% |
Note: Small cNFs were defined as ≤5 mm in diameter, large cNFs were defined as >5 mm in diameter. The MDD for each imaging system was defined as at least twice the CV. MDD were used to propose treatment response thresholds to detect a change in cNF size in future clinical studies. The following colours represent thresholds for MDD: orange = 21%–25%; yellow = 16%–20%; blue = 11%–15% and green = ≤ 11%.
Abbreviations: cNF, cutaneous neurofibroma; CV, coefficient of variation; MDD, minimal detectable difference.
3.5. Time for image acquisition and analysis, and cost of hardware and software
Time taken for image acquisition and analysis, and cost of the three imaging systems were reported in a recent review.19 To summarise, Cherry Imaging required the longest time for image acquisition (0.5–2 min) and 3D model generation (1.0–6.5 min), whereas Vectra H1 software required the longest time for analysis (5.0–9.5 min). Cumulatively, LifeViz Micro had the shortest total image acquisition and analysis time (∼4.5 min) compared to Vectra H1 (∼7.5 min) and Cherry Imaging (∼6.5 min). Cherry Imaging was the most expensive system (∼$34 000), followed by LifeViz Micro (∼$28 000) and Vectra H1 (∼$17 000).
4. DISCUSSION
This is the first study to compare different 3D imaging systems to identify the most suitable imaging system and cNF measurement parameter for evaluation of cNFs in clinical trials. The three 3D imaging systems investigated in this study were all effective and reliable tools for providing quantitative measurements of cNF size. Each device possesses distinct strengths and limitations that may impact their utility when used in a clinical setting. Based on our findings and experience with each device, the LifeViz Micro demonstrated best precision for surface area measurements, and the shortest time for image acquisition and analysis. For quantitative analysis in clinical trials, change in cNF surface area is recommended as the primary endpoint, since this was one of the most consistent and reliable measurement parameters examined.
4.1. Comparison of 3D imaging system accuracy, reliability and precision
Significant differences between the Vectra H1, LifeViz Micro and Cherry Imaging devices were found for width, volume and height measurements (p < 0.001). It was only for length and surface area that no significant differences were found (p > 0.05) suggesting these are the optimal parameters to use in future studies for measuring cNFs.
All three devices demonstrated ‘excellent’ intra‐ and inter‐rater reliability for image acquisition, with ICC > 0.9 for all measurement parameters. These findings are consistent with previous reports for the Vectra H1 17 , 18 and the LifeViz Micro. 21
Similar to Thalheimer et al., 17 small cNF measurements in this study displayed greater variability compared to larger cNFs, suggesting that larger cNFs are more suitable target lesions for investigation in clinical trials as 3D imaging may not be sensitive to treatment‐induced changes in small cNFs. Volume measurements demonstrated the most variability compared to surface area and linear measurements similar to another validation study using Cherry Imaging. 22
MDD thresholds to detect a change in cNF size ranged between 10% and 15% for length and width, 15%–20% for height, 10%–15% for surface area and 15%–25% for volume measurements, depending on the device and cNF size. These thresholds were lower than those proposed by Thalheimer et al. who proposed thresholds of 25% for linear and surface area measurements, and 40% for volume measurements of large cNFs ≥5 mm. 17
4.2. Feasibility and practicality of each 3D imaging system
The Vectra H1 and LifeViz Micro acquire images rapidly and require little training to operate. The main difference between the two cameras is that the Vectra H1 captures a larger image area, allowing for the assessment of multiple cNFs in a single image. On the other hand, the LifeViz Micro provides a higher model resolution due to its smaller image size, which may increase the precision when measuring minute changes in cNF size.
In contrast, Cherry Imaging is an excellent asset for scanning faces and curved parts of the body, but imaging can take several minutes to perform. This can be inconvenient if wanting to perform multiple scans of a patient in one sitting, due to the accumulative wait times. Additionally, it is contraindicated for use on patients with epilepsy or severe migraines and must be connected physically to a dedicated laptop or computer while in use.
Another point of consideration is the software capability of each imaging system for cNF analysis. Both LifeViz Micro and Cherry Imaging could provide simultaneous data for all measurement parameters (length, width, height, surface area and volume) after highlighting a cNF for analysis using the software (Figure 2). This made cNF analysis very efficient compared to the Vectra software, which required almost twice the amount of time. Additionally, Vectra software by default calculates volume using an interpolated base surface, which underestimates cNF volume compared to the other imaging systems. Hence in this study, we had to use multiple functions to select and ‘fill’ each cNF model for analysis on Vectra, after which its volume could be calculated appropriately.
Furthermore, 3D models generated by all imaging systems were distorted by bodily hair. As such, studies utilising 3D imaging should avoid selecting cNFs on or near the hairline, or request patients to shave or use hair removal preparations before imaging.
4.3. Recommendations for cNF analysis in clinical trials and practice
For future studies, we recommend selecting at least five cNFs per patient for 3D modelling and quantitative analysis. Suitable lesions ideally should be non‐pedunculated >5 mm in diameter with a clearly defined border, and not be obscured by hair or clothing. 2D photography should be used to document cNF locations to ensure that the same cNF is measured throughout the study.
Reliable and reproducible imaging is crucial for multisite clinical trials. Although the three imaging systems used here showed similar reliability, working with the same system across sites is most desirable, and ensuring local systems are in place for troubleshooting and maintenance is an important consideration.
4.4. Study limitations and future research
Whilst we have proposed MDD thresholds to detect size changes of small and large cNFs in Table 3, these may need to be refined after further investigation by studies with a larger sample size of cNFs. Future studies could also investigate how patient factors such as skin colour and cNF phenotype may affect the quality of 3D images. However, further investigation of new devices may be recommended as 3D technologies evolve and refine their capabilities. A recent review of imaging modalities for cNFs identified several techniques including digital callipers, 3D whole body photography, high‐frequency ultrasound sonography, spatial frequency domain imaging and optical coherence tomography.19 Further research comparing these techniques is needed to identify the most appropriate modalities for use in international, multi‐centre cNF therapeutic trials.
5. CONCLUSION
The use of 3D imaging is a reliable and effective tool for quantitative analysis of cNFs, enabling objective assessment of treatment outcomes. We identify cNF surface area as the most suitable primary endpoint for cNF imaging. The Vectra H1, LifeViz Micro and Cherry Imaging each possess distinct strengths and limitations that need to be considered.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Northern Sydney Local Health District Human Research Ethics Committee (REGIS: 2019/ETH08177). Written consent was obtained from all patients prior to participation in the study.
ACKNOWLEDGEMENTS
We would like to thank the individuals with NF1 for taking the time to contribute to this study. Medical writing services provided by Holly Smith from WriteSource Medical were funded by Northern Sydney Local Health District in accordance with Good Publication Practice (GPP3) guidelines (http://ismpp.org/gpp3). This study received funding support from the Neurofibromatosis Therapeutic Acceleration Program and NSW Office of Health and Medical Research.
Lau JCL, Fleming J, Good M, et al. Comparing 3D imaging devices for the measurement of cutaneous neurofibromas in patients with Neurofibromatosis Type 1. Skin Res Technol. 2024;30:e70020. 10.1111/srt.70020
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, B.D.Y., upon reasonable request.
REFERENCES
- 1. Uusitalo E, Leppävirta J, Koffert A, et al. Incidence and mortality of neurofibromatosis: a total population study in Finland. J. Invest. Dermatol. 2015;135:904. [DOI] [PubMed] [Google Scholar]
- 2. Legius E, Messiaen L, Wolkenstein P, et al. Revised diagnostic criteria for Neurofibromatosis Type 1 and Legius syndrome: an international consensus recommendation. Genet. Med. 2021;23(8):1506‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huson S, Harper P, Compston D. Von Recklinghausen neurofibromatosis: a clinical and population study in south‐east Wales. Brain. 1988;111:1355‐1381. [DOI] [PubMed] [Google Scholar]
- 4. Chamseddin BH, Le LQ. Management of cutaneous neurofibroma: current therapy and future directions. Neurooncol Adv. 2020;2:i107‐i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ortonne N, Wolkenstein P, Blakeley JO, et al. Cutaneous neurofibromas: current clinical and pathologic issues. Neurology. 2018;91:S5‐S13. [DOI] [PubMed] [Google Scholar]
- 6. Bettegowda C, Upadhayaya M, Evans DG. et al. Genotype‐phenotype correlations in neurofibromatosis and their potential clinical use. Neurology. 2021;97:S91‐S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamseddin BH, Hernandez LN, Solorzano D, Vega J, Le LQ. Robust surgical approach for cutaneous neurofibroma in Neurofibromatosis Type 1. JCI Insight. 2019;5:e128881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levine SM, Levine E, Taub PJ, Weinberg H. Electrosurgical excision technique for the treatment of multiple cutaneous lesions in Neurofibromatosis Type I. J Plast Reconstr Aesthet Surg. 2008;61:958‐962. [DOI] [PubMed] [Google Scholar]
- 9. Méni C, Sbidian E, Moreno JC, et al. Treatment of neurofibromas with a carbon dioxide laser: a retrospective cross‐sectional study of 106 patients. Dermatology. 2015;230:263‐268. [DOI] [PubMed] [Google Scholar]
- 10. Peltonen S, Jannic A, Wolkenstein P. Treatment of cutaneous neurofibromas with carbon dioxide laser: technique and patient experience. Eur J Med Genet. 2022;65:104386. [DOI] [PubMed] [Google Scholar]
- 11. Kim S‐H, Roh S‐G, Lee N‐H, Yang K‐M. Radiofrequency ablation and excision of multiple cutaneous lesions in Neurofibromatosis Type 1. Arch plas surg. 2013;40:57‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elwakil TF, Samy NA, Elbasiouny MS. Non‐excision treatment of multiple cutaneous neurofibromas by laser photocoagulation. Lasers Med Sci. 2008;23:301‐306. [DOI] [PubMed] [Google Scholar]
- 13. Kriechbaumer LK, Susani M, Kircher SG, Distelmaier K, Happak W. Comparative study of CO2‐ and Er:YAG laser ablation of multiple cutaneous neurofibromas in von Recklinghausen's disease. Lasers Med Sci. 2014;29:1083‐1091. [DOI] [PubMed] [Google Scholar]
- 14. Slopis JM, Arevalo O, Bell CS, et al. Treatment of disfiguring cutaneous lesions in Neurofibromatosis‐1 with everolimus: a Phase II, open‐label, single‐arm trial. Drugs R. D. 2018;18:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cannon A, Chen M‐J, Li P, et al. Cutaneous neurofibromas in Neurofibromatosis Type I: a quantitative natural history study. Orphanet. J. Rare. Dis. 2018;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raffin D, Zaragoza J, Georgescou G, et al. High‐frequency ultrasound imaging for cutaneous neurofibroma in patients with Neurofibromatosis Type I. Eur J Dermatol. 2017;27:260‐265. [DOI] [PubMed] [Google Scholar]
- 17. Thalheimer RD, Merker VL, Ly KI, et al. Validating Techniques for Measurement of Cutaneous Neurofibromas: Recommendations for Clinical Trials. Neurology. 2021;97:S32‐S41. [DOI] [PubMed] [Google Scholar]
- 18. Almadori A, Speiser S, Ashby I, et al. Portable three‐dimensional imaging to monitor small volume enhancement in face, vulva, and hand: a comparative study. J Plast Reconstr Aesthet Surg. 2022;75:3574‐3585. [DOI] [PubMed] [Google Scholar]
- 19. Li Y, Blakeley JO, Ly I, et al. Current and emerging imaging techniques for Neurofibromatosis Type 1‐associated cutaneous neurofibromas. J Invest Dermatol. 2023;143(8):1397‐1405. [DOI] [PubMed] [Google Scholar]
- 20. Horovitz T, Salameh F, Shehadeh W, Koren A, Artzi O. Painting CROSS TCA technique: modification of the CROSS method for the treatment of atrophic acne scars—case series. J Cosmet Dermatol. 2022;21:327‐330. [DOI] [PubMed] [Google Scholar]
- 21. Petit L, Zugaj D, Bettoli V, et al. Validation of 3D skin imaging for objective repeatable quantification of severity of atrophic acne scarring. Skin Res Technol. 2018;24:542‐550. [DOI] [PubMed] [Google Scholar]
- 22. Hendel K, Ortner VK, Fuchs CS, Eckhouse V, Haedersdal M. Dermatologic scar assessment with stereoscopic imaging and digital three‐dimensional models: a validation study. Lasers Surg. Med. 2021;53:1043‐1049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, B.D.Y., upon reasonable request.


