Abstract
Background
Psychiatric comorbidities are common in patients with epilepsy. Reasons for the co-occurrence of psychiatric conditions and epilepsy remain poorly understood.
Aim
We aimed to triangulate the relationship between epilepsy and psychiatric conditions to determine the extent and possible origins of these conditions.
Methods
Using nationwide Swedish health registries, we quantified the lifetime prevalence of psychiatric disorders in patients with epilepsy. We then used summary data from genome-wide association studies to investigate whether the identified observational associations could be attributed to a shared underlying genetic aetiology using cross-trait linkage disequilibrium score regression. Finally, we assessed the potential bidirectional relationships using two-sample Mendelian randomisation.
Results
In a cohort of 7 628 495 individuals, we found that almost half of the 94 435 individuals diagnosed with epilepsy were also diagnosed with a psychiatric condition in their lifetime (adjusted lifetime prevalence, 44.09%; 95% confidence interval (CI) 43.78% to 44.39%). We found evidence for a genetic correlation between epilepsy and some neurodevelopmental and psychiatric conditions. For example, we observed a genetic correlation between epilepsy and attention-deficit/hyperactivity disorder (rg=0.18, 95% CI 0.09 to 0.27, p<0.001)—a correlation that was more pronounced in focal epilepsy (rg=0.23, 95% CI 0.09 to 0.36, p<0.001). Findings from Mendelian randomisation using common genetic variants did not support bidirectional effects between epilepsy and neurodevelopmental or psychiatric conditions.
Conclusions
Psychiatric comorbidities are common in patients with epilepsy. Genetic correlations may partially explain some comorbidities; however, there is little evidence of a bidirectional relationship between the genetic liability of epilepsy and psychiatric conditions. These findings highlight the need to understand the role of environmental factors or rare genetic variations in the origins of psychiatric comorbidities in epilepsy.
Keywords: neuropsychiatry, psychiatry
WHAT IS ALREADY KNOWN ON THIS TOPIC
Although psychiatric comorbidities are common in patients with epilepsy, the reasons for the co-occurrence of psychiatric conditions and epilepsy remain poorly understood.
WHAT THIS STUDY ADDS
Almost half of the 94 435 individuals diagnosed with epilepsy in Sweden were also diagnosed with psychiatric conditions during their lifetime.
Some psychiatric conditions share an underlying genetic architecture with epilepsy, which may be more pronounced in focal epilepsy.
Mendelian randomisation analyses did not support the hypothesis that epilepsy causally increases the risk of psychiatric conditions and vice versa.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The lack of widespread genetic correlation and effects in either direction suggests that environmental factors may explain psychiatric comorbidities in epilepsy.
Introduction
Epilepsy co-occurs with psychiatric conditions throughout life and contributes to the challenges faced by people with epilepsy. For example, Swedish register-based studies have suggested that the lifetime prevalence of a psychiatric diagnosis among people with epilepsy is as high as 40%, compared with ∼10% of the general population.1 Individuals with epilepsy are more likely to have neurodevelopmental disorders (eg, autism, attention-deficit/hyperactivity disorder, tic disorders and obsessive-compulsive disorders) as well as other psychiatric conditions (eg, anorexia nervosa, anxiety, bipolar disorder, depression and schizophrenia). However, the reasons for these co-occurrences remain largely unknown.2–4
Few large-scale studies have examined the population-wide comorbidity of epilepsy and psychiatric disorders,5 6 possible genetic correlations between comorbidities7–9 or the direction of the effects using Mendelian randomisation (MR).10 11 Psychiatric comorbidities in epilepsy may be a function of shared genetic aetiology, bidirectional relationships, shared environmental aetiology, chance and artefactual processes or a combination of these.2 A better understanding of the mechanisms underlying psychiatric comorbidities in epilepsy may help identify targets for the prevention or treatment of these comorbidities.
It is often suggested that the psychiatric comorbidities in epilepsy are explained by bidirectional relationships,12 where psychiatric conditions increase the risk of epilepsy, and vice versa, that epilepsy increases the risk of psychiatric conditions. This notion is supported by a series of neurobiological pathogenic mechanisms (eg, induced abnormalities in cortical and subcortical structures).13 Yet, observational studies contributing to this hypothesis12 have typically been limited in sample size, liable to confounding factors and/or have been restricted to clinical cohorts with limited generalisability.
In this study, we used novel and standard methods to triangulate the relationship between epilepsy and psychiatric conditions (online supplemental figure 1). First, using Swedish health registers, we characterised the rate of comorbidity of different psychiatric conditions in the largest nationwide cohort of individuals with epilepsy. Second, we investigated whether any comorbidities could be attributed to a shared underlying genetic architecture by assessing genetic correlations using cross-trait linkage disequilibrium score regression.14 15 Finally, we examined the possibility of bidirectional effects by using MR, a design that leverages the random assortment of genotypes associated with epilepsy and psychiatric disorders, and allows for the exploration of the direction of effects.16
gpsych-2023-101201supp001.pdf (1MB, pdf)
Methods
All research was performed in accordance with the relevant guidelines and regulations.
Swedish population-based registries
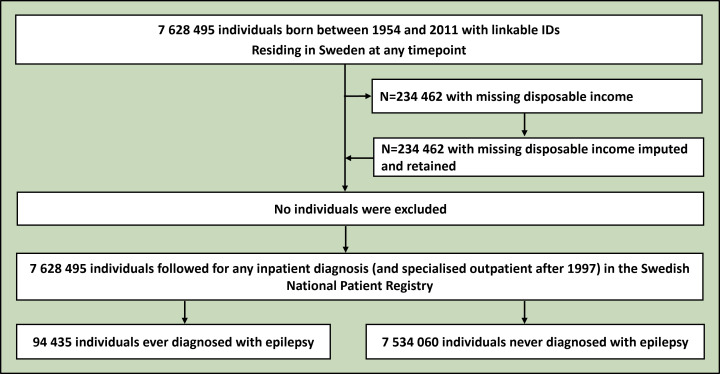
To estimate the population-level co-occurrence of epilepsy and psychiatric conditions, we derived a nationwide cohort of 7 628 495 individuals born between 1954 and 2011 from the Swedish population registries (figure 1). Using the Total Population Registry and the National Patient Registry (initiation 1969, end of follow-up 2016), we collected information on vital statistics, immigration status (born in or outside Sweden), birth year and any inpatient diagnosis (and specialised outpatient after 1997) of the investigated psychiatric conditions and epilepsy (see online supplemental table 1 for International Classification of Diseases (ICD) 8/9/10 codes). We also identified disposable income quintiles for each year between 1968 and 2016 and retained the highest quintile obtained over a person’s lifetime from the Income and Taxation Registry, which we conceptualised as both a socioeconomic proxy and a measure of resilience to morbidity manifestation. Individuals born after 1996 with missing disposable income values were designated as a distinct category, as they were probably too young to have accumulated disposable income.
Figure 1.
Flowchart depicting the derivation of the analytical sample from the Swedish registry data. No individuals were excluded from the analysis. Those missing disposable income were imputed, as they were either too young to have accumulated any disposable income, or this information was missing. Further details are available in the text.
The National Patient Registry records the date of diagnosis and not the date of onset. Therefore, we focused solely on estimating lifetime comorbidities of epilepsy and psychiatric disorders.
Statistical methods
Logistic regression was used to estimate the crude and conditional odds ratio (OR) and 95% confidence interval (CI) of a lifetime diagnosis of psychiatric conditions among individuals ever diagnosed with epilepsy (n=94 435) compared with those who were never diagnosed (n=7 534 060), controlling for birth year, sex, highest disposable income achieved and immigration status. We also estimated the crude and counterfactual probabilities of the outcomes (holding all covariates at their initial values). Hereafter, we refer to this quantity as the adjusted lifetime prevalence. This is because it captures the estimated prevalence among those with epilepsy if they had the same covariate distribution as those without epilepsy, and vice versa. In the primary analysis, we did not separate the types of epilepsy, as they are often not well characterised in the National Patient Registry and because many people with epilepsy often have multiple seizure types. However, in a complementary analysis, we classified individuals based on the most recent instance in which they received either a focal (n=24 430, 25.9%) or generalised epilepsy diagnosis (n=19 199, 20.3%), although some individuals remained uncharacterised (either unspecific (n=32 468, 34.4%) or were never coded with sufficient detail (n=18 079, 19.1%)). We chose not to classify 259 (0.3%) individuals who were discharged with both focal and generalised ICD codes on their most recent visit. In principle, there should be no missing data in the analysed variables (reporting mandated by Swedish law). However, there was some missing data in disposable income (≈3%), and we treated these as a distinct income category.
Genome-wide association data sources and phenotypes
Summary statistics were retrieved from the largest European ancestry genome-wide association study (GWAS) of epilepsy performed by the International League Against Epilepsy (ILAE) Consortium on Complex Epilepsies.7 In brief, all epilepsy cases were classified according to ILAE standard and all phenotypes were assessed by epilepsy specialists at individual sites. Controls (persons without epilepsy) were derived from the available population-based datasets within the Consortium on Complex Epilepsies and partially screened for the presence of neurological conditions. Extended phenotypic information is available in the original publication.7 To reduce bias from population stratification, we limited our investigation to a European ancestry sample, which was identified using principal component analysis in the original GWAS.7 Given the phenotypic and genetic heterogeneity of epilepsies, we retrieved summary statistics separately for epilepsy (all epilepsies) (n cases=27 559), focal epilepsies (n cases=14 939) and genetic generalised epilepsies (n cases=6952) (table 1) per the diagnoses of epilepsy specialists in the original GWAS.7
Table 1.
The investigated phenotypes, number of cases and controls contributing to the GWAS and the number of single-nucleotide polymorphisms qualifying as instruments for Mendelian randomisation
| Phenotype | Study/Consortium | N cases | N controls | Number of instruments (mean F-statistic) |
| Epilepsy (broadly defined)* | ILAE7 | 27 559 | 42 436 | 36–42 (25.38–25.54) |
| Generalised epilepsy | ILAE7 | 6952 | 42 436 | 65–80 (27.63–27.93) |
| Focal epilepsy | ILAE7 | 14 939 | 42 436 | 24–28 (23.06–23.15) |
| Anorexia nervosa | Watson et al 17 | 16 992 | 55 525 | 39 (24.33) |
| Anxiety disorder | Otowa et al 18 | 7016 | 14 745 | 3 (26.21) |
| Attention-deficit/hyperactivity disorder | Demontis et al 19 | 38 691 | 186 843 | 102 (27.19) |
| Autism | Grove et al 20 | 18 381 | 27 969 | 21 (24.26) |
| Bipolar disorder | Mullins et al 21 | 41 917 | 371 549 | 187 (27.14) |
| Depression | Als et al 22 | 176 143 | 528 770 | 174 (27.85) |
| Intelligence | Savage et al 23 | 269 867 | – | 487 (30.96) |
| OCD | IOCDF-GC and OCGAS24 | 2688 | 7037 | 7 (22.04) |
| Schizophrenia | Trubetskoy et al 25 | 67 390 | 94 015 | 489 (32.35) |
| Suicide attempts | Mullins et al 26 | 26 590 | 492 022 | 29 (24.32) |
| Tic-disorders (Tourette syndrome) | Yu et al 27 | 4819 | 9488 | 9 (23.81) |
*For epilepsy, the number of instruments and mean F-statistic varied (past the decimal) due to individual harmonisation to outcome GWAS (a procedure employed to maximise power), reported values represent the minimum and maximum values.
GWAS, genome-wide association studies; ILAE, International League Against Epilepsy; IOCDF-GC, International Obsessive Compulsive Disorder Foundation Genetics Collaborative; OCD, obsessive-compulsive disorder; OCGAS, OCD Collaborative Genetics Association Studies.
We then extracted summary statistics from the European ancestry GWAS of commonly studied psychiatric comorbidities of epilepsy (table 1). The recent ILAE GWAS7 performed linkage disequilibrium score regression (LDSC) on some psychiatric phenotypes. However, we extended this analysis by using larger GWAS datasets and investigating a broader spectrum of psychiatric conditions. These included GWAS on anorexia nervosa,17 anxiety,18 attention-deficit/hyperactivity disorder,19 autism,20 bipolar disorder,21 depression,22 intelligence,23 obsessive-compulsive disorder,24 schizophrenia,25 suicide attempts26 and tic-disorders (Tourette syndrome).27 Detailed descriptions of the phenotypes and GWAS analyses can be found in the original publications.17–27
Genetic correlations—cross-trait linkage disequilibrium score regression
To quantify the genetic correlation between epilepsy and psychiatric comorbidities, we employed cross-trait LDSC.14 15 LDSC leverages the pattern of linkage disequilibrium among common genetic variants to estimate the genetic correlation across phenotypes.15 We obtained precomputed linkage disequilibrium scores based on the 1000 Genomes Project European Reference Panel. We estimated the LDSC using an unconstrained intercept to allow for sample overlap and population stratification. Univariate LDSC criteria for consistent estimation appeared to be generally satisfied (heritability Z-score >1.5, mean χ2 >1.02, single-nucleotide polymorphism (SNP) heritability intercept 0.9 to 1.1).28 A Bonferroni correction of 11×3 test was applied for the genetic correlation analysis (α=0.001515).
Mendelian randomisation
To assess the potential effect of epilepsy on psychiatric conditions and these conditions on epilepsy, we employed bidirectional two-sample MR. MR is based on the principles of instrumental variable analysis using common genetic variants that are robustly associated with a phenotype (exposure) of interest as instruments, yielding an estimate of the causal effect of genetic liability for a phenotype on the outcome of interest if the genetic variant is a valid instrument.16 A genetic variant is a valid instrument if (i) it is robustly associated with the exposure (relevance), (ii) it is not associated with any measured/unmeasured confounders of the exposure-outcome associations (independence) and (iii) it does not affect the outcome via pathways other than the potential effect on the exposure of interest (exclusion restriction).29 Importantly, as genetic variants are fixed at conception and randomly assorted during meiosis, MR is considered robust against reverse causality and many forms of environmental confounding factors.
In two-sample MR, SNP-exposure and SNP-outcome estimates are extracted from two independent GWASs of the same underlying population.29 In bidirectional MR, the procedure is repeated in both postulated directions in separate analyses.
To derive the instruments for our MR analyses, we pooled exposure and outcome GWAS data and excluded non-overlapping SNPs. To retain the highest number of SNPs, we repeated this procedure for each psychiatric GWAS separately. From these datasets, we then extracted all variants with a p value <5×10−06, a threshold employed to maximise power. This inclusive approach may, under some scenarios, lead to a conservative estimate in the two-sample MR because of weak instruments (so-called weak instrument bias), assuming the absence of sample overlap between GWASs. The same p value threshold has been previously employed in studies of psychiatric phenotypes.30 The identified variants were clumped using PLINK LD-clumping, with an R2 <0.01 and a 10 000 kb window, based on the 1000 Genome European phase III reference panel. SNPs from the epilepsy GWAS were then extracted from the GWAS of each outcome and harmonised to ensure that the effect estimates were expressed on the same allele.31
For the primary analysis, we employed the inverse-variance-weighted (IVW) method, which estimates the causal effect under the assumption that all instruments are valid.29 Specifically, the IVW method is the ratio of SNP-exposure and SNP-outcome associations weighted by their relative precision. Because the assumptions of IVW are strong32 (eg, no horizontal pleiotropy), we performed a series of standard sensitivity analyses to assess the robustness of the IVW causal effect estimates (see online supplemental material). A Bonferroni correction of 11×3×2 test was applied for the MR (α=0.00075758).
Results
Population-wide lifetime comorbidity
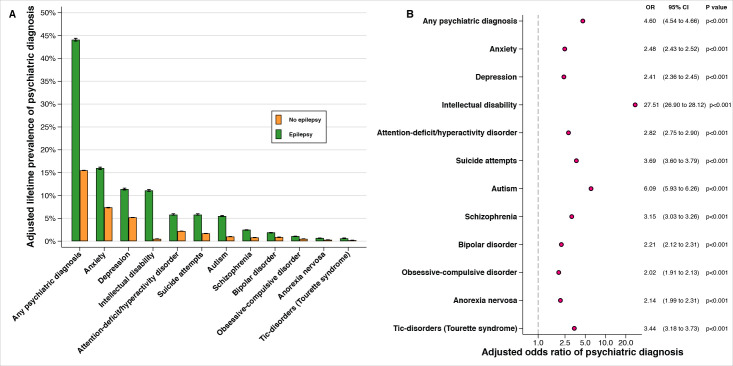
We observed substantial psychiatric morbidity among patients with epilepsy. Almost half of all individuals diagnosed with epilepsy were also diagnosed with a (neuro-)psychiatric disorder in their lifetime (adjusted lifetime prevalence (%) 44.09, 95% CI 43.78 to 44.39; OR 4.60, 95% CI 4.54 to 4.66, p<0.001) (figure 2A), compared with 15.52% among those with no diagnosis of epilepsy (adjusted lifetime prevalence (%) 15.52, 95% CI 15.50 to 15.55) (for extended results, see online supplemental table 2). Each of the considered psychiatric disorders was more common among those diagnosed with epilepsy (figure 2B), than among those who were not, although the absolute lifetime prevalence of specific psychiatric disorders varied according to the underlying prevalence in the population. There was no apparent distinction between the co-occurrence of neurodevelopmental and psychiatric disorders; both forms of comorbidity were more common among those with epilepsy. The highest relative occurrence was observed for autism (OR 6.09, 95% CI 5.93 to 6.26) and intellectual disability. Intellectual disability was 27 times more common among those with epilepsy (adjusted lifetime prevalence (%) 11.12, 95% CI 10.94 to 11.30; OR 27.51, 95% CI 26.9 to 28.12), compared with those not diagnosed with epilepsy (adjusted lifetime prevalence (%) 0.51, 95% CI 0.51 to 0.52).
Figure 2.
The population-wide co-occurrence of epilepsy and psychiatric morbidities. (A) The adjusted (marginal) lifetime prevalence of psychiatric diagnoses among those with (n=94 435) and without an epilepsy diagnosis (n=7 534 060). (B) The adjusted (conditional) OR of psychiatric diagnoses comparing those with and without an epilepsy diagnosis. All analyses are adjusted for birth year, sex, highest achieved disposable income and immigration status. Both panels are sorted according to the adjusted lifetime prevalence among those with an epilepsy diagnosis. Black lines indicate 95% CIs in both panels (CIs are plotted in (B) but they are smaller than the marker size, reflecting our large sample size). CI, confidence interval; OR, odds ratio.
The estimated lifetime comorbidity did not materially differ before or after controlling for covariates, although all estimates were elevated in crude analysis (unadjusted lifetime prevalence of any psychiatric diagnosis (%) 48.61, 95% CI 48.29 to 48.93; OR 5.16, 95% CI 5.09 to 5.23, p<0.001) (online supplemental figure 2 and online supplemental table 2). Lifetime comorbidity did not vary materially between men and women (online supplemental figure 3) or between focal and generalised epilepsy (online supplemental figure 4) and was not limited to recent birth cohorts (online supplemental figure 5).
Genetic correlation
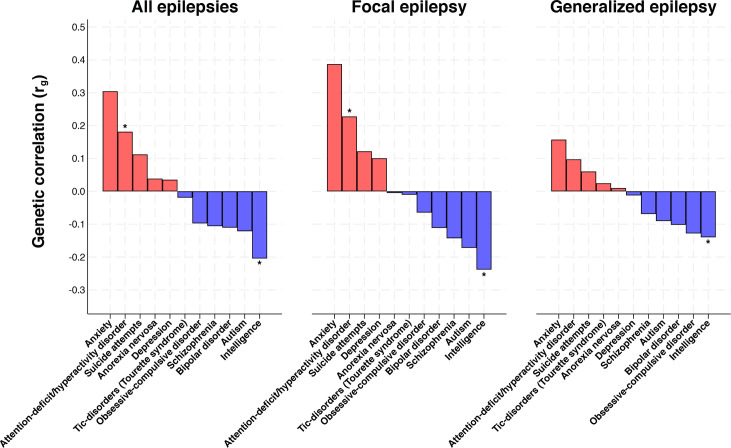
We observed a positive genetic correlation between epilepsy and attention-deficit/hyperactivity disorder (rg=0.18, 95% CI 0.09 to 0.27, p<0.001) (figure 3) (for extended results see online supplemental tables 3–5). The genetic correlation between epilepsy and attention-deficit/hyperactivity disorder was more pronounced for focal epilepsy (rg=0.23, 95% CI 0.09 to 0.36, p<0.001). Furthermore, we identified a negative genetic correlation between epilepsy and intelligence (rg=−0.20, 95% CI −0.28 to −0.13, p<0.001), which was also more pronounced for focal epilepsy (rg=−0.24, 95% CI −0.36 to −0.12, p<0.001). The negative genetic correlation between epilepsy and intelligence may be consistent with the observational association between epilepsy and intellectual disability, as the intelligence scale is inversely related to intellectual disability. We observed modest-to-weak genetic correlations between the remaining psychiatric disorders and epilepsy, regardless of the type of epilepsy (figure 3).
Figure 3.
The genetic correlation between epilepsy (all epilepsies, generalised and focal) and common psychiatric comorbidities of epilepsy. Estimates represent the genetic correlation obtained from cross-trait linkage disequilibrium score regression. Asterisks reflect a p value lower than the Bonferroni-corrected threshold (α=0.001515).
Mendelian randomisation
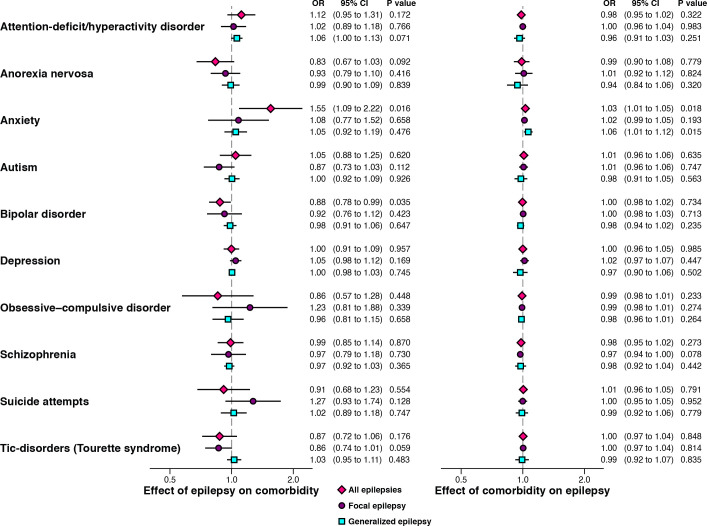
In the MR analyses, limited evidence existed of an association between genetic liability to epilepsy and any of the considered psychiatric conditions after Bonferroni correction (figure 4) (for extended results, see online supplemental tables 3–5). The largest observed association was for genetic liability to epilepsy and anxiety disorder, which did not pass Bonferroni correction (p>0.000758). Specifically, genetic liability to epilepsy was associated with an increased likelihood of anxiety disorder (OR 1.55, 95% CI 1.09 to 2.22, p=0.016); a finding that was not replicated in sensitivity analysis (online supplemental methods and online supplemental table 7).
Figure 4.
The bidirectional relationship between genetic liability to epilepsy (all epilepsies, generalised and focal) and genetic liability to common psychiatric comorbidities of epilepsy. The estimates are ORs obtained from an inverse-variance weighted regression, which assumes that all variants are valid instruments. Findings for intelligence are presented in-text, as intelligence is measured on a continuous scale and therefore not scale-comparable to the other traits. Both x-axes are plotted on the natural logarithmic scale. CI, confidence interval; OR, odds ratio.
Limited evidence existed of an association between genetic liability to most psychiatric conditions and epilepsy (figure 4). One exception was intelligence. Genetic predisposition to higher intelligence reduced the likelihood of epilepsy (OR 0.89, 95% CI 0.85 to 0.93, p<0.001), and the focal (OR 0.88, 95% CI 0.84 to 0.92, p<0.001) and generalised subtypes of epilepsy (OR 0.87, 95% CI 0.87 to 0.94, p<0.001).
Although there were strong assumptions in our primary MR analysis (IVW), our findings were mostly consistent across sensitivity analyses (online supplemental table 7). Generally, our sensitivity analysis was inconsistent with widespread horizontal pleiotropy in our MR analysis (online supplemental figure 6 and online supplemental table 6). That is, the MR-Egger intercepts did not suggest that the selected genes were invalid instruments through an independent effect on the outcome (violation of the third MR assumption: exclusion restriction).
Discussion
Main findings
Using data from nationwide health registries in Sweden, we found that individuals with epilepsy had a substantially higher prevalence of neurodevelopmental and psychiatric comorbidities. Using cross-trait linkage disequilibrium analysis, we found genetic correlations between epilepsy and certain psychiatric conditions, which, in some cases, such as attention-deficit/hyperactivity disorder, were more pronounced in focal epilepsy. The majority of genetic correlations observed between epilepsy and psychiatric conditions were modest, supporting the partial role of a genetic basis for these comorbidities. Finally, by leveraging common genetic variations, we did not find strong evidence to suggest that these comorbidities are explained by the effects of epilepsy on psychiatric conditions or psychiatric conditions on epilepsy. Taken together, these findings suggest that there are important genetic correlations between epilepsy and some psychiatric conditions. However, these are unlikely to explain the full extent of psychiatric comorbidities in epilepsy.
Our finding that the genetic correlation between epilepsy and attention-deficit/hyperactivity disorder is more pronounced in focal epilepsy is novel, although the general correlation between epilepsy and attention-deficit/hyperactivity disorder is consistent with recent work.9 33 For example, a recent study on the co-aggregation of epilepsy and attention-deficit/hyperactivity disorder in Swedish families found a strikingly similar genetic correlation (0.21, 95% CI 0.02 to 0.40),33 although they were unable to explore subtypes of epilepsy. Our findings suggest that focal epilepsy is the main cause of this association. This contrasts with the aim of a previous study that examined generalised epilepsy because of its higher heritability.9 However, using updated GWAS data, we also confirmed the identification of a shared genetic overlap between epilepsy and attention-deficit/hyperactivity disorder.9 Although focal epilepsy has a lower heritability than generalised epilepsy,7 our findings suggest that pleiotropic genes are distinctly associated with focal epilepsy and attention-deficit/hyperactivity disorder. However, we noted that there was no substantial difference in the population-wide prevalence of psychiatric comorbidities between focal and generalised epilepsy (online supplemental figure 4), although subtype characterisation of epilepsy in Swedish registries may not be accurately recorded, and many individuals may have multiple seizure types.
Consistent with the population rate of intellectual disability among those with epilepsy (OR 27.5, p<0.001), we found that epilepsy and intelligence had a negative genetic correlation, and that genetic liability to higher intelligence was associated with a lower risk of epilepsy. Our findings are consistent with a shared genetic aetiology of intellectual disability and epilepsy. However, it is important to acknowledge that intelligence and intellectual disability are two distinct phenotypes. We were only able to perform MR and genetic correlation analyses of intelligence because of the absence of GWAS data for intellectual disabilities.
Intriguingly, using MR, we were unable to confirm the common assumption that epilepsy is a ‘cause’ of an increased risk of psychiatric disorders, or that these disorders are a ‘cause’ for an increased risk of epilepsy.12 This finding should be interpreted in light of the MR assumptions being met and the possibility of the role of rare genetic variations not captured within the GWAS data which comprise common variants.
However, this finding may also indicate the importance of environmental factors in the origin of psychiatric comorbidities in epilepsy. Future work could help identify the causal role of such factors (eg, prenatal or perinatal events unique to the individual, or discrimination, stigma and life events during the individual’s lifetime, which are common risk factors for several neurological and psychiatric phenotypes2 33 34), ideally using causal triangulation across study designs with orthogonal sources of bias.
Limitations
An important limitation to note is that existing epilepsy and psychiatric conditions GWAS are not sufficiently large, potentially leading to reduced power in identifying genetic correlations, and necessitating an inclusive threshold (p<5×10−6) to identify instruments for MR analyses. Although this threshold has been widely employed in previous studies, the possibility of reduced specificity in instrument selection, leading to weak instrument bias, cannot be ruled out. Analyses like ours, repeated using a larger GWAS, when available, may provide stronger evidence. Furthermore, rare genetic variants are known to play an important role in the aetiology of epilepsy,7 and their contribution to psychiatric comorbidities in epilepsy cannot be excluded. Although rare genetic variants may not be sufficiently common in the population to explain the high rates of psychiatric conditions reported in observational studies including our nationwide study, they may still be common among individuals with epilepsy. Nonetheless, future studies will help clarify the role of rare genetic variants in the overlap between psychiatric conditions and epilepsy. We were also unable to perform any age-stratified or sex-stratified cross-trait genetic analysis; however, this could be a target for future analysis if sex-stratified GWAS data were available. Another limitation of the Swedish data analysis is that our study relied on the accuracy of the epilepsy and psychiatric diagnoses recorded in the registers. Validation studies of epilepsy35 and a range of psychiatric and neurodevelopmental conditions suggest a high validity of these recorded diagnoses. However, the possibility of measurement bias in the data cannot be excluded. This limitation is common across large-scale epidemiological studies. Further large-scale efforts to estimate the burden of psychiatric conditions among people with epilepsy are warranted, with particular emphasis on including diverse and heterogeneous populations. Future work based on high-resolution phenotyping of smaller representative cohorts will shed further light on the complexities of the relationships studied.
Implications
Our study confirms the notable prevalence of psychiatric comorbidities among individuals with epilepsy and provides mechanistic insights into this co-occurrence. These findings underscore the importance of comprehensive patient care that acknowledges and addresses associated conditions during epilepsy diagnosis and treatment. This is particularly important, as psychiatric comorbidities notably impact the prognosis of epilepsy, influencing mortality and life expectancy.36 37 While our study revealed some genetic correlations suggesting a potential genetic contribution to comorbidities, it did not strongly support bidirectional relationships. Consequently, our study emphasises the importance of exploring alternative factors, including environmental influences and rare genetic variations, to unravel the origins of psychiatric comorbidities in epilepsy. Understanding the underlying causes of comorbidities is crucial, as it can guide treatment decisions and offer more effective strategies for managing both epilepsy and associated psychiatric conditions. This may enhance the overall quality of care for individuals affected by epilepsy.
In conclusion, psychiatric comorbidities are common in patients with epilepsy. Genetic correlations may partly explain some comorbidities; however, little evidence exists of a bidirectional relationship between the genetic liability of epilepsy and psychiatric conditions. These findings highlight the need to understand the role of environmental factors or rare genetic variations in the origins of psychiatric comorbidities in epilepsy.
Acknowledgments
This work was conducted in part using the computational facilities of the Advanced Computing Research Centre, University of Bristol (http://www.bris.ac.uk/acrc/). Certain figures were created using BioRender.com.
Biography
Mr. Viktor H. Ahlqvist obtained his Master of Medical Sciences degree from the Karolinska Institutet in Stockholm, Sweden, in 2019. He enrolled as a doctoral student in 2019 in the Department of Global Public Health at the Karolinska Institutet. His research centres on epilepsy and its related comorbidities, with a specific focus on the factors associated with pregnancy and their influence on the health of both mothers and their offspring. His doctoral project investigates the benefits and potential risks associated with the use of antiseizure medications during pregnancy. In pursuit of these insights, Mr. Ahlqvist employs registry-based pharmacoepidemiological surveillance and causal modelling. One key aspect of his research involves exploring whether epilepsy is independently linked to the occurrence of various conditions observed in the offspring of mothers with epilepsy. Through his work, Mr. Ahlqvist aims to enhance our understanding of epilepsy and its implications, ultimately striving to improve the health outcomes of both mothers and their children.

Footnotes
Twitter: @AhlqvistViktor
Contributors: VHA (CA) and DR conceptualised the study. VHA performed the formal analysis, drafted the original manuscript, had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, and accepts full responsibility for the work as a guarantor. DR supervised the study. BKL, KL, CN, DR, NMD, CM and TT were involved in funding acquisition. ML performed data curation of Swedish registries. C Dardani, PMD, HF, JR, CZ, RMG, C Dalman, DB, and all other authors, critically revised the manuscript for important intellectual content and contributed to the development of the study methodology.
Funding: This study was funded by the National Institutes of Health (1R01NS107607-01A1), Erik and Edith Fernström Foundation for Medical Research (2020-00321), Karolinska Institutet (2020-00160, 2020-01172) and the Swedish Society for Medical Research (RM21-0005). This study was also supported by the NIHR Biomedical Research Centre at the University of Bristol and University Hospitals Bristol and the Weston NHS Foundation Trust. The Medical Research Council (MRC) and the University of Bristol supported the MRC Integrative Epidemiology Unit (MC_UU_00011/1). NMD was supported by the Norwegian Research Council (grant number 295989). The Swedish Research Council (523-2010-1052) supports the (Psychiatry Sweden) register linkage.
Disclaimer: The funders had no role in the design of the study, data collection, data analysis or interpretation of the findings.
Competing interests: TT has received support from Eisai, GSK, UCB, Bial, Sanofi, GW Pharma, Teva, Angelini Pharma, and personal fees from Eisai, Sanofi, Sun Pharma, UCB and Angelini Pharma outside the submitted work. The remaining authors have no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. All genetic data produced in this study are publicly available in their original publication and upon reasonable request from the authors. Swedish privacy laws prohibit the authors from making registry data publicly available. The data supporting these findings were used under licence and ethical approval for this study. Readers interested in obtaining microdata or replicating this study may seek similar approval and enquiries from Statistics Sweden. For further advice, see https://www.scb.se/en/services/guidance-for-researchers-and-universities/, or contact Statistics Sweden at mikrodata@scb.se.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The Swedish Ethical Review Authority (DNR 2010/1185-31/5) gave ethical approval for the registry-based analysis and waived the need for informed consent. All genome-wide association studies-based analyses were performed using publicly available data and were covered by ethical approval in the original studies.
References
- 1. Fazel S, Wolf A, Långström N, et al. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet 2013;382:1646–54. 10.1016/S0140-6736(13)60899-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 2016;15:106–15. 10.1016/S1474-4422(15)00225-2 [DOI] [PubMed] [Google Scholar]
- 3. Shimizu H, Morimoto Y, Yamamoto N, et al. Overlap between epilepsy and neurodevelopmental disorders: insights from clinical and genetic studies. In: Czuczwar SJ, ed. Epilepsy. Brisbane (AU): Exon Publications, 2022: 41–54. [PubMed] [Google Scholar]
- 4. Chow J, Jensen M, Amini H, et al. Dissecting the genetic basis of comorbid epilepsy phenotypes in neurodevelopmental disorders. Genome Med 2019;11:65. 10.1186/s13073-019-0678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tellez-Zenteno JF, Patten SB, Jetté N, et al. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia 2007;48:2336–44. 10.1111/j.1528-1167.2007.01222.x [DOI] [PubMed] [Google Scholar]
- 6. Rai D, Kerr MP, McManus S, et al. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia 2012;53:1095–103. 10.1111/j.1528-1167.2012.03500.x [DOI] [PubMed] [Google Scholar]
- 7. International League Against Epilepsy Consortium on Complex Epilepsies . GWAS meta-analysis of over 29,000 people with epilepsy identifies 26 risk Loci and subtype-specific genetic architecture. Nat Genet 2023;55:1471–82. 10.1038/s41588-023-01485-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell C, Cavalleri GL, Delanty N. Exploring the genetic overlap between psychiatric illness and epilepsy: a review. Epilepsy Behav 2020;102:106669. 10.1016/j.yebeh.2019.106669 [DOI] [PubMed] [Google Scholar]
- 9. Wu Y, Li Y, Zhu J, et al. Shared genetics and causality underlying epilepsy and attention-deficit hyperactivity disorder. Psychiatry Res 2022;316:114794. 10.1016/j.psychres.2022.114794 [DOI] [PubMed] [Google Scholar]
- 10. Yuan S, Tomson T, Larsson SC. Modifiable risk factors for epilepsy: a two-sample Mendelian randomization study. Brain Behav 2021;11:e02098. 10.1002/brb3.2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li G, Wang M, Zheng M, et al. Causal effect of psychiatric disorders on epilepsy: A two-sample Mendelian randomization study. Brain Behav 2023;13:e2939. 10.1002/brb3.2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanner AM, Ribot R, Mazarati A. Bidirectional relations among common psychiatric and neurologic comorbidities and epilepsy: do they have an impact on the course of the seizure disorder. Epilepsia Open 2018;3:210–9. 10.1002/epi4.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanner AM. Can neurobiological pathogenic mechanisms of depression facilitate the development of seizure disorders. Lancet Neurol 2012;11:1093–102. 10.1016/S1474-4422(12)70201-6 [DOI] [PubMed] [Google Scholar]
- 14. Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet 2015;47:1236–41. 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bulik-Sullivan BK, Loh P-R, Finucane HK, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 2015;47:291–5. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease Int J Epidemiol 2003;32:1–22. 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 17. Watson HJ, Yilmaz Z, Thornton LM, et al. Genome-wide association study identifies eight risk Loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet 2019;51:1207–14. 10.1038/s41588-019-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otowa T, Hek K, Lee M, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry 2016;21:1391–9. 10.1038/mp.2015.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demontis D, Walters GB, Athanasiadis G, et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet 2023;55:198–208. 10.1038/s41588-022-01285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grove J, Ripke S, Als TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 2019;51:431–44. 10.1038/s41588-019-0344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mullins N, Forstner AJ, O’Connell KS, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet 2021;53:817–29. 10.1038/s41588-021-00857-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Als TD, Kurki MI, Grove J, et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat Med 2023;29:1832–44. 10.1038/s41591-023-02352-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savage JE, Jansen PR, Stringer S, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet 2018;50:912–9. 10.1038/s41588-018-0152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS) . Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry 2018;23:1181–8. 10.1038/mp.2017.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trubetskoy V, Pardiñas AF, Qi T, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022;604:502–8. 10.1038/s41586-022-04434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mullins N, Kang J, Campos AI, et al. Dissecting the shared genetic architecture of suicide attempt, psychiatric disorders, and known risk factors. Biol Psychiatry 2022;91:313–27. 10.1016/j.biopsych.2021.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu D, Sul JH, Tsetsos F, et al. Interrogating the Genetic Determinants of Tourette's Syndrome and Other Tic Disorders Through Genome-Wide Association Studies. Am J Psychiatry 2019;176:217–27. 10.1176/appi.ajp.2018.18070857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng J, Erzurumluoglu AM, Elsworth BL, et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 2017;33:272–9. 10.1093/bioinformatics/btw613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37:658–65. 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi KW, Chen C-Y, Stein MB, et al. Assessment of Bidirectional Relationships Between Physical Activity and Depression Among Adults: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 2019;76:399–408. 10.1001/jamapsychiatry.2018.4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hemani G, Zheng J, Elsworth B, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carter AR, Fraser A, Howe LD, et al. Why caution should be applied when interpreting and promoting findings from Mendelian randomisation studies. Gen Psychiatr 2023;36:e101047. 10.1136/gpsych-2023-101047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brikell I, Ghirardi L, D’Onofrio BM, et al. Familial Liability to Epilepsy and Attention-Deficit/Hyperactivity Disorder: A Nationwide Cohort Study. Biol Psychiatry 2018;83:173–80. 10.1016/j.biopsych.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gu W, Zhao Q, Yuan C, et al. Impact of adverse childhood experiences on the symptom severity of different mental disorders: a cross-diagnostic study. Gen Psychiatr 2022;35:e100741. 10.1136/gpsych-2021-100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sveinsson O, Andersson T, Carlsson S, et al. The incidence of SUDEP: A nationwide population-based cohort study. Neurology 2017;89:170–7. 10.1212/WNL.0000000000004094 [DOI] [PubMed] [Google Scholar]
- 36. Dreier JW, Laursen TM, Tomson T, et al. Cause-specific mortality and life years lost in people with epilepsy: a Danish cohort study. Brain 2023;146:124–34. 10.1093/brain/awac042 [DOI] [PubMed] [Google Scholar]
- 37. Moon HJ, Lee H, Yoon D, et al. Premature Mortality and Causes of Death Among People With Epilepsy: A Nationwide Population-Based Incident Cohort Study. Neurology 2023;100:e2060–70. 10.1212/WNL.0000000000207212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2023-101201supp001.pdf (1MB, pdf)
Data Availability Statement
No data are available. All genetic data produced in this study are publicly available in their original publication and upon reasonable request from the authors. Swedish privacy laws prohibit the authors from making registry data publicly available. The data supporting these findings were used under licence and ethical approval for this study. Readers interested in obtaining microdata or replicating this study may seek similar approval and enquiries from Statistics Sweden. For further advice, see https://www.scb.se/en/services/guidance-for-researchers-and-universities/, or contact Statistics Sweden at mikrodata@scb.se.