Abstract
Tuberculosis (TB) is an important cause of morbidity and mortality globally. About 3–4% of hospitalized TB patients require admission to the intensive care unit (ICU); the mortality in these patients is around 50–60%. There is limited literature on the evaluation and management of patients with TB who required ICU admission. The Indian Society of Critical Care Medicine (ISCCM) constituted a working group to develop a position paper that provides recommendations on the various aspects of TB in the ICU setting based on available evidence. Seven domains were identified including the categorization of TB in the critically ill, diagnostic workup, drug therapy, TB in the immunocompromised host, organ support, infection control, and post-TB sequelae. Forty-one questions pertaining to these domains were identified and evidence-based position statements were generated, where available, keeping in focus the critical care aspects. Where evidence was not available, the recommendations were based on consensus. This position paper guides the approach to and management of critically ill patients with TB.
How to cite this article
Chacko B, Chaudhry D, Peter JV, Khilnani G, Saxena P, Sehgal IS, et al. isccm Position Statement on the Approach to and Management of Critically Ill Patients with Tuberculosis. Indian J Crit Care Med 2024;28(S2):S67–S91.
Keywords: Antituberculous treatment, Critically ill, Immunocompromised host, Infection prevention and control, Organ support, Tuberculosis
Introduction
Tuberculosis (TB) is an important cause of morbidity and mortality globally. In 2021, around 10.6 million people were estimated to be infected with TB and 1.6 million died.1 This important communicable disease is more prevalent among marginalized socio-economic status communities with limited access to healthcare.2 In India, 2.77 million people were estimated to be affected with TB in 2022 with a mortality of 0.32 million and an incidence of 196 per 100,000 population.3 About 3–4% of hospitalized TB patients require admission to the intensive care unit (ICU); the mortality in this subset of patients is around 50–60%.4,5 Despite the burden of TB and the likelihood of such patients needing ICU care, there is limited literature on the management of patients with TB who require ICU admission. The Indian Society of Critical Care Medicine (ISCCM) position paper aims to bridge this gap by providing recommendations on the various aspects of TB in the ICU setting.
Methods
The ISCCM convened a conclave meeting on 16th July 2023 to identify the writing group for TB and to set out the objectives for the position statement. The working group comprised 19 members including a chair and co-chair and 4 women with considerable experience in TB, who are working in academic and private institutions across India in critical care, respiratory medicine, infectious disease, neurology, and microbiology. The domains were deliberated and outlined at the conclave and broad questions were constructed. Seven subgroups were created, each with a coordinator, to refine and finalize the questions and work on the domains. The domains were as follows:
Categorization of TB in critically ill.
Diagnostic workup.
Drug therapy.
TB in the immunocompromised host.
Organ support.
Infection control, and
Post-TB sequelae.
Relevant articles on the subject were identified by a literature search undertaken by each of the subgroups, using search terms pertaining to the domains allocated to them. A tutorial on how to perform a PubMed® search was done at the conclave to ensure that authors had a clear understanding on how to perform a thorough literature search using the appropriate search terms. The recommendations of the subgroups were made based on available evidence. Grading of recommendations was not done as there was limited research on this topic in terms of randomized clinical trials, and hence it was decided at the conclave that this would be a position paper and not a guideline. Where evidence was not available, the recommendations were based on consensus. Although a Delphi process would have been ideal to make the recommendations where evidence was not available, this was not undertaken. The recommendations of each of the subgroups were reviewed by a three-member core group (DC, BC, JVP), and position statements and the commentary were refined, ensuring that they were accurate and reflected current evidence. Care was taken to ensure that the recommendations focused on the approach to, the evaluation and management of a critically ill patient with TB. The executive summary provides a quick reference guide on the approach to and management of critically ill patients with TB.
Executive summary
| No. | Question | Position statement |
|---|---|---|
| Categorization of TB | ||
| 1 | How should TB be categorized in the critically ill? | In the critically ill patient, TB should be categorized as pulmonary and extrapulmonary TB. |
| 2 | When should TB be suspected in critically ill patients? | TB should be considered in ICU patients with risk factors for developing TB, particularly in high endemic areas, in the setting of nonresolving community-acquired pneumonia (CAP), acute respiratory distress syndrome (ARDS), meningitis, intracranial or bony lesions, pleural or pericardial effusion, psoas abscess, and multiorgan failure. |
| 3 | What are the reasons for ICU admission in patients with TB? | Patients with TB are usually admitted to the ICU with a process that is directly related to TB or due to secondary processes. |
| 4 | What should the diagnostic approach be in patients with suspected pulmonary TB? | Patients with a high risk of TB, with compatible clinical features and radiological abnormalities, should undergo tests for microbiological confirmation of TB. |
| 5 | What should the diagnostic approach be in patients with suspected CNS TB? | The diagnostic approach of a critically ill patient with suspected CNS TB is based on clinical features, imaging, and cerebrospinal (CSF) analysis. |
| 6 | When would you suspect drug-resistant TB in ICU patients with suspected TB? | Given the high level of drug resistance to TB, drug resistance must be ruled out in all patients who are admitted to the ICU with suspected TB. Multidrug resistance must be particularly considered in patients >40 years, those with prior history of TB, poor compliance, or failure to TB treatment, and in HIV co-infection. |
| Diagnostic workup | ||
| 7 | Is there a role of microscopy in the diagnosis of TB? | Microscopy remains the primary method for diagnosing M. tuberculosis infection and is important in detecting patients with high bacterial load. |
| 8 | What is the role of molecular testing in the diagnosis of TB in critically ill? | Molecular testing (Xpert MTB/Rif or Xpert Ultra) should be performed initially on ALL samples obtained from pulmonary or extrapulmonary sites to arrive at a rapid diagnosis of TB and for detecting rifampicin-resistance. LPA, recommended by the guidelines for the PMDT in India, must be performed to further evaluate for drug resistance. The current reference for drug resistance is phenotypic DST using solid (LJ) medium and liquid (MGIT) medium cultures. |
| 9 | What is the role of cultures in the diagnosis of TB? | Culture remains the gold standard for TB diagnosis for pulmonary and extrapulmonary TB as it enables accurate identification and subsequent susceptibility testing. However, the turnaround time is high. |
| 10 | Is there a role for ADA in the diagnosis of extrapulmonary TB? | ADA is a useful adjunct test in the diagnosis of extrapulmonary TB. It must be used in conjunction with other tests to diagnose TB. |
| 11 | What is the role of invasive diagnostic tests for TB? | Invasive tests such as bronchoscopic procedures, biopsy techniques, and conventional surgery may be required to diagnose pulmonary and extrapulmonary TB when noninvasive and less invasive tests fail to give a diagnosis |
| 12 | What is the role of imaging in the diagnosis of TB? | Chest radiography is the mainstay imaging for pulmonary parenchymal TB. CT and MRI are used for the evaluation of pulmonary and extrapulmonary TB. |
| Drug therapy | ||
| 13 | When should anti-TB be initiated in a critically ill patient? | Timely initiation of anti-TB drugs is essential for treatment success, particularly in those with high disease severity. |
| 14 | Which anti-TB regimen should be initiated in a critically ill patient? | As per the National TB Eradication Program (NTEP), a weight based, standard anti-TB regimen with four drugs should be initiated unless drug resistance is suspected or demonstrated. Higher doses of anti-TB drugs may be considered in severe disease. |
| 15 | Do enterally administered anti-TB drugs achieve therapeutic levels in critically ill patients? | Absorption of enterally administered anti-TB drugs is reduced in the critically ill patient and if administered when continuous enteral feeds are given. Therapeutic levels may be achieved with enteral pyrazinamide but not with INH, rifampicin, and ethambutol. |
| 16 | Is there a role for higher doses of anti-TB drugs or IV anti-TB drugs in TB meningitis and severe illness? | Higher doses of anti-TB drugs or intravenous preparations where available, may be considered in TB meningitis and severe illness. |
| 17 | What is the role of other intravenous antibiotics in TB? | Although several IV antibiotics such as aminoglycosides, quinolones, linezolid, and carbapenems are effective against TB, these second-line drugs should be used only in the setting of DR-TB, life-threatening TB, and in those who do not tolerate first-line anti-TB drugs. |
| 18 | How should DR-TB be treated in the ICU? | Patients with DR-TB should be enrolled in the NTEP if not already enrolled. The NTEP has adopted bedaquiline-based DR-TB regimen in India based on WHO guidelines. Treatment should be started in consultation with respiratory physicians and NTEP. The recommended drugs and duration of treatment in such patients are guided by program management of DR-TB in India. |
| 19 | Is there a risk of drug-drug interaction with commonly used anti-TB drugs? | There are significant drug-drug interactions with the commonly used anti-TB drugs. Clinicians need to be familiar with the common interactions in the ICU setting. |
| 20 | What is the role of steroids in TB? | Corticosteroids may be effective in reducing clinically relevant adverse outcomes in some forms of TB. |
| 21 | Is there a role for TDM in DR-TB? | There is insufficient evidence to recommend routine TDM during treatment of DR-TB. |
| 22 | How do risk prediction scores perform in TB? | Risk prediction scores should not be relied on for mortality prediction in TB. |
| TB in the immunocompromised host | ||
| 23 | Does the immunocompromised state predispose an individual to reactivation of latent TB or the acquisition of TB? | Since immunocompromised patients are at risk for TB, the clinical suspicion should be high in patients admitted to the ICU. The interpretation of tests for latent TB infection (such as TST and IGRA are likely to be influenced by immunosuppression and the severity of critical illness. |
| 24 | Is the clinical presentation of TB in the immunocompromised individual similar to that of the immunocompetent host? | In an immunocompromised host, the clinical presentation of TB may be more insidious and likely to be disseminated. TB should be suspected when there is unexplained worsening of clinical status. The surveillance for atypical presentations of TB should be robust in order to pick them up early. |
| 25 | What should the diagnostic work be in the immunocompromised host who is suspected to have TB infection? | Taking into consideration the indolent nature of TB in the immunocompromised patient, cross sectional imaging should be considered early to direct diagnostic sampling. Early invasive sampling including bronchial lavage and/or biopsy is advocated in these patients. |
| 26 | What would the approach be to the management of TB in the setting of intestinal malabsorption? | In patients with anticipated poor enteral uptake of ATT (short bowel, gut GVHD, severe multiorgan dysfunction with high-dose vasoactive medications), parenteral ATT may be considered along with TDM. |
| 27 | What would the approach be to IRIS in TB? | IRIS should be suspected when there is paradoxical worsening of symptoms, typically seen when immunosuppression is reduced or reversed. IRIS can also occur in the immunocompetent host. A course of steroids should be considered. |
| Organ support | ||
| 28 | Is the ventilatory management of respiratory failure due to TB different from the ventilatory management of respiratory failure due to other infective etiologies? | There is limited data on the management of respiratory failure in patients with TB. The management of respiratory failure in patients with TB should be along the same lines of management as with other infective etiologies of hypoxemic and hypercapnic respiratory failure. |
| 29 | Should noninvasive respiratory support be considered in the management of acute respiratory failure due to TB? | The use of noninvasive respiratory support including HFNC is challenging given their tendency to cause aerosol dispersion and spread of infection to HCWs. It is preferable to use them in isolation facilities with adequate precautions by staff. |
| 30 | Is there a role for ECMO support in TB? | There is limited evidence for the use of ECMO in TB. It may be considered in TB in the setting of refractory respiratory or cardiac failure. |
| 31 | How should massive hemoptysis be managed? | Patients with massive or life-threatening hemoptysis should be managed in an ICU with focus on airway and hemodynamic stabilization, evaluation of the source of bleed by CT angiogram or bronchoscopy and consideration of bronchial artery embolization as an early treatment option. Nebulized and/or intravenous tranexamic acid may reduce the volume of hemoptysis. |
| 32 | When should tracheostomy be considered in patients ventilated for TB? | The indications and timing of tracheostomy in patients admitted to the ICU with TB is similar to that of other patients admitted to critical care. There is inconclusive evidence to recommend early tracheostomy in patients with TB. |
| 33 | What is the optimal management of hypotension due to adrenal insufficiency and septic shock with mycobacteremia (Landouzy sepsis)? | In TB, shock may be due to adrenal insufficiency, secondary bacterial sepsis, or mycobacteremia. Shock should be managed along the same lines as the management of septic shock. Early initiation of anti-TB treatment is important. Steroids are indicated for refractory septic shock, adrenal insufficiency, and shock due to mycobacteremia. |
| 34 | What is the optimal management of renal failure in patients with TB? | Renal failure in TB should be managed along the same lines as the approach to the management of acute kidney injury in the setting of other infectious diseases. Drug dosing should be optimized for renal function, and nephrotoxic agents should be avoided. |
| 35 | Is there a role for first line anti-TB drugs in patients with liver involvement in TB? | Patients with liver involvement due to TB should be started on standard four-drug regimen, irrespective of baseline liver function tests. |
| 36 | Is there a role for low-volume PLEX in patients with liver dysfunction due to TB or DILI? | In patients with ALF or ACLF due to anti-TB drugs-induced DILI, low-volume plasma exchange may be considered especially in those who fulfill the King's College criteria for liver transplantation but do not have access to the same. |
| Infection control | ||
| 37 | What are the recommended isolation systems for patients with presumed or documented infectious TB in the ICU? | Respiratory isolation of individuals with confirmed or suspected infectious TB is advised, ideally in an AIIR equipped with a HEPA filter and negative pressure. Closed suctioning is recommended for intubated patients. The application of a bacterial filter to the ventilator tubing's expiratory limb may reduce the transmission of infection. |
| 38 | What are the key IPC measures for HCWs taking care of patients with suspected or confirmed tuberculosis in the ICU? | Healthcare professionals entering rooms of patients with suspected or confirmed contagious TB disease must wear a properly sized N95 disposable respirator. |
| 39 | When can respiratory isolation precautions be discontinued for critically ill patients with pulmonary TB? | For sputum-positive rifampicin susceptible TB patients, a minimum of 2 weeks of isolation is suggested, while on effective anti-TB treatment. |
| 40 | Is there a role for routine testing and surveillance of HCWs managing infectious TB patients? | The role and benefit of regular testing and surveillance for TB infection among HCWs in the ICU, using TST or IGRA, in endemic countries like India is unclear. |
| 41 | Post TB sequelae: What are the reasons for ICU admission? | Post-TB sequelae patients may present to the ICU with respiratory failure due to exacerbation of obstructive airway disease or restrictive fibrotic lung disease, pulmonary hypertension, hemoptysis due to bronchiectasis, aspergillosis or vascular causes or with secondary infections. There are currently no evidence-based recommendations for the investigation and management of PTLA. |
ACLF, acute on chronic liver failure; ADA, adenosine deaminase; AIIR, airborne infection isolation room; ALF, acute liver failure; ARDS, acute respiratory distress syndrome; ATT, antituberculous treatment; CAP, community-acquired pneumonia; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; DILI, drug-induced liver disease; DR-TB, drug resistant TB; DST, drug sensitivity testing; ECMO, extracorporeal membrane oxygenation; GVHD, graft vs host disease; HCW, healthcare worker; HEPA, high efficiency particulate air; HFNO, high frequency nasal oxygen; HIV, human immunodeficiency virus; IGRA, interferon gamma release assay; IRIS, immune reconstitution inflammatory syndrome; ICU, intensive care unit; ICP, intracranial pressure; IV, intravenous; LJ, Lowenstein-Jensen medium; LPA, line probe assay; MGIT, Mycobacteria growth indicator tube; MRI, magnetic resonance imaging, NTEP, National Tuberculosis Eradication Program; TB, tuberculosis, TDM, therapeutic drug monitoring; TST, tuberculin skin test; PMDT, programmatic management of drug-resistant tuberculosis; PTLA, post-TB lung abnormality; PLEX, plasma exchange; WHO, World health organization
Categorization of TB in Critically Ill
Q1: How should TB be Categorized in the Critically ill?
Position statement: In critically ill patients, TB should be categorized as either pulmonary or extrapulmonary TB.
Commentary: TB is generally categorized as pulmonary and extrapulmonary TB.6,7 The same classification is applicable to the critically ill.8
Q2: When should TB be Suspected in Critically Ill Patients?
Position statement: TB should be considered in ICU patients with risk factors for developing TB, particularly in high endemic areas, in the setting of nonresolving community-acquired pneumonia (CAP), acute respiratory distress syndrome (ARDS), meningitis, intracranial or bony lesions, pleural or pericardial effusion, psoas abscess, and multiorgan failure.
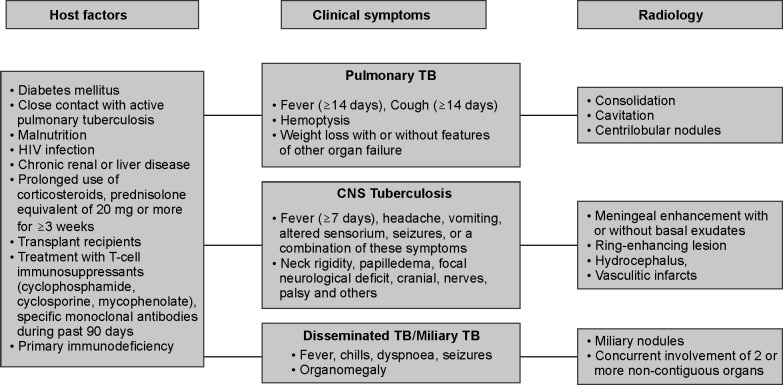
Commentary: Patients with pulmonary TB can present acutely or sub-acutely. Tuberculosis should be suspected in a patient who presents with fever and cough of ≥14 days with hemoptysis, weight loss, or features of organ dysfunction, particularly in high endemic areas and in those with risk factors for developing TB (Fig. 1). Tuberculosis can present as CAP9 or ARDS10–12 or life-threatening hemoptysis. The presence of miliary or centrilobular nodules, consolidation with or without cavitation, and pleural effusion should raise the clinical suspicion of TB as a cause of respiratory failure.13 Patients with extrapulmonary TB may present with acute respiratory failure, septic shock, and multiorgan failure.14 Those with central nervous system (CNS) TB (meningitis, meningoencephalitis, space-occupying lesion, epidural abscess causing cord compression, or a combination of these) and pericardial TB may require ICU admission.8,9 Although isolated involvement of other organs such as the liver,15 gastrointestinal,16 or urogenital17 is uncommon in TB patients admitted to the ICU, such patients may require ICU care postoperatively if surgical intervention is required or if they develop organ dysfunction. Disseminated TB should be suspected when there is concurrent involvement of two or more noncontiguous sites; miliary pattern on chest X-ray is a common finding.18
Fig. 1.
When to suspect TB in the critically ill. The figure demonstrates an algorithmic approach to the diagnosis of tuberculosis (TB) in the critically ill. Several risk factors (host factors) predispose to the development of TB. The symptoms in pulmonary, central nervous system (CNS), and disseminated/miliary TB as well as the radiological features in these forms of TB are listed
Q3: What are the Reasons for ICU Admission in Patients with TB?
Position statement: Patients with TB are usually admitted to the ICU with a process that is directly related to TB or due to secondary processes.
Commentary: Patients with TB may require admission to the ICU for a process that is directly related to TB (pulmonary or extrapulmonary manifestations) or due to secondary processes (Table 1). In pulmonary TB, patients may present with massive hemoptysis that requires airway protection and intubation, severe hypoxia due to superimposed pneumonia or ARDS, or pneumothorax. Patients with pulmonary or extrapulmonary TB can also present with respiratory failure due to pulmonary embolism.19 Isolated massive pleural effusion as a cause of hypoxia is uncommon in TB. Patients with extrapulmonary TB may present with seizures, electrolyte abnormalities, features of hydrocephalus, pericardial tamponade, sepsis, multiorgan dysfunction, or complications related to drug therapy.20,21 In a series of 48 patients admitted to the ICU with TB meningitis, 47 patients were referred because of neurological deterioration; 22 were comatose at admission; 36 patients (75%) required ventilatory support, and 16 needed neurosurgery.22
Table 1.
Indications for ICU admission in tuberculosis8
| Site | Primary process | Secondary process* |
|---|---|---|
| Parenchymal lung disease | Nonresolving community-acquired pneumonia (CAP) TB acute respiratory distress syndrome (ARDS) Fibro-cavitary disease Miliary TB TB bronchopneumonia |
Superimposed bacterial infection Fungal infection (e.g., Aspergillosis) TB sequelae (fibrosis, cavitation) IRIS Hemoptysis (Rasmussen's aneurysm) |
| Pleural disease | Massive pleural effusion TB empyema |
Pneumothorax |
| CNS disease | Subacute or chronic TB meningitis Basal arachnoiditis due to TB Tuberculoma TB brain abscess Spinal arachnoiditis |
Coma or altered conscious state (multi-factorial causes) Noncommunicating hydrocephalus Focal neurologic signs (cranial nerve palsy, hemiparesis) Seizures Electrolyte abnormalities |
| Pericardial disease | Pericardial tamponade Constrictive pericarditis |
Heart failure Organ dysfunction due to heart failure |
| Abdominal TB | Massive ascites Intestinal obstruction or perforation (ileocecal TB) |
Secondary bacterial peritonitis Sepsis |
| Disseminated TB | Respiratory failure due to miliary TB Multiorgan dysfunction Hypotension due to adrenal involvement |
Secondary sepsis |
| Musculoskeletal TB | Psoas abscess | Paraplegia Superimposed bacterial infection |
ARDS, acute respiratory distress syndrome; CAP, community-acquired pneumonia; CNS, central nervous system; ICU, intensive care admission; IRIS, immune reconstitution inflammatory syndrome; TB, tuberculosis. *In addition patients on treatment for tuberculosis (TB) may present with drug toxicity due to antitubercular drugs, intercurrent infections, pulmonary embolism, cardiac or neurological events unrelated to the primary TB process
Q4: What should the Diagnostic Approach be in Patients with Suspected Pulmonary TB?
Position statement: Patients with a high risk of TB, with compatible clinical features and radiological abnormalities, should undergo tests for microbiological confirmation of TB.
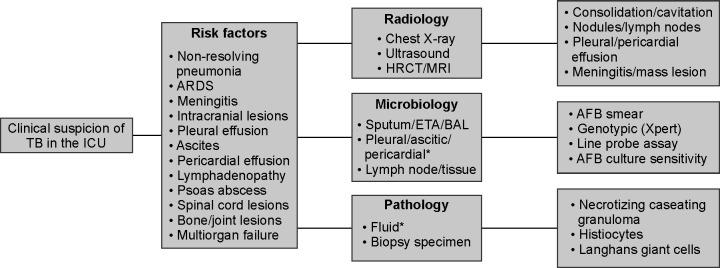
Commentary: The guidelines followed in the radiological and microbiological evaluation and confirmation of TB are similar to those of nonICU patients.23 Patients with a high risk of TB with compatible clinical and radiological abnormalities should be investigated further to confirm the diagnosis of TB. For microbiological confirmation, sputum, endotracheal aspirate, bronchial washing, or bronchoalveolar lavage should be sent for microscopy, molecular tests, and cultures (Figs 1 and 2). Biopsy may be required in some situations to confirm the diagnosis. In an intubated patient, microbiological sampling of the lower respiratory tract, by means of bronchoalveolar lavage, is likely to improve the yield and facilitate the diagnosis of pulmonary TB.24,25
Fig. 2.
Diagnostic approach to TB in the critically ill. The figure outlines a diagnostic approach to tuberculosis (TB) in the intensive care unit (ICU). Several clinical features would raise the suspicion of TB. TB may be confirmed with a combination of tests that include radiology, microbiology, and histopathology. ARDS acute respiratory distress syndrome, HRCT high resolution computed tomography, MRI magnetic resonance imaging, ETA endotracheal aspirate, BAL bronchoalveolar lavage, AFB acid fast bacilli. * Pleural, pericardial, or ascitic fluid can be sent for microbiology and histopathology
The role of tuberculin skin test (TST) and interferon-gamma release assay (IGRA) in the diagnosis of TB merit mention. These tests are used for diagnosing latent TB infection (LTBI).26 Neither of the tests predicted the subsequent development of active TB among household contacts of pulmonary TB during follow-up.26 Its role in the diagnosis of LTBI in high-prevalence areas and its relevance in the ICU are questionable.
Q5: What should the Diagnostic Approach be in Patients with Suspected CNS TB?
Position statement: The diagnostic approach of a critically ill patient with suspected CNS TB is based on clinical features, imaging, and cerebrospinal (CSF) analysis.
Commentary: The evaluation of a patient with suspected CNS TB is the same as a nonICU patient. Any patient presenting with fever, headache, altered sensorium, seizures, focal neurological deficits, or a combination of these symptoms for more than 1 week should be investigated for CNS TB. Clinical examination may reveal neck rigidity, cranial nerve palsy, focal neurological deficits, and signs of raised intracranial pressure (ICP), including papilledema. Patients presenting with features of meningitis could also have abnormalities on chest imaging. The diagnostic approach would involve imaging (computed tomography [CT] and/or magnetic resonance imaging [MRI] with contrast), CSF analysis, and microbiological confirmation (Figs 1 and 2).27–29
Although molecular diagnostic tests such as the Xpert/Rif can enable rapid diagnosis of CNS TB, CSF culture is likely to increase the sensitivity of TB meningitis diagnosis.30 Since cultures take 6–8 weeks, anti-TB treatment should be initiated immediately if there is a high probability of TB even when molecular tests are negative as it does not rule out CNS TB given their modest sensitivity. When the index of suspicion is high, it is important to also look for evidence of active TB elsewhere. In immune-deficiency states, it is important to evaluate for a concomitant cryptococcal meningitis.31
Q6: When would you Suspect Drug-resistant (DR)-TB in Patients Admitted to ICU with Suspected TB?
Position statement: Given the high prevalence of DR-TB, drug resistance must be ruled out in all patients who are admitted to the ICU with suspected TB. Multidrug resistance must be particularly considered in patients >40 years, those with prior history of TB, poor compliance, or failure to TB treatment, and in human immunodeficiency virus (HIV) co-infection.
Commentary: In 2022 TB was reported as the biggest DR airborne epidemic.32 The term DR-TB33,34 encompasses (a) mono resistance to a first-line anti-TB drug, (b) multidrug-resistant TB (MDR-TB) that are resistant to isoniazid and rifampicin, (c) pre-extensively drug-resistant TB (pre-XDR-TB MDR/RR-TB) that are also resistant to a fluoroquinolone, and (d) extensively drug-resistant TB (XDR-TB) where the isolates are resistant to other important medications including bedaquiline and/or linezolid.
In India, DR-TB was found to be prevalent in 24.9% of newly diagnosed patients and in 58.4% of patients who had received prior treatment; 18.8% of patients with HIV and TB co-infection had drug resistance.35 Multidrug-resistant ranged from 3.5 to 26.7% in treatment naïve and in patients who had received prior treatment respectively.35 A significant increase in MDR (p < 0.001) of 12% over time was reported from a tertiary care hospital in New Delhi (4.7% in 2000 to 19.8% in 2012).36 The global pooled prevalence of MDR-TB was 11.6% (95% CI, 9.1–14.5) in a review of 148 studies37 with substantial heterogeneity (I2 99.6) and a sample size of 3,18,430 individuals.
The risk factors for MDR-TB vary depending on the area. An increased risk of MDR-TB was found in a meta-analysis among patients ≥ 40 years of age who were unemployed, did not have health insurance, had a positive smear, had not completed or failed their TB treatment, had an adverse drug reaction, were nonadherent, had HIV, COPD, or had an infection with M. tuberculosis Beijing.38 Male gender and a history of prior TB therapy are risk factors for drug resistance in extrapulmonary TB.39
Diagnostic Workup
Q7: Is there a Role of Microscopy in the Diagnosis of TB?
Position statement: Microscopy remains the primary method for diagnosing M. tuberculosis infection and is important in detecting patients with high bacterial load.
Commentary: The sensitivity of smear and microscopy for diagnosing M. tuberculosis ranges from 20 to 80% and is low in pediatric TB, extra-pulmonary TB, or TB with HIV infection.40 Ziehl-Neelsen stain involving carbol fuchsin for acid-fast staining is rapid, cheap, and affordable in resource-limited settings. Microscopy is important in detecting patients with pulmonary TB with high bacterial load and high risk of transmission. Fluorescent microscopy enhances the sensitivity of smear microscopy as it increases productivity with light-emitting diodes.41,42 Microscopy cannot distinguish between viable and dead bacilli and is not useful for early detection of treatment failure or drug resistance.
Q8: What is the Role of Molecular Testing in the Diagnosis of TB in the Critically Ill?
Position statement: Molecular testing (Xpert MTB/Rif or Xpert Ultra) should be performed initially on all samples obtained from pulmonary or extrapulmonary sites to arrive at a rapid diagnosis of TB and to detect rifampicin-resistance. Line probe assays (LPAs), recommended by the guidelines for the Programmatic Management of Drug-Resistant Tuberculosis (PMDT) in India, must be performed to further evaluate for drug resistance. The current reference for drug resistance is phenotypic drug susceptibility testing (pDST) using solid Lowenstein-Jensen (LJ) medium and the liquid mycobacteria growth indicator tube (MGIT) medium cultures.
Commentary: In a critically ill patient, time is highly crucial so early diagnosis and initiation of treatment is important. In adults with suspected pulmonary TB, Xpert MTB/RIF or Xpert MTB/RIF Ultra or TrueNAT should be used as the initial diagnostic test for TB and for detecting rifampicin resistance in sputum.43,44 Xpert Ultra performs slightly better than Xpert MTB/Rif (Table 2).45
Table 2.
Performance of the Xpert test in various forms of TB
| Type of TB | Number of studies/specimens | Comparator | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| Pulmonary TB (Xpert)45 | 7 | Culture | 84.7% (78.6–89.9) | 98.4% (97.0–99.3) |
| Pulmonary TB (Xpert Ultra)45 | 7 | Culture | 90.9% (86.2–94.7) | 95.6% (93.0–97.4) |
| TB meningitis (Xpert)45 | 5 | CRS | 37% (25–50) | 100% |
| TB meningitis (Xpert Ultra)46 | 6 | CRS | 64% (45–80) | 100% |
| Cochrane review | ||||
| Pleural (Xpert)48 | 27 | Culture | 50.9% (39.7–62.8) | 99.2% (98.2–99.7) |
| Pleural tissue (Xpert) | 3 | Culture | 30.5% (3.5–77.8) | 97.4% (92.1–99.3) |
| CSF (Xpert)48 | 29 | Culture | 71.1% (60.9–80.4) | 98.0% (97.0 to 98.8) |
| Lymph node aspirate (Xpert) | 17 | Culture | 87.6% (81.7–to 92.0) | 86.0% (78.4–91.5) |
| Urine (Xpert)48 | 13 | Culture | 82.7% (69.6–91.1) | 98.7% (94.8–99.7) |
| Peritoneal (Xpert) | 16 | Culture | 59.2% (45.2–73.5) | 97.9% (96.2–99.1) |
| Pericardial (Xpert)48 | 7 | Culture | 65.7% (46.3–81.4) | 96.0% (85.8–99.3) |
| Genitourinary–urine (Xpert) | 13 | Culture | 82.7% (69.6 to 91.1) | 98.7% (94.8–99.7) |
| Adults | ||||
| Extrapulmonary TB (Xpert)47 | 572* | CRS | 75% (70–80) | 98% (97–100) |
| Lymph node (Xpert)47 | 279 | CRS | 90% (86–94) | 71.5 (60–83) |
| CSF (Xpert) | 45 | CRS | 53% (28 to 79) | 100% |
| Pleural (Xpert) | 159 | CRS | 30% (17–44) | 100% |
| Peritoneal (Xpert)47 | 80 | CRS | 32% (12–51) | 100% |
| Children | ||||
| Extrapulmonary TB (Xpert)47 | 8 (652*) | CRS | 71% (63–79) | 97% (95–99) |
| Lymph node (Xpert)47 | 6 | CRS | 80% (70–88) | 94% (89–97) |
| CSF (Xpert)47 | 5 | CRS | 42% (22–63) | 99% (95–100) |
CI, confidence interval/credible interval; CRS, composite reference standard. *number of specimens
The performance of Xpert for samples obtained from pulmonary and extrapulmonary sites, when compared with culture or composite reference standard (CRS), is summarized in Table 2.45–48 In adults and children with signs and symptoms of TB meningitis, the World Health Organization (WHO) strongly recommends that Xpert MTB/RIF or Xpert Ultra should be used in CSF as an initial diagnostic test for TB meningitis rather than smear microscopy and culture.49 In a meta-analysis of six studies that used a CRS, Xpert Ultra had a pooled sensitivity of 64% and specificity of 100% for TB meningitis.46 Although Xpert Ultra seems to represent a step forward in TB meningitis diagnosis, it cannot fully exclude TB meningitis.49 The sensitivity of Xpert was high for lymph node samples (>80%) and low for pleural (30–50.9%) and peritoneal fluid (32–59.2%), while the specificity was generally above 85% for all extrapulmonary sites (Table 2).47,48
Testing for resistance to isoniazid (INH) and fluoroquinolone is important in settings where the prevalence of resistance is >5%.50 Both Xpert MTB/RIF and Xpert Ultra perform similarly in detecting rifampicin resistance.45 With regards to picking up multidrug resistance, the Xpert MTB/XDR assay51 has high diagnostic accuracy and meets the WHO's minimum target product profile criteria for a next-generation DST. The performance of the various tests to detect drug resistance is summarized in Table 3. It is necessary to consider the reference sample under examination and the diagnostic accuracy of the test when interpreting the results of the quick diagnostic genomic DST.
Table 3.
Performance of molecular tests against culture-based DST to detect drug-resistant TB
| Test | Number of studies/specimens | Sensitivity | Specificity |
|---|---|---|---|
| Near patient technology-automated or semi-automated NAAT assay | |||
| Xpert MTB/RIF54 | 48 studies, 8020 participants | Rifampicin resistance: 96% (94–97%) |
Rifampicin resistance: 98% (98–99%) |
| Truenat MTB55 | 1 study, 250 participants | 89.3% (66.0–87.4) | 92.2% (87.2–95.6) |
| Truenat MTB55 | 1 study, 250 participants | 81.7% (70.7–89.8) | 94.9% (89.8–97.9) |
| Xpert MTB/XDR Cochrane review56 |
Isoniazid resistance 6 cohorts, 1083 participants |
94.2% (87.5–97.4) | 98.5% (92.6–99.7) |
| Fluoroquinolone resistance 6 cohorts, 1021 participants |
93.2% (88.1–96.2) | 98.0% (90.8–99.6) | |
| Ethionamide resistance: 4 cohorts, 434 participants |
98.0% (74.2–99.9) | 99.7% (83.5–100.0) | |
| Amikacin resistance 4 cohorts, 490 participants | 86.1% (75.0–92.7) | 98.9% (93.0–99.8) | |
| Xpert MTB/XDR57 | 1 study 497 participants |
Isoniazid 96.8% Fluoroquinolones 90.0% Kanamycin, 70.0% Amikacin 83.3% Capreomycin 70.0% Ethionamide 61.8% |
Isoniazid 95.8% Fluoroquinolones 98.3% Kanamycin 99.6% Amikacin 99.6% Capreomycin 99.6% Ethionamide 99.6% |
| Line probe assays | |||
| GenoType® MTBDR assay58INH and Rifampicin resistance | 10 studies, 3,349 specimens | INH 84.3% (76.6–89.8) Rifampicin 98.1%, (95.9–99.1) |
INH Resistance 99.5% Rifampicin resistance 98.7% |
| GenoType® MTBDR assay59 Fluoroquinolone resistance |
9 studies, 1,771 participants | 86.2% (74.6–93.0%) | |
| GenoType® MTBDR assay59 Second line injectable drugs |
8 studies, 1,639 participants | 87.0% (38.1–98.6%) | 99.5 (93.6–100.0%) |
| GenoType® MTBDR assay59 XDR TB |
6 studies, 1,420 participants | 69.4 (38.8–89.0%) | 99.4 (95.0–99.3%) |
Values in parentheses indicate confidence intervals (CI) unless specified.
Line probe assays are recommended to confirm and further evaluate drug resistance. The LPA test offers an advantage as it is directly performed on clinical samples, has a short turnaround time of 24–48 hours, and detects DR not only to first-line but also to second-line anti-TB drugs.52,53 It has the advantage of rapid screening for MDR-TB in areas where the prevalence of DR-TB is high and enables timely initiation of appropriate anti-TB drugs. The performance of LPAs against the gold standard is provided in Table 3.54–59
The current reference method for assessing DR is pDST,60 which is a sensitive diagnostic technique that uses cultured isolates exposed to bacterial growth in the presence of antibiotics. Phenotypic drug susceptibility test is available using both solid (LJ) medium and liquid (MGIT) medium but critical concentrations are limited and available only for rifampicin, isoniazid, levofloxacin, moxifloxacin, bedaquiline, linezolid, clofazimine, amikacin, and streptomycin.61 In general, a liquid culture medium is preferred. However, the disadvantages of cultures include a long turnaround time, an extremely labor-intensive and complex procedure, and the need for specialized infrastructure, which restricts its impact and accessibility.
Whole genome sequencing is a promising tool that is currently being evaluated to guide individualized clinical decisions for the most complicated TB-resistant cases, predict resistance to first- and second-line anti-TB, characterize hetero-resistance and mixed infections, study taxonomy and transmission dynamics, and discover targets involved in phenotypic resistance and resistance-conferring mutations.62 Its role in the ICU setting is currently limited.
Q9: What is the Role of Cultures in the Diagnosis of TB?
Position statement: Culture remains the gold standard for TB diagnosis for pulmonary and extrapulmonary TB as it enables accurate identification and subsequent susceptibility testing. However, the turnaround time is long.
Commentary: Cultures should be done on all samples even if a rapid diagnosis of TB is made on microscopy or molecular tests, since it enables accurate identification and subsequent susceptibility testing. The traditional LJ medium, the prototype of the traditional solid medium, is cost-effective and less likely to get contaminated. Liquid culture is faster and offers improved sensitivity.63 The MGIT 960 technique is an advanced and semi-automated method for the cultivation of M. tuberculosis in a controlled and monitored system. In a study involving 14,597 isolates, 41% were positive by the MGIT 960 TB system while 24% grew on the conventional LJ medium within 6 weeks.64 Typically, all positive MGIT cultures grow both M. tuberculosis complex (MTBC) and most nontuberculous mycobacteria (NTM). These need to be further characterized. Cultures increase the sensitivity in the diagnosis of extrapulmonary TB.27,48
Q10: Is there a Role for Adenosine Deaminase (ADA) in the Diagnosis of Extrapulmonary TB?
Position statement: Adenosine deaminase is a useful adjunct test in the diagnosis of extrapulmonary TB. It must be used in conjunction with other tests to diagnose TB.
Commentary: Pleural fluid ADA is a useful diagnostic test for tuberculous pleural effusion. In a systematic review of 174 publications, the sensitivity, specificity, and diagnostic odds ratio were 92, 90, and 0.97%, respectively. The threshold used for ADA in the studies ranged from 40 to >65 IU/l. All studies however showed a high risk of bias.65 In CNS TB, ADA has low to moderate diagnostic utility.66,67 In one study, the widely used cut-off value of 10 U/l had a specificity of 82% and a sensitivity of 50%.67 In abdominal TB, a meta-analysis of 24 articles demonstrated a pooled sensitivity and specificity of 93% and 95%, respectively.68 In a study involving 105 patients with TB pericarditis, the sensitivity and specificity for ADA were 82.1 and 92.4%, respectively when a threshold of 40 U/l was used.69
Q11: What is the Role of Invasive Diagnostic Tests for TB?
Position statement: Invasive tests such as bronchoscopic procedures, biopsy techniques, and conventional surgery may be required to diagnose pulmonary and extrapulmonary TB when noninvasive and less invasive tests fail to give a diagnosis.
Commentary: In the diagnosis of pulmonary TB, if a diagnosis cannot be made based on sampling of the respiratory tract, bronchoscopic procedures such as (BAL) may be required, targeting areas of radiographic abnormality.70 Bronchoalveolar lavage is likely to improve the yield and facilitate the diagnosis of pulmonary TB.24,25 If endobronchial lesions are observed, biopsies may be taken.70 Transbronchial lung biopsies have now been largely replaced with endobronchial ultrasound (EBUS)-guided needle aspiration of lung lesions as well as paratracheal, subcarinal, and hilar lymphadenopathy.70 Image-guided or surgical biopsy may be required for peripheral lung lesions.70
In extrapulmonary TB involving the pleura, pericardium, lymph nodes, bones and joints, bowel, peritoneum, kidney, fallopian tubes, and epididymis, noninvasive techniques may not be feasible and other methods may not be diagnostic. Many of these sites may be amenable to percutaneous aspiration and/or biopsy.71 In disseminated TB, a diagnosis may also be obtained through bone marrow, lung, or liver biopsy.71 Samples must be subject to histopathological examination and cultures.
Q12: What is the Role of Imaging in the Diagnosis of TB?
Position statement: Chest radiography is the mainstay imaging for pulmonary parenchymal TB. Computed tomography and MRI are used for the evaluation of pulmonary and extrapulmonary TB.
Commentary: In parenchymal disease of the lung due to TB, chest radiography remains the main process for the evaluation of TB. Computed tomography is more sensitive than radiography for the detection of lymphadenopathy.72 Magnetic resonance imaging is superior to CT in the detection and assessment of CNS TB.72 Contrast MRI is superior to CT for the evaluation of meningitis and its complications, including hydrocephalus as well as characterizing homogeneous or ring-enhancing tuberculoma, and miliary TB involving the brain.72 Abdominal lymph nodes are best evaluated on CT.72 F-FDG18 PET may help differentiate active and inactive pulmonary TB and assess treatment response.73 Since tubercular lesions demonstrate high F-FDG18 uptake, it has the potential to be used for the diagnosis of spinal infections and monitoring treatment response in select cases of extrapulmonary TB.72
Drug Therapy for TB
Q13: When should Anti-TB be Initiated in a Critically ill Patient?
Position statement: Timely initiation of anti-TB drugs is essential for treatment success, particularly in those with high disease severity.
Commentary: Early diagnosis and treatment are likely to improve outcomes in all forms of TB. In one study of TB patients with septic shock, early and appropriate anti-TB treatment appeared to improve mortality.74
Q14: Which Anti-TB Regimen should be Initiated in a Critically Ill Patient?
Position statement: As per the National TB Eradication Program (NTEP), a weight based, standard anti-TB regimen with four drugs should be initiated unless drug resistance is suspected or demonstrated. Higher doses of anti-TB drugs may be considered in severe disease.
Commentary: As per the NTEP guidelines, a weight-based, standard anti-TB regimen with four drugs (rifampicin, isoniazid, pyrazinamide, and ethambutol) should be initiated unless drug resistance is suspected or demonstrated (Table 4). Higher doses of anti-TB drugs may be considered in severe disease.14,75,76 For both adults and children, the WHO recommends an intensive treatment regimen with four drugs for 2 months, followed by a continuation regimen (rifampicin, isoniazid) for 4 months except for CNS TB and bone or joint TB.77 However, the current RNTCP guideline advocates a three-drug regimen in the continuation phase (rifampicin, isoniazid, ethambutol) because of the risk of isoniazid mono-resistance.78 Streptomycin is recommended instead of ethambutol in the intensive phase if vision is impaired or cannot be assessed.78
Table 4.
Overview of anti-tubercular drugs and their relevance to critical care14
| Drug | Dosing | IV | Comments | CSF |
|---|---|---|---|---|
| Isoniazid | Adults: 5 mg/kg/d Children: 10 mg/kg/d (max. 300 mg/d) |
Available* | Enteral absorption is reduced in the setting of sepsis, respiratory failure75,83 | 100% |
| Rifampicin | Adults: 10 mg/kg/d (max. 600 mg/d) Children: 15–20 mg/kg/d (max. 600 mg/d) |
Available* | In critically ill patients with TB and respiratory failure, Cmax below recommended level (>8 mg/L) when given orally.83 Higher oral dose (35 mg/kg) or 20 mg/kg IV achieves CSF MIC in >93% of patients87 | 10–20% |
| Ethambutol | Adults: 15 mg/kg/d Children: 20–30 mg/kg/d (max. 1200 mg/d) |
Available* | In TB meningoencephalitis and HIV infection IV INH and ethambutol resulted in better clinical outcomes as compared with oral treatment.89 | 25–50% |
| Pyrazinamide | Adults: 25 mg/kg/d Children: 35 mg/kg/d (max. 2000 mg) |
Not available | Absorption rapid after oral administration, peak concentration in 1–2 hours. Minor changes in bioavailability when taken with meal. Serious adverse events are frequent, particularly in elderly.91 | 100% |
| Moxifloxacin | Adults: < 30 kg, 400 mg once daily 30–50 kg 600 mg once daily > 60 kg, 800 mg once daily Children: 10–15 mg/kg once daily |
Available | Second-line drug; majority of trials, treatment success not in favor of quinolones over standard regimens.93 Survival benefit for TB meningitis in one trial.93 | AUC for CSF to plasma 0.82 at 400 mg once day94 |
| Amikacin | Adults: 15 mg/kg/day | Available | Effective for MDR-TB; Among 437 MDR-TB of whom 288 were HIV co-infected, 270 (62%) developed hearing loss.92 | Mean (SD) 1.65 (1.6) mg/L children92 |
| Carbapenems | Imipenem, ertapenem and meropenem used in studies. | Available | Bactericidal activity greater with meropenem at 6 gm daily than with 3 gm daily.96 In vitro studies suggested that activity of carbapenems increases when used with clavulanate.97 | Low; 79% undetectable98 |
| Linezolid | Adults: 600 mg/day | Available | Risk-benefit ratio favors 600 mg/day.98 Adverse events less and longer tolerance to drug when switched from 600 to 300 mg per day of linezolid.100 | ~ 30%102 |
*Not available in India; AUC, area under the curve; CSF, cerebrospinal fluid; IV, intravenous; MDR, multi-drug resistant; MIC, minimum inhibitory concentration
In critically ill patients, first-line enterally administered drugs appear to be superior to second-line parenteral drugs plus ethambutol. In a study of 178 critically ill patients, an alternative regimen that included IV levofloxacin plus oral ethambutol plus IM streptomycin or IV amikacin, without rifampicin and isoniazid was associated with a significantly (p = 0.011) higher (63.5%) mortality when compared with the mortality of 51.4% among 284 critically ill patients who were administered of rifampicin, INH, pyrazinamide, and ethambutol enterally.79 There is some evidence that higher doses of the usual anti-TB drugs (e.g., rifampicin up to 35 mg/kg/d) are safe and may reduce the time to culture conversion and may be associated with better survival in severe disease.80,81
Q15: Do Enterally Administered Anti-TB Drugs Achieve Therapeutic Levels in Critically Ill Patients?
Position statement: Absorption of enterally administered anti-TB drugs is reduced in critically ill patients and if administered when continuous enteral feeds are given. Therapeutic levels may be achieved with enteral pyrazinamide but not with INH, rifampicin, and ethambutol.
Commentary: Multiple factors alter drug pharmacokinetics in critical illness and include reduced absorption, increased volume of distribution, augmented renal clearance, genetic variations in drug metabolization, and poor penetration into infected compartments.76 Pharmacokinetic studies in 10 critically ill patients showed that a fixed-dose combination of rifampicin, INH, pyrazinamide, and ethambutol, given according to weight via a nasogastric tube resulted in sub-therapeutic rifampicin plasma concentrations in a majority of patients, while other drugs had a more favorable pharmacokinetic profile.82 In a study of eight critically ill patients with TB and acute respiratory failure, blood samples were obtained at steady state.83 The Cmax of pyrazinamide was above the recommended concentration of >20 mg/L; for rifampicin, the Cmax was below the recommended level of >8 mg/L and the Cmax of INH was below the recommended levels of >3 mg/L.83 In another study involving 20 critically ill patients, rifampicin concentrations were low in all patients receiving continuous enteral feeding.84 In another study of 81 HIV patients hospitalized for sepsis, with or without meningitis, who were initiated on first-line anti-TB drugs, 49 completed pharmacokinetic studies.75 Serum Cmax targets were achieved in 8.2% with 450 mg of rifampicin, 0% with 300 mg of INH, 89.8% with 1500 mg of pyrazinamide, and 63.2% with 1200 mg of ethambutol.75 Enteral administration of pyrazinamide results in rapid absorption with peak concentration achieved within 1–2 hours.85 Only minor changes in bioavailability occur when it is taken with a meal.
Q16: Is there a Role for Higher Doses of Anti-TB Drugs or Intravenous Anti-TB Drugs in TB Meningitis and Severe Illness?
Position statement: Higher doses of anti-TB drugs or intravenous (IV) preparations, where available, may be considered in TB meningitis and severe illness.
Commentary: Given the reduced bioavailability of enterally administered anti-TB drugs INH, ethambutol, and rifampicin, it may be preferable to administer these drugs at higher doses, or intravenously when they are available, along with enteral pyrazinamide in critically ill patients.76 In one of the earliest phase II trials of patients with TB meningitis, a higher oral dose of rifampicin (13 mg/kg) was found to be safe and possibly associated with survival benefits in severe disease.81 In another study, a higher dose of oral rifampicin (17 mg/kg and 20 mg/kg) resulted in approximately similar AUC0–24, but lower plasma Cmax values compared with 600 mg IV over 1.5 hours.86 In a phase II study, which was predominantly done on HIV individuals, standard dosing of rifampicin (10 mg/kg) was able to achieve CSF concentrations above minimal inhibitory concentration (MIC) in only 11% (2/18) of patients when compared with 93% (14/15) in those receiving 20 mg/kg IV of rifampicin and 95% (18/19) in those receiving 35 mg/kg of rifampicin.87 In another study of 46 HIV individuals, the AUC0-24 for rifampicin was 42.9 µg.h/mL for the standard (10 mg/kg) oral dose, 295.2 µg. h/mL for high (35 mg/kg) oral dose and 206.5 µg.h/mL for IV administration.88 In one study of patients with TB meningoencephalitis and HIV infection, IV INH and ethambutol resulted in higher sputum conversion, clinical improvement, and improved mortality when compared with oral treatment.89 In another study of 152 patients with pulmonary TB, 65 patients received parenteral INH, ethambutol, and rifamycin.90 After the intensive phase, sputum conversion was significantly higher (p < 0.05) in patients treated parenterally (100%) when compared with 71% among controls.90 Given these observations, IV formulations of INH, rifampicin, and ethambutol should be administered where available14 or a higher dose of enterally administered rifampicin may be justified in the critically ill patient with TB, when IV formulations are not available. Since pyrazinamide is not available as an IV preparation and has a favorable pharmacokinetic profile, a standard dose of pyrazinamide can be administered enterally in the critically ill patient. Serious adverse events are however frequent, particularly in the elderly.91 The first- and second-line anti-TB drugs and dosing are given in Table 4. Intravenous preparations of rifampicin, INH, and ethambutol are not available in India. Further studies are required to evaluate the safety and efficacy of higher doses of enterally administered anti-TB drugs in the critically ill.
Q17: What is the Role of Other Intravenous Antibiotics in TB?
Position statement: Although several IV antibiotics such as aminoglycosides, quinolones, linezolid, and carbapenems are effective against TB, these second-line drugs should be used only in the setting of DR-TB, life-threatening TB and in those who do not tolerate first-line anti-TB drugs.
Commentary: Several IV antibiotics commonly used in the treatment of gram-positive and gram-negative organisms are effective against TB (Table 4). However, they are considered only in the setting of drug-resistant TB, intolerance to first-line drugs, or in patients with life-threatening TB. These include aminoglycosides (such as streptomycin, amikacin, and kanamycin), quinolones (such as moxifloxacin, levofloxacin, and gatifloxacin) linezolid and carbapenems (such as ertapenem, imipenem, and meropenem).
IV amikacin is effective in the treatment of MDR-TB. Among 437 MDR-TB of whom 288 were HIV co-infected, 228 (73%) had a good outcome in terms of cure or treatment completion; 270 (62%) developed hearing loss.92 Quinolones are available as IV formulations and used as second-line drugs in TB. In most trials, treatment success was not in favor of quinolones over standard regimens.93 Survival benefit for TB meningitis was observed in only one of three published trials.93,94
In a systematic review of the use of carbapenems in TB that included two studies on ertapenem, one on imipenem and four on meropenem, that included patients with XDR-TB, the authors concluded that culture conversion rates ranged between 60 and 94.8% with the proportion of adverse events attributable to carbapenems below 15%.95 The bactericidal activity of meropenem was greater with 6 gm daily than with 3 gm daily.96 Although β-lactam antibiotics have achieved overall success, their usage for treating TB is limited due to inherent resistance induced by the existence of a chromosomally-encoded gene (BLaC) in M. tuberculosis, which encodes for a Class A Ambler β-lactamase (BlaC). In vitro studies suggested that the activity of carbapenems against M. tuberculosis was increased when used in combination with clavulanate, a BLaC inhibitor.97 In TB meningitis, CSF levels were undetectable in 79% of samples.98
Linezolid may be effective in treating XDR or pre-XDR-TB. In a recently published study, of the 181 participants enrolled, 88% had XDR or pre-XDR-TB.99 A favorable outcome was observed in 84–93% of participants who were administered bedaquiline-pretomanid-linezolid (BPaL) combination. The overall risk-benefit ratio favored the group that received a lower dose of linezolid (600 mg/d) for 26 weeks compared with the higher dose (1200 mg/d) for 26 weeks or 600 mg for 9 weeks.99 In another study involving 69 patients, better treatment outcomes, fever recurring adverse events, and tolerance to longer duration of linezolid were observed when patients were switched from 600 to 300 mg per day of linezolid.100 There was pharmacokinetic evidence of the potential usefulness of linezolid in TB meningitis,101 with the extent of CSF penetration around 30% of plasma exposure and correlating with CSF protein concentrations.102
It must be kept in mind that the indiscriminate use of these antibiotics as empiric treatment for fever of unknown origin could potentially mask an underlying tubercular infection and may delay its diagnosis.
Q18: How Should Drug-resistant TB (DR-TB) be Treated in the ICU?
Position statement: Patients with DR-TB should be enrolled in The National TB Elimination Programme (NTEP) if not already enrolled. The NTEP has adopted a bedaquiline-based DR-TB regimen in India based on WHO guidelines. Treatment should be started in consultation with respiratory physicians and NTEP. The recommended drugs and duration of treatment in such patients are guided by the program management of DR-TB in India.
Commentary: Patients with MDR-TB need a multi-disciplinary approach. The recommended anti-TB drug regimen is based on the findings from the NiX-TB,103 ZeNiX-TB99 and TB-PRACTECAL trials.104 In the NiX-TB trial, the safety and efficacy of an all-oral three-drug BPaL regimen administered over 6 months in patients with treatment-intolerant or nonresponsive MDR-TB or XDR-TB was assessed. With this regimen, 90% (95% CI, 83–95%) of patients had satisfactory outcomes; nevertheless, the daily high dose of linezolid was associated with a significant incidence of side effects, with 48% developing anemia and/or thrombocytopenia and 81% developing peripheral neuropathy.
Linezolid at lower daily doses was better tolerated with fewer adverse events.99,100,104 Three 24-week BPaL-based therapy regimens with linezolid (BPaL, BPaLC (with clofazimine), and BPaLM (with moxifloxacin) were compared to standard-of-care treatment in the TB-PRACTECAL trial to assess their efficacy and safety. At 72 weeks, 89% of patients in the BPaLM arm reported treatment success, compared to 52% of patients in the standard-of-care arm.
The WHO updated its DR-TB guidelines in 2022,33 recommending the BPaLM regimen as the preferred regimen for patients with MDR/RR-TB when fluoroquinolone susceptibility is presumed or documented, and BPaL alone for patients with additional fluoroquinolone resistance (pre-XDR-TB).
Q19: Is there a Risk of Drug-drug Interaction with Commonly Used Anti-TB Drugs?
Position statement: There are significant drug-drug interactions with the commonly used anti-TB drugs. Clinicians need to be familiar with the common interactions in the ICU setting.
Commentary: The commonly used anti-TB drugs have significant drug interactions by virtue of inhibition or activation of enzymes. The mechanism of action of the anti-TB drugs, the mechanism of drug-drug interaction, and the important drug interactions are summarized in Table 5. Clinicians must be familiar with the common interactions in the ICU setting, particularly with rifampicin (Table 6). Therapeutic drug monitoring (TDM) may be considered, where available, when significant drug interactions are likely to lead to toxicity or sub-therapeutic effect. For more information, the readers may refer to the exhaustive review of the drug interactions published in 2020.105
Table 5.
Important drug-drug interactions and mechanisms105
| Drug | Mechanism of action/mechanism of drug interaction | Important drugs interactions |
|---|---|---|
| Isoniazid | Destruction of cell wall, generating RO Drug interaction: Inhibits cytochrome P450 system and acts as a mild monoamine oxidase inhibitor |
Disulfiram, acetaminophen, antacids, rifampicin, oral hypoglycemic agents, anticonvulsants, theophylline |
| Rifampicin | Inhibits DNA-dependent RNA polymerase; decreases affinity of RNA polymerase for short RNA transcripts. Drug interaction: Induces P450 cyto-chrome oxidases (CYP3A4, CYP2A, CYP2B, CYP2C, CYP3A), and human P glycoprotein ABC transporter |
Morphine, antacids, antiarrhythmics, several antibiotics, anticoagulants, anticonvulsants, antidepressants, INH, pyrazinamide, bronchodilators, steroids, tacrolimus, cyclosporin, retrovirals, thyroid hormones |
| Ethambutol | Inhibits arabinosyl transferases involved in synthesis of mycobacterial cell wall | Coadministration with aluminum salts delays and reduces the absorption of ethambutol. Other drugs potentially causing optic neuritis should be avoided |
| Pyrazinamide | Nicotinamide analog; inhibits the coenzyme A biosynthesis | Concomitant administration of pyrazinamide with isoniazid and/or rifampin is associated with an increased risk of hepatotoxicity |
| Fluroquinolones | Inhibits DNA gyrase | Antacids, antiarrhythmics, rifampicin, warfarin, insulin, bronchodilators, cyclosporin, tacrolimus |
| Amikacin | Blocks 30S ribosomal subunit | Diuretics, cephalosporins, cyclosporin, colistimethate sodium, tacrolimus |
| Carbapenems | Inhibiting BlaC beta-lactams | Caution in patients with TB meningitis and when coadministered with ganciclovir and valproic acid |
| Linezolid | Binds 50S subunit of prokaryotic ribosome, prevents formation of initiation complex, inhibits protein synthesis Drug interaction: Reversible inhibitor of monoamine oxidases A and B and serotonin agonists |
Rifampicin, warfarin, antidepressants (SSRI like fluoxetine, sertraline), fentanyl, morphine derivatives |
ROS, reactive oxygen species; SSRI, selective serotonin reuptake Inhibitor
Table 6.
Drugs frequently prescribed in the ICU and their interaction with rifampicin
| Drugs | Comment |
|---|---|
| Oral anticoagulants | Avoid newer oral anticoagulants (dabigatran, rivaroxaban, apixaban), warfarin may be used with international normalized ratio monitoring (dose escalation likely required) |
| Glucocorticoids | Increase dose of glucocorticoid 2–3-fold |
| Azoles | If possible, avoid (voriconazole, itraconazole, posaconazole). Fluconazole has less reduction in serum concentration compared to other azoles. Monitor for response |
| Atorvastatin | Monitor lipid panel; increased dose will likely be needed |
| Phenytoin | Monitor serum phenytoin concentrations and seizure activity, dose escalation likely required |
| Midazolam | Preferably avoid with rifampin, use alternatives |
| Tacrolimus | Monitor serum tacrolimus concentrations and clinical response, increased dose may be required, use alternatives if possible |
| Cyclosporine | Monitor serum cyclosporine concentrations, dose escalation likely required |
| Anti-retroviral drugs | Use dolutegravir-based regimen, double the dose of dolutegravir |
Multiple drug interactions, particularly with calcineurin inhibitors (CNI), mTOR (mammalian target of rapamycin) inhibitors, and glucocorticoids106 can occur in post-transplant patients on immunosuppression. These can lead to an increased risk of graft rejection, up to 25%.107 Based on these, modifications of immunosuppressant medication doses have been suggested to the tune of a three to five-fold increase in CNI or mTOR inhibitors, with doubling of steroid dose.108 Though some investigators have demonstrated adequate serum levels,109 other investigators have reported the relative impossibility of such attempts.110,111
Q20: What is the Role of Corticosteroids in TB?
Position statement: Corticosteroids may be effective in reducing clinically relevant adeverse outcomes in some forms of TB.
Commentary: Corticosteroids may be effective in reducing clinically relevant outcomes in some forms of TB (Table 7).112–116 In patients with pulmonary TB admitted to ICU with acute respiratory failure (ARF) (n = 124), steroids were associated with reduced 90-day mortality (OR 0.47; 95% CI, 0.22–0.98; p = 0.049) using inverse probability treatment weighting (IPTW) method.117 Steroids were prescribed in this study for ARDS, shock, wheeze, or disseminated TB. On TB pleural effusions, six trials (n = 590) were summarized in a Cochrane review.112 The risk of residual pleural effusion on chest X‐ray reduced by 45% at 8 weeks and 65% at 24 weeks.112 In TB meningitis, a systematic review of nine RCTs (n = 1337), showed a reduction in death (RR 0.75, 95% CI, 0.65–0.87); however, there was no effect on disabling neurological deficits.113 In HIV-negative individuals, steroids may reduce the risk of death (4 trials, n = 660, RR 0.80, 95% CI, 0.59–1.09), and the need for pericardiocentesis (RR 0.85, 95% CI, 0.70–1.04) in patients with TB pericardial effusions; there is uncertainty on the effect of steroids on constriction and in HIV individuals.118 In a small study of 13 patients, high dose methylprednisolone (500–1000 mg/d for 3 days) was associated with higher 3-month survival in miliary TB complicated by ARDS.114 Steroids may also be indicated in immune reconstitution inflammatory syndrome (IRIS) and adrenal insufficiency. The role of steroids in the various forms of TB is summarized in Table 7.
Table 7.
Steroids for tuberculosis
| Type of TB | Steroid type and dose | Comments and supporting evidence |
|---|---|---|
| Pulmonarya | Adults: Prednisolone 50 mg or equivalent; duration unclear | Pulmonary TB admitted to ICU with ARF (n = 124), steroids reduced 90-day mortality (OR 0.47; 95% CI, 0.22–0.98; p = 0.049) using IPTW method.117 Steroids prescribed for ARDS, shock, wheeze or disseminated TB. Meta-analysis suggested that steroids could be effective in reducing mortality for all forms, including pulmonary TB.115 |
| TB pleural effusion | Prednisolone 1 mg/kg reduced after 1–2 weeks, total duration max up to 3 months | Six trials (n = 590) summarized in a Cochrane review. Risk of residual pleural effusion on chest X‐ray reduced by 45% at 8 weeks and by 65% at 24 weeks.112 |
| TB meningitis | Dexamethasone 0.6 mg/kg children, 0.4 mg/kg adults intravenously to be tapered on a weekly basis by 0.1 mg/kg—then oral therapy starting at 4 mg per day decreasing by 1 mg every 7 days;119 Prednisolone 2–4 mg/kg children, adults 2.5 mg/kg for 6–8 weeks and then taper116 |
Systematic review of nine RCTs (n = 1337), reduction in death (RR 0.75, 95% CI, 0.65–0.87); no effect on disabling neurological deficits.113 |
| Pericardial | Adults: prednisone 60 mg/day (or equivalent) for 4 weeks, followed by 30 mg/day for 4 weeks, 15 mg/day for 2 weeks, and 5 mg/day for 1 week | In HIV-negative individuals, may reduce risk of death (4 trials, n = 660, RR 0.80, 95% CI, 0.59–1.09), need for pericardiocentesis (RR 0.85, 95% CI, 0.70–1.04); uncertainty on effect on constriction and in HIV individuals.118 |
| TB-associated IRIS | Prednisone 40 mg per day for 14 days, followed by 20 mg per day for 14 days | RCT on prophylactic prednisolone to reduce IRIS in patients with TB and HIV infection who were ART naïve was associated with lower incidence of TB-associated IRIS than placebo, without evidence of an increased risk of severe infections or cancers.143 |
| Adrenal insufficiency | Hydrocortisone 50–100 mg 6–8 hourly acute phase; maintenance prednisolone | Adrenal insufficiency can be confirmed by diminished response to synthetic adrenocorticotropin. Should be suspected when there is hypotension needing high-dose vasoactive agents |
aincludes pulmonary and tuberculosis (TB) acute respiratory distress syndrome (ARDS); ART, anti-retroviral therapy; HIV, human immunodeficiency virus; ICU, intensive care unit; IPTW, inverse probability of treatment weighted; RCTs, randomized controlled trials; RR, risk ratio; IRIS, immune reconstitution inflammation syndrome;
Q21: Is there a Role for TDM in DR-TB?
Position statement: There is insufficient evidence to recommend routine TDM during the treatment of DR-TB.
Commentary: There is currently little evidence to support TDM for medications used to treat MDR/RR-TB. Even though there is good reason to use TDM, it is unclear if this enhances treatment outcomes and lowers resistance risk. However, given that rifampicin exhibits a significant amount of pharmacokinetic variability and that the exposures resulting from the recommended doses lie on the steep side of the dose-response curve, ensuring adequate drug concentrations, either through TDM or higher dosing, may play a significant role in DR-TB prevention strategies.119 TDM may be considered in patients who are slow to respond to treatment, have drug-resistant TB, are at risk of drug-drug interactions, or have concurrent disease states that significantly complicate the clinical situation.120
Q22: How do Risk Prediction Scores Perform in TB in the Critically Ill?
Position statement: Risk prediction scores should not be relied on for mortality prediction in TB.
Commentary: Tuberculosis requiring ICU admission has high mortality. In a systematic review of 17 studies, APACHE II, SOFA, and SPAS II scores underestimated mortality in pulmonary TB.121 Factors associated with negative outcomes included hospital-acquired infections, need of mechanical ventilation and vasopressors, delay in initiation of anti-TB treatment, more than one organ involvement, and higher severity score.
Tuberculosis in the Immunocompromised Host
Among patients admitted to the ICU with TB, immunocompromised patients comprise 6.9–68.7% of the cases.122,123 The umbrella term of “immunocompromised individual” includes patients with acquired or primary immune deficiency, patients on glucocorticoids, other immunosuppressant drugs for the treatment of connective tissue disorders, post solid organ transplant, and hematological malignancies in the pre- and post-transplant periods.124 The increasing efficacy of treatment for these diseases has resulted in a change in the scenario from uniformly fatal diagnoses to treatable or manageable chronic comorbid conditions. At the same time, the presence of immune deficiency of a chronic nature and the ubiquitous presence of TB bacilli (especially in countries with high prevalence of TB) makes the risk of infection with TB several-fold higher.122 Understanding the risks of infection and clinical syndromes that tuberculosis manifests with, in the immunocompromised critically ill individual is essential to diagnose TB and to provide optimal care.
Q23: Does the Immunocompromised State Predispose an Individual to Reactivation of Latent TB or the Acquisition of TB?
Position statement: Since immunocompromised patients are at risk for TB, the clinical suspicion should be high in patients admitted to the ICU. The interpretation of tests for latent TB infection (such as the TST and IGRA) are likely to be influenced by immunosuppression and the severity of critical illness.
Commentary: Immunocompromised hosts have a 10–110-fold higher risk of latent TB reactivation in HIV infection and 20 times higher risk in solid organ transplants than the general population.125,126 While latent TB screening by TST or IGRA is advised before the use of immunosuppressive drugs such as anti-TNF alpha, and prior to solid organ and hematological transplants,125,126 in severely ill patients with coexisting immunological insufficiency (acquired or primary), these tests may yield more ambiguous results.127,128 After recovery from critical illness, 48% of results remain ambiguous.127 These results were related with higher severity of critical illness and lower blood albumin levels.
Solid organ transplant recipients have the highest TB risk compared to allogenic hematopoietic stem cell transplant (HSCT) recipients and the general population.129 Lung transplant recipients have a 5.6-fold higher risk of TB than other stem cell recipients.130 Allogenic transplant with unrelated donor (RR 23.9), prior total body irradiation (RR 4.9), and persistent GVHD (RR 3.6) should raise clinical suspicion for TB in post-HSCT patients.131
Tuberculosis symptoms typically appear in the first year after solid organ transplantation, albeit the timing varies.130 Up to 30% of patients may acquire TB later in the post-transplant phase, with renal transplants causing symptoms later than lung and liver transplants.130 Pulmonary was the most common site in renal transplant recipients, with a median presentation period of 46–196 days. Post-allogenic HSCT recipients average 150 days of symptoms,129 with a median gap of 6–7 weeks between symptoms and diagnosis.132
Q24: Is the Clinical Presentation of TB in the Immunocompromised Individual Similar to that of the Immunocompetent Host?
Position statement: In an immunocompromised host, the clinical presentation of TB may be more insidious and likely to be disseminated. TB should be suspected when there is unexplained worsening of clinical status. The surveillance for atypical presentations of TB should be robust in order to pick them up early.
Commentary: In the immunocompetent host, the most common reason for admission due to TB is acute respiratory failure, followed by septic shock with multiorgan dysfunction.4 In contrast, the clinical presentation of TB infection in the immunocompromised patient is more insidious. The symptoms are limited in intensity even though TB may be of a disseminated nature.122 In patients with HIV co-infection, lower CD4 counts are associated with a higher prevalence of extrapulmonary infections and miliary TB, which signifies hematogenous dissemination.133
In the immunocompromised post-solid organ transplant recipient, about one-third to one-half of all cases of active TB are disseminated or occur at extrapulmonary sites. Atypical presentation of TB such as pyomyositis, cutaneous ulcers or abscess, and tenosynovitis also occurs.108,134 Clinical suspicion for TB should be high in patients with respiratory failure after treatment for acute rejection.124,135
The absence of fever in about 20–31% of patients could confound the diagnostic paradigm.124,135 As in other groups of immunocompromised patients, clinical presentation is indolent. Atypical presentations including diffuse alveolar hemorrhage have been reported.131
Q25: What should the Diagnostic Work be in the Immunocompromised Host Who is Suspected to have TB Infection?
Position statement: Taking into consideration the indolent nature of TB in the immunocompromised patient, cross-sectional imaging should be considered early to guide diagnostic sampling. Early invasive sampling including bronchial lavage and/or biopsy is advocated in these patients.
Commentary: In post-renal transplant patients, the pattern of pulmonary involvement was found to be miliary nodules (40%), cavitation and centrilobular tree-in-bud nodules (22.5%), ground-glass attenuation and consolidation (15%), mediastinal lymph node enlargement (12.5%) and pleural effusion (10%) constituted the major HRCT patterns.136
In post-HSCT patients, most common pattern of pulmonary involvement of the chest X-ray was that of consolidation (100%) and nodules (80%).82 On cross-sectional imaging, the most common imaging findings were consolidation (100%), nodules (71%), tree-in-bud appearance (43%), and ground-glass opacity (43%).136 In contrast, cavitations were only found in 14% of patients and lymphadenopathies in 71% of patients.136
In post-renal transplant recipients, respiratory samples yielded microbiological evidence of TB, but the culture of at least one sample from other areas was positive in 53% of patients.124 In a study of patients with HIV co-infection, invasive sampling in the form of BAL and organ biopsy were needed in 11% and 25% of the patients respectively.137
Q26: What would the Approach be in the Management of TB in the Setting of Intestinal Malabsorption?
Position statement: In patients with anticipated poor enteral uptake of antituberculous treatment (ATT) [short bowel, gut graft vs host disease (GVHD), severe multiorgan dysfunction with high-dose vasoactive medications], parenteral ATT may be considered along with TDM.
Commentary: Data is lacking on ATT in patients with anticipated poor enteral uptake of ATT. This includes critically ill patients on organ support, short bowel syndrome, and gut GVHD. Case reports and expert opinions are the sources of information for these patients.138,139 Expert opinion has advocated pragmatically to initially use an IV regimen in critically ill patients.76 The suggested regimens include rifampicin along with moxifloxacin and amikacin,76 IV fluoroquinolones with oral anti-TB drugs,140 or higher doses of rifampicin (15 mg/kg/day) along with fluoroquinolone (levofloxacin 20 mg/kg/day).81
Q27: What would the Approach be to IRIS in TB?
Position statement: IRIS should be suspected when there is paradoxical worsening of symptoms, typically seen when immunosuppression is reduced or reversed. IRIS can also occur in the immunocompetent host. A course of steroids should be considered.
Commentary: IRIS describes the paradoxical worsening of a pre-existing infectious process, typically seen when immunosuppression is reduced/reversed. Although it is seen in patients with HIV-TB co-infection, it can also be seen in immunocompetent patients diagnosed with TB, solid organ transplant, and post-HSCT. In one study, the prophylactic use of prednisolone in patients with TB and HIV infection who were ART naïve was associated with a lower incidence of TB-associated IRIS than placebo, without evidence of an increased risk of severe infections or cancers.141 However, evidence or treatment protocols for solid organ transplants are nonexistent, with case reports suggesting the continuation of ATT142 and high-dose corticosteroids,143 with some advocating the use of NSAIDS and tocilizumab.144
Organ Support
Q28: Is the Ventilatory Management of Respiratory Failure due to TB Different from the Ventilatory Management of Respiratory Failure due to Other Infective Etiologies?
Position statement: There is limited data on the management of respiratory failure in patients with TB. The management of respiratory failure in patients with TB should be along the same lines of management as with other infective etiologies of hypoxemic and hypercapnic respiratory failure.
Commentary: In a case series published in 1987, of the 15 patients admitted with respiratory failure due to TB, 11 required mechanical ventilation and 4 patients had evidence of hypercapnic respiratory failure.145 The radiological features ranged from lobar pneumonia, pleural effusion, bronchopneumonia, and miliary involvement to the destruction of lungs; 33% died in ICU.145,146 In a study from North India21 involving 63 patients, 90.5% were ventilated and the mortality was 44.4%. In a larger study of 212 patients, 87.7% were ventilated and the mortality was 50%.8
In the absence of studies that have focused on invasive mechanical ventilation for respiratory failure due to TB, the current guidelines for safe ventilatory strategies that were summarized in the recent 2023 European Society of Intensive Care Medicine (ESICM) should be followed.147 This includes low tidal volume (LTV: 4–8 mL/kg predicted body weight), titration of positive end-expiratory pressure (PEEP), maintenance of a plateau pressure of <30 cm H20, avoidance of prolonged high-pressure recruitment maneuvers (airway pressures maintained ≥35 cm H20 for at least 1 minute), judicious use of continuous infusion of neuromuscular blocking agents and the use of prone positioning in patients with moderate-severe ARDS (defined as PaO2/FiO2 < 150 and PEEP ≥ 5 cm H20), despite optimization of ventilation settings.147
Q29: Should Noninvasive Respiratory Support be Considered in the Management of Acute Respiratory Failure Due to TB?
Position statement: The use of noninvasive respiratory support including HFNC is challenging given their tendency to cause aerosol dispersion and spread of infection to HCWs. It is preferable to use this in isolation facilities with adequate precautions by staff.
Commentary: A recent study examined the extent of exhaled air dispersion with the use of noninvasive ventilation (NIV) devices.148 They observed that continuous positive airway pressure (CPAP) dispersed air up to 366 mm in the sagittal plane, with the use of positive end-expiratory pressure (PEEP) up to 20 cm H2O via nasal pillows, whereas HFNO dispersed up to 205 mm with flow up to 60 L/min. The dispersion on HFNO increased to 620 mm when the interface became loose. A meta-analysis showed that NIV was a significant risk factor for transmission of acute respiratory infections, with an odds ratio of 3.1 (1.4–6.8), while HFNO was not.149 However, these studies were done in the non-TB setting.
In a small case series of 3 patients, published in 2005, NIV was used to successfully treat patients with moderate-severe ARDS due to TB with PF ratios in the range of 110–120.150 In another study of 58 patients with acute exacerbation of pulmonary TB sequelae, 86.2% were successfully weaned off supports.151 In a more recent study of 44 patients with TB and respiratory failure who required mechanical ventilation, the use of NIV post-extubation was associated with a significant (p < 0.001) reduction in the duration of ventilation (–36.2 hours, 95% CI, –53.6 to –18.8 hours) and a significant reduction in the risk (RR 0.44, 95% CI, 0.24–0.83) of ventilatory associated pneumonia.152
Q30: Is there a Role for Extracorporeal Membrane Oxygenation (ECMO) Support in TB?
Position statement: There is limited evidence for the use of ECMO in TB. It may be considered in TB in the setting of refractory respiratory or cardiac failure.
Commentary: A systematic review of case reports on the use of ECMO in 43 cases of TB over 47 years, was published in 2024.153 The indications for ECMO were ARDS in 83.4% (n = 30) and cardiac failure in 9 patients. Twenty-seven patients were exclusively on veno-venous ECMO (VV ECMO), while 5 patients were exclusively on veno-arterial ECMO (VA ECMO). In 4 patients, VA ECMO was commenced for respiratory failure coupled with hemodynamic instability. At the end of the observation period, 81.4% survived. The authors concluded that ECMO could have a positive effect on overcoming ARDS or bridging the time until response to anti-TB drugs.153
Q31: How Should Massive Hemoptysis be Managed?
Position statement: Patients with massive or life-threatening hemoptysis should be managed in an ICU with a focus on airway and hemodynamic stabilization, evaluation of source of bleed by CT angiogram or bronchoscopy, and consideration of bronchial artery embolization as an early treatment option. Nebulized and/or intravenous tranexamic acid may reduce the volume of hemoptysis.
Commentary: Patients presenting with massive hemoptysis should be initially stabilized with a focus on airway, breathing and circulation and correction of coagulopathy. The evaluation of the source of bleeding should be done by bronchoscopy or CT angiogram. In one study of 28 patients with massive hemoptysis of diverse etiology, bronchoscopy was essential in making the diagnosis in only 10.7% of cases and it was comparable with radiographs in 82% of the patients.154 Although bronchoscopy and CT were comparable in identifying the site of bleeding (73 vs 70%), CT was more efficient in identifying the cause of bleeding.155 If the airway is compromised, intubation will be required; selective intubation of one bronchus and single lung ventilation using double lumen endotracheal tube may be required to prevent aspiration of blood into the normal lung.156
Nebulized and/or intravenous tranexamic acid at the dose of 500 mg thrice daily may be associated with a reduction in the volume of hemoptysis. Inhaled tranexamic acid is safe157 and may be associated with a reduction in the volume of hemoptysis in nonmassive hemoptysis.158 Its role in massive hemoptysis is unclear.
The definitive management for life-threatening hemoptysis is bronchial artery embolization. In a systematic review of the role of bronchial arterial embolization for hemoptysis, 22 studies were included.159 The common indications included TB, post-TB sequelae, and aspergillomas. The overall success of embolization, defined as complete cessation of bleeding, ranged from 70 to 99%; recurrence rates were high at 10–57% and attributed to incomplete initial embolization, recanalization of previously embolized arterial, and the development of new collaterals.159
Q32: When Should Tracheostomy Considered in Patients Ventilated for TB?
Position statement: The indications and timing of tracheostomy in patients admitted to the ICU with TB are similar to those of other patients admitted to critical care. There is inconclusive evidence to recommend early tracheostomy in patients with TB.
Commentary: A proportion of patients admitted to the ICU for the management of TB may require tracheostomy. In one study published in 2018, of the 63 patients admitted to the ICU, 9 patients (14.3%) required tracheostomy.21 In another study of patients with TB-ARDS, 5 out of 47 (10.4%) required tracheostomy.10 There are no studies that have addressed the question of timing of tracheostomy in TB patients admitted to the ICU. However, two recent systematic reviews on tracheostomy in critically ill patients, which have included 15 studies with 3,003 patients160and 17 studies enrolling 3,145 patients,161 respectively, showed that early tracheostomy (≤7 days) was associated with a reduced in ventilator-associated pneumonia (VAP); however, there was significant heterogeneity of treatment effects (I2 > 60%). The duration of ventilation was reduced by about 1 day with early tracheostomy. There was no effect of early tracheostomy on mortality. In the Indian setting, where there may be financial constraints on prolonged hospitalization and the challenges of managing a tracheostomy at home, the decision on tracheostomy should be made with much deliberation.
Q33: What is the Optimal Management of Hypotension due to Adrenal Insufficiency and Septic Shock with Mycobacteremia (Landouzy Sepsis)?
Position statement: In TB, shock may be due to adrenal insufficiency, secondary bacterial sepsis, or mycobacteremia. Shock should be managed along the same lines as the management of septic shock. Early initiation of anti-TB treatment is important. Steroids are indicated for refractory septic shock, adrenal insufficiency, and shock due to mycobacteremia.
Commentary: Adrenal insufficiency can occur in the setting of TB due to involvement of the adrenal gland due to TB or due to relative adrenal insufficiency during critical illness. It can be confirmed by diminished response to synthetic adrenocorticotropin. Adrenal insufficiency should be suspected when there is hypotension requiring high-dose vasoactive agents. In the acute setting, replacement should be done with IV hydrocortisone. Subsequently, replacement doses of oral steroids (prednisolone) may be required.
Very few case reports of mycobacterial septicemia exist, and most are documented in those with HIV.162 Immunocompetent patients may also present with fulminant multiorgan failure and vasoplegic shock, indistinguishable from bacterial sepsis. The overall management of this condition is the same as that of septic shock, in general. Anecdotal reports on the use of pulse steroids exist.163 However, the disease is almost always fatal.162–164
Q34: What is the Optimal Management of Renal Failure in Patients with TB?
Position statement: Renal failure in TB should be managed along the same lines as the approach to managing acute kidney injury in the setting of other infectious diseases. Drug dosing should be optimized for renal function, and nephrotoxic agents should be avoided.
Commentary: Renal failure may occur as part of the multiorgan dysfunction syndrome in TB or as a result of secondary infections. It should be managed along standard guidelines for the management of renal failure. Drug dosing would need to be adjusted in the setting of renal failure. Isoniazid and rifampicin do not require any dose adjustment in renal failure.165 Although pyrazinamide is largely cleared by the liver, due to possible delayed elimination of the drug and its metabolite, a dose of 25–30 mg/kg thrice weekly is recommended in stage IV and V chronic kidney disease (CKD) and in patients on hemodialysis.165 The dose of ethambutol needs to be reduced to 15–25 mg/kg thrice weekly in CKD stages IV and V.165
Q35: Is there a Role for First-line Anti-TB Drugs in Patients with Liver Involvement in TB?
Position statement: Patients with liver involvement due to TB should be started on standard four-drug regimen, irrespective of baseline liver function tests.
Commentary: Patients with liver involvement due to TB (based on imaging or biopsy) should be started on standard four-drug regimen, irrespective of baseline liver function tests. In case of subsequent clinical or biochemical worsening, close monitoring, and possible biopsy is warranted to differentiate between hepatic TB and drug-induced liver injury (DILI) and TB-related IRIS. If DILI is suspected, hepatotoxic anti-TB drugs should be discontinued and rechallenged. If the diagnosis is TB-related IRIS, the drugs may be continued with close monitoring and steroid cover. A meta-analysis published in 2015 showed inferior outcomes in patients with hepatic TB treated with monotherapy or nonhepatoxic regimens compared to those with full-dose multi-drug therapy with INH, rifampicin, pyrazinamide, and ethambutol.166
Q36: Is there a Role for Low-volume Plasma Exchange (PLEX) in Patients with Liver Dysfunction due to TB or DILI?
Position statement: In patients with acute liver failure (ALF) or acute on chronic liver failure (ACLF) due to anti-TB drugs induced DILI, low-volume plasma exchange may be considered especially in those who fulfill the King's College criteria for liver transplantation but do not have access to the same.
Commentary: There is emerging evidence that shows that low-volume PLEX in specific sub-populations of liver failure may provide a mortality benefit over standard medical therapy, especially in patients with limited access to liver transplantation. A recent meta-analysis has shown promising results in ALF and ACLF, not specific to TB or TB-DILI. Preliminary data from a prospective trial conducted to assess the role of PLEX in DILI (which had 14% of patients with TB-DILI) showed survival benefit over standard medical care and improvement in hepatic encephalopathy. Based on this, it may be reasonable to consider PLEX as an option in ALF and ACLF, especially in areas without access to liver transplantation.167–170
Infection Control
There is evidence that primary transmission of drug-resistant bacteria is the main mechanism for the global spread of drug resistant TB, rather than acquired resistance. So, identifying the source of infection quickly and halting its spread by effective infection prevention and control (IPC) measures are therefore imperative. Infection prevention and control strategies focus on lowering the concentration in airborne particles and the duration of exposure among healthcare providers.
Healthcare workers (HCWs) have an increased risk of contracting M. tuberculosis infection when compared with the general population.171 It is therefore critical to implement and maintain effective strategies for controlling TB infections.172 Administrative control implementation,173,174 utilizing triage, and patient isolation, are the fundamental and essential components of any IPC strategy. Furthermore, the strategies prioritize the implementation of respiratory hygiene and the timely initiation of effective treatment. Additional methods encompass engineering and environmental controls. While the evidence for the effectiveness of IPC measures is limited, the deployment of numerous measures has shown a definite decrease in the risk of TB occurrence among HCWs and those visiting healthcare facilities.174
Q37: What are the Recommended Isolation Systems for Patients with Presumed or Documented Infectious TBs in the ICU?
Position statement: Respiratory isolation of individuals with confirmed or suspected infectious TB is advised, ideally in an airborne infection isolation room (AIIR) equipped with a high-efficiency particulate air (HEPA) filter and negative pressure. Closed suctioning is recommended for intubated patients. The application of a bacterial filter to the ventilator tubing's expiratory limb may reduce the transmission of infection.
Commentary: Patients diagnosed or suspected to have pulmonary TB should be isolated in an AIIR to mitigate the potential transmission of TB particles. It was found that respiratory isolation of patients with putative or confirmed infectious TB reduced the risk of active TB infection among HCWs by 2% and latent TB infection by 45%,172 respectively. It is recommended that AIIR implement negative pressure ventilation, with a minimum of 6 air changes per hour (ACH) and a maximum of 12 per day.175 High-efficiency particulate air filters are recommended to handle the exhaust from the room. Therapeutic interventions and diagnostic assessments that necessitate patients to be transferred from the isolation facility must be limited and preferably performed within the AIIR as far as possible.
Although animal models have demonstrated a reduction in latent TB infection in rooms treated with upper room ultraviolet germicidal irradiation (UVGI) with ultraviolet-C light to kill or inactivate airborne M. tuberculosis,176 the evidence is scant, and its efficacy may be compromised when the relative humidity in the room exceeds 50–60%.177
Nonventilated patients diagnosed or suspected of having TB who are coughing should be provided with surgical masks.175 Although studies have demonstrated a decline in latent TB infection among HCWs,178 there is no evidence indicating a reduction in active TB infection. Drawing a parallel from the patients wearing masks to decrease transmission, we recommend that closed suctioning is used for intubated patients to reduce the dispersal of respiratory secretions that contain infectious M. tuberculosis. Furthermore, to prevent the spread of pathogens, endotracheal tubes or the expiratory side of the ventilator may be equipped with a bacterial filter capable of capturing particles as small as 0.3 µm with an efficacy of at least 95%. Standard environmental cleaning of the ICU room should be sufficient to disinfect the room given that M. tuberculosis is transmitted exclusively through airborne transmission and not through surface contact.179
Q38: What are the Key IPC Measures for HCWs Taking Care of Patients with Suspected or Confirmed Tuberculosis in the ICU?
Position statement: Healthcare professionals entering rooms of patients with suspected or confirmed contagious TB disease should wear a properly sized N95 disposable respirator.
Commentary: Although surgical masks can reduce the number of infectious particulates in the air, their filtration efficiency is only 50% and they fail to form a secure seal on the face; therefore, they are unsuitable for HCWs. Specific respiratory protection, including properly fitted N95 masks, filters at least 95% of particulate 1 µm in diameter when utilized appropriately and with a snug facial seal. In order to achieve this, medical facilities that treat TB patients should establish protocols for periodic respirator fit testing, initial respirator fit testing, and annual respirator training.175
Sputum induction and nebulization therapy provoke coughing, heightening the risk of transmission of M. tuberculosis.180,181 While doing procedures that generate aerosols, such as intubation, bronchoscopy, or sputum induction, HCWs should wear at the barest minimum, N95 respirators.182 However, for these procedures, it is advisable to use a higher level of respiratory protection, such as an elastomeric full-facepiece respirator or a powered air-purifying respirator (PAPR), instead of a N95 disposable respirator.175,182
Q39: When can Respiratory Isolation Precautions be Discontinued for Critically Ill Patients with Pulmonary TB?
Position statement: For sputum-positive rifampicin susceptible TB patients, a minimum of 2 weeks of isolation is suggested, while on effective anti-TB treatment.
Commentary: There is a lack of distinct, unequivocal guidelines and insufficient evidence regarding the de-isolation of patients with sputum-positive pulmonary tuberculosis.183 The 2 weeks of isolation is based on a review article by Rouillon et al. in the 1970s, which suggested that with effective treatment, patients may lose infectivity in probably less than 2 weeks.184 Dharmadhikari et al. found that patients on successful treatment lose infectivity, even if they are AFB microscopy and culture positive.185 There is evidence to suggest that isolation may be needed for patients with negative smears, as they too possess the capacity to transmit the infection; research has shown that smear-negative culture-positive tuberculosis is responsible for approximately 17% of TB transmission.186
Q40: Is there a Role for Routine Testing and Surveillance of HCWs Managing Infectious TB Patients?
Position statement: The role and benefit of regular testing and surveillance for TB infection among HCWs in the ICU, using TST or IGRA, in endemic countries like India is unclear.
Commentary: At present, there is no gold standard test for establishing the diagnosis of LTBI. Two screening tests, TST and IGRAs, are recommended for diagnosing LTBI. Although IGRA may aid in the early detection and mitigation of potential exposures in high-income countries with low TB prevalence, their application in low- and middle-income countries with high endemicity lacks definitive guidelines. Data from high endemic countries have yielded mixed conclusions with regards to active screening. While a study from South India suggested a potential role for screening of HCWs based on a higher annual risk of TB infection,187 a recent individual patient meta-analysis of individual patients found that the efficacy of both IGRA and TST was inferior in countries with a high incidence of TB.188,189
Post-tuberculosis Sequelae
The term post-tuberculosis describes the range of pathological conditions experienced by TB survivors involving various organ systems.190 These post-TB sequelae mainly arise from the disease process or the side effects related to TB treatment.
Q41: Post-TB Sequelae: What are the Reasons for ICU Admission?
Position statement: Post-TB sequelae patients may present to the ICU with respiratory failure due to exacerbation of obstructive airway disease or restrictive fibrotic lung disease, pulmonary hypertension, hemoptysis due to bronchiectasis, aspergillosis or vascular causes or with secondary infections. There are currently no evidence-based recommendations for the investigation and management of post TB lung abnormality (PTLA).
Commentary: One of the most common post-TB sequelae is post-TB lung illness, which can result in respiratory failure and the need for ICU admission. This can happen because of post-TB issues linked to the vascular system, parenchyma, pleura, mediastinum, or airway. Airflow limitation, restrictive diseases, or a combination of both, occasionally linked to respiratory failure, are functional conditions affecting patients.
Studies have shown that up to 50% of patients experience health issues due to PTLA.191,192 Fibrosis accounts for 25.0–70.4%, cavitation for 8.3–83.7%, and bronchiectasis for 4.3–11.2% of the imaging-defined PTLA.193 Younger individuals, patients without HIV and people from high endemic areas are at risk of developing PTLA. In a review of 17 studies among HIV-infected and uninfected individuals, HIV individuals with low CD4 counts and TB were four times more likely to have normal chest radiographs and less cavitation than individuals with higher CD4 counts.194 The extent of impairment in lung functions is more in DR-TB when compared with drug-sensitive TB.195
Exacerbations of chronic obstructive pulmonary disease (COPD), bronchiectasis, and pneumonia are also more frequent after pulmonary TB.196,197 Population-based studies PLATINO and BOLD suggest that prior TB doubles the risk of developing COPD.196,198 According to a recent review, persons over 40 years of age who had a history of TB were significantly more likely to get COPD (odds ratio (OR) 3.05, 95% CI, 2.42–3.85).197 Lung involvement in post-TB COPD is heterogeneous199,200 and may not respond as well to bronchodilators as a regular COPD patient; the use of inhaled corticosteroids in post-TB COPD may increase the risk of reactivation of TB.201
A cross-sectional study of 100 patients indicated a 2.13-fold increase in the chances of pulmonary hypertension post-TB with each further TB episode (CI: 1.17–3.88; p = 0.013).202 Post-TB studies have shown that the prevalence of pulmonary hypertension ranges from 6.3% in outpatients to 42.4% in hospitalized patients and 67% in patients with chronic respiratory failure.203
Patients with a history of TB may present with hemoptysis either from airway-related broncholiths, bronchiectasis, pulmonary aspergillosis, and vascular causes from rupture of Rasmussen's aneurysm or from dilated bronchial arteries. Chronic pulmonary aspergillosis commonly complicates treated pulmonary TB with residual cavitation. Other presentations of aspergillosis that may complicate PTLA include aspergilloma, allergic bronchopulmonary aspergillosis, invasive and chronic pulmonary aspergillosis or asymptomatic colonization. Based on symptoms of cough, weight loss, hemoptysis, CT chest features of cavitation, a fungus ball, pleural thickening, and pulmonary fibrosis, and positive IgG to A. fumigatus, a cross-sectional prospective study from Uganda found that 4.9% of patients who had finished TB treatment 2 years prior had “definite” and another 2.3% had “probable” chronic pulmonary aspergillosis. This increased to 26% among patients with a cavity on CXR.204
Future Directions
There is paucity of good trials on TB in the critically ill. Given that an increasing number of patients are admitted to ICUs with TB and its complications in our country, it is incumbent on ICU practitioners to not only be aware of the problem but also channel efforts to reduce mortality. It is important to focus on studies that will explore rapid diagnostic tests in the ICU setting, particularly for extrapulmonary TB where delays in diagnosis and treatment may result in worse outcomes. Studies on the use of IV anti-TB drugs and/or the use of higher doses of anti-TB drugs in ICU patients is important to address the problems of subtherapeutic concentrations of drugs which are common due to a multiplicity of factors. Therapeutic drug monitoring would be another vital area that would need to become integral to the management of TB in the critically ill. Awareness and knowledge on the key aspects of TB and its management will go a long way in improving outcomes of TB in the critically ill.
Conclusion
Tuberculosis continues to be a major problem in developing countries including India. The more recent increase has been attributed to the COVID-19 pandemic, due to the disruption of services because of the lockdown and diversion of healthcare resources to tackling the pandemic. Newer therapies for the management of cancers and immune disorders, particularly the use of highly potent immunosuppressive agents, have contributed to re-activation of TB in these patient cohorts. These factors have resulted in an increase in the number of patients with TB in the ICU setting. Mortality continues to be unacceptably high among patients with TB admitted to the ICU at 50–60%. Newer diagnostic tests with faster turnaround are likely to help with the early diagnosis of TB including drug-resistant TB. The possibility of use of higher doses of anti-TB drugs in critically ill patients, the availability of IV preparations and the options of TDM of anti-TB drugs is likely to improve diagnostics and management in the ICU patients. More studies are required in the ICU setting to inform decision making in critically ill patients.
Orcid
Binila Chacko https://orcid.org/0000-0002-1609-2208
Dhruva Chaudhry https://orcid.org/0000-0001-5138-2908
John V Peter https://orcid.org/0000-0002-3423-1830
Gopi C Khilnani https://orcid.org/0000-0003-0820-0624
Prashant Saxena https://orcid.org/0009-0000-6735-9833
Inderpaul S Sehgal https://orcid.org/0000-0003-0516-9350
Kunal Ahuja https://orcid.org/0009-0006-6666-088X
Camilla Rodrigues https://orcid.org/0000-0002-6105-6660
Manish Modi https://orcid.org/0000-0002-5494-3704
Anand Jaiswal https://orcid.org/0009-0004-3523-8083
Joel Jasiel G https://orcid.org/0009-0004-7565-4129
Shrikant Sahasrabudhe https://orcid.org/0000-0002-6074-8569
Prithviraj Bose https://orcid.org/0000-0003-4522-0261
Aman Ahuja https://orcid.org/0000-0003-0307-3427
Vineela Suprapaneni https://orcid.org/0000-0003-0726-4474
Brijesh Prajapat https://orcid.org/0000-0002-1854-8408
Abi Manesh https://orcid.org/0000-0001-9970-9674
Rajesh Chawla https://orcid.org/0000-0003-2659-2430
Randeep Guleria https://orcid.org/0000-0002-6258-2160
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Bagcchi S. WHO's Global Tuberculosis Report 2022. Lancet Microbe. 2023;4:e20. doi: 10.1016/S2666-5247(22)00359-7. [DOI] [PubMed] [Google Scholar]
- 2.Litvinjenko S, Magwood O, Wu S, Wei X. Burden of tuberculosis among vulnerable populations worldwide: an overview of systematic reviews. Lancet Infect Dis. 2023;23:1395–1407. doi: 10.1016/S1473-3099(23)00372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandal S, Rao R, Joshi R. Estimating the Burden of Tuberculosis in India: A Modelling Study. Indian J Community Med Off Publ Indian Assoc Prev Soc Med. 2023;48:436–442. doi: 10.4103/ijcm.ijcm_160_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry D, Tyagi D. Tuberculosis in Intensive Care Unit. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2021;25:S150–S154. doi: 10.5005/jp-journals-10071-23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erbes R, Oettel K, Raffenberg M, Mauch H, Schmidt-Ioanas M, Lode H. Characteristics and outcome of patients with active pulmonary tuberculosis requiring intensive care. Eur Respir J. 2006;27:1223–1228. doi: 10.1183/09031936.06.00088105. [DOI] [PubMed] [Google Scholar]
- 6.Barss L, Connors WJA, Fisher D. Chapter 7: Extra-pulmonary tuberculosis. Can J Respir Crit Care Sleep Med. 2022;6:87–108. doi: 10.1080/24745332.2022.2036073. [DOI] [Google Scholar]
- 7.Diagnostic Standards and Classification of Tuberculosis in Adults and Children This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 8.Thomas L, Chacko B, Jupudi S, Mathuram A, George T, Gunasekaran K, et al. Clinical Profile and Outcome of Critically Ill Patients with Tuberculosis. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2021;25:21–28. doi: 10.5005/jp-journals-10071-23503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dheda K, Makambwa E, Esmail A. The Great Masquerader: Tuberculosis Presenting as Community-Acquired Pneumonia. Semin Respir Crit Care Med. 2020;41:592–604. doi: 10.1055/s-0040-1710583. [DOI] [PubMed] [Google Scholar]
- 10.Sudarsan TI, Thomas L, Samprathi A, Chacko B, Mathuram A, George T, et al. Tuberculous ARDS is associated with worse outcome when compared with non-tuberculous infectious ARDS. J Crit Care. 2021;61:138–143. doi: 10.1016/j.jcrc.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Muthu V, Dhooria S, Aggarwal AN, Behera D, Sehgal IS, Agarwal R. Acute respiratory distress syndrome due to tuberculosis in a respiratory ICU over a 16-year period. Crit Care Med. 2017;45:e1087–e1090. doi: 10.1097/CCM.0000000000002479. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Experience with ARDS caused by tuberculosis in a respiratory intensive care unit. Intensive Care Med. 2005;31:1284–1287. doi: 10.1007/s00134-005-2721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SK, Mohan A. Miliary Tuberculosis. Microbiol Spectr. 2017:5. doi: 10.1128/microbiolspec.TNMI7-0013-2016. [DOI] [PubMed] [Google Scholar]
- 14.Otu A, Hashmi M, Mukhtar AM, Kwizera A, Tiberi S, Macrae B, et al. The critically ill patient with tuberculosis in intensive care: Clinical presentations, management and infection control. J Crit Care. 2018;45:184–196. doi: 10.1016/j.jcrc.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez SZ. Hepatobiliary tuberculosis. J Gastroenterol Hepatol. 1998;13:833–839. doi: 10.1111/j.1440-1746.1998.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 16.Malikowski T, Mahmood M, Smyrk T, Raffals L, Nehra V. Tuberculosis of the gastrointestinal tract and associated viscera. J Clin Tuberc Mycobact Dis. 2018;12:1–8. doi: 10.1016/j.jctube.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muneer A, Macrae B, Krishnamoorthy S, Zumla A. Urogenital tuberculosis - epidemiology, pathogenesis and clinical features. Nat Rev Urol. 2019;16:573–598. doi: 10.1038/s41585-019-0228-9. [DOI] [PubMed] [Google Scholar]
- 18.Khan FY. Review of literature on disseminated tuberculosis with emphasis on the focused diagnostic workup. J Fam Community Med. 2019;26:83–91. doi: 10.4103/jfcm.JFCM_106_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Mrigpuri P, Faye A, Bandyopadhyay D, Singla R. Pulmonary tuberculosis - An emerging risk factor for venous thromboembolism: A case series and review of literature. Lung India Off Organ Indian Chest Soc. 2017;34:65–69. doi: 10.4103/0970-2113.197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthu V, Agarwal R, Dhooria S, Aggarwal AN, Behera D, Sehgal IS. Outcome of critically ill subjects with tuberculosis: Systematic review and meta-analysis. Respir Care. 2018;63:1541–1554. doi: 10.4187/respcare.06190. [DOI] [PubMed] [Google Scholar]
- 21.Behera D, Prasad K, Aggarwal AN. Profile of atients with active tuberculosis admitted to a respiratory Intensive Care Unit in a Tertiary Care Center of North India. Indian J Crit Care Med. 2018;22:63–66. doi: 10.4103/ijccm.IJCCM_491_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdon R, Chevret S, Laissy JP, Wolff M. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis Off Publ Infect Dis Soc Am. 1996;22:982–988. doi: 10.1093/clinids/22.6.982. [DOI] [PubMed] [Google Scholar]
- 23.Behr MA, Lapierre SG, Kunimoto DY, Lee RS, Long R, Sekirov I, et al. Chapter 3: Diagnosis of tuberculosis disease and drug-resistant tuberculosis. Can J Respir Crit Care Sleep Med. 2022 doi: 10.1080/24745332.2022.2035638. [DOI] [Google Scholar]
- 24.Bai W, Liu L, Wu L, Chen S, Wu S, Wang Z, et al. Assessing the utility of the Xpert Mycobacterium tuberculosis/rifampin assay for analysis of bronchoalveolar lavage fluid in patients with suspected pulmonary tuberculosis. J Clin Lab Anal. 2022;36:e24154. doi: 10.1002/jcla.24154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neves CP, Costa AG, Safe IP, de Souza Brito A, Jesus JS, Kritski AL, et al. The role of mini-bronchoalveolar lavage fluid in the diagnosis of pulmonary tuberculosis in critically ill patients. BMC Infect Dis. 2020;20:229. doi: 10.1186/s12879-020-04954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma SK, Vashishtha R, Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-burden setting. PLOS ONE. 2017;12:e0169539. doi: 10.1371/journal.pone.0169539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dian S, Ganiem AR, van Laarhoven A. Central nervous system tuberculosis. Curr Opin Neurol. 2021;34:396–402. doi: 10.1097/WCO.0000000000000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonard JM. Central nervous system tuberculosis. Microbiol Spectr. 2017:5. doi: 10.1128/microbiolspec.TNMI7-0044-2017. [DOI] [PubMed] [Google Scholar]
- 29.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10:803–812. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 30.Nhu NTQ, Heemskerk D, Thu DDA, Chau TTH, Mai NTH, Nghia HDT, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52:226–233. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis J, Cresswell FV, Rhein J, Ssebambulidde K, Boulware DR. Cryptococcal meningitis and tuberculous meningitis co-infection in HIV-infected Ugandan Adults. Open Forum Infect Dis. 2018;5:ofy193. doi: 10.1093/ofid/ofy193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulson T. Epidemiology: A mortal foe. Nature. 2013;502:S2–S3. doi: 10.1038/502S2a. [DOI] [PubMed] [Google Scholar]
- 33.WHO consolidated guidelines on drug-resistant tuberculosis treatment n.d https://www.who.int/publications-detail-redirect/9789241550529 Available from: (Accessed May 21, 2024)
- 34.Meeting report of the WHO expert consultation on drug-resistant tuberculosis treatment outcome definitions n.d https://www.who.int/publications-detail-redirect/9789240022195 Available from: (Accessed May 21, 2024)
- 35.Lohiya A, Abdulkader RS, Rath RS, Jacob O, Chinnakali P, Goel AD, et al. Prevalence and patterns of drug resistant pulmonary tuberculosis in India-A systematic review and meta-analysis. J Glob Antimicrob Resist. 2020;22:308–316. doi: 10.1016/j.jgar.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Raveendran R, Oberoi JK, Wattal C. Multidrug-resistant pulmonary & extrapulmonary tuberculosis: A 13 years retrospective hospital-based analysis. Indian J Med Res. 2015;142:575–582. doi: 10.4103/0971-5916.171285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salari N, Kanjoori AH, Hosseinian-Far A, Hasheminezhad R, Mansouri K, Mohammadi M. Global prevalence of drug-resistant tuberculosis: a systematic review and meta-analysis. Infect Dis Poverty. 2023;12:57. doi: 10.1186/s40249-023-01107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pradipta IS, Forsman LD, Bruchfeld J, Hak E, Alffenaar JW. Risk factors of multidrug-resistant tuberculosis: A global systematic review and meta-analysis. J Infect. 2018;77:469–478. doi: 10.1016/j.jinf.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Diriba G, Tola HH, Alemu A, Yenew B, Gamtesa DF, Kebede A. Drug resistance and its risk factors among extrapulmonary tuberculosis in Ethiopia: A systematic review and meta-analysis. PLoS ONE. 2021;16:e0258295. doi: 10.1371/journal.pone.0258295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y, Ai L, Wang X, Sun Z, Wang F. Review and updates on the diagnosis of tuberculosis. J Clin Med. 2022;11:5826. doi: 10.3390/jcm11195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fluorescent light-emitting diode (LED) microscopy for diagnosis of tuberculosis: Policy Statement. Geneva: World Health Organization; 2011. ISBN-13: 978-92-4-150161-3. [PubMed] [Google Scholar]
- 42.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: Part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn. 2006;6:423–432. doi: 10.1586/14737159.6.3.423. [DOI] [PubMed] [Google Scholar]
- 43.Rapid Communication: Molecular assays as initial tests for the diagnosis of tuberculosis and rifampicin resistance n.d https://www.who.int/publications-detail-redirect/9789240000339 Available from: (Accessed May 21, 2024)
- 44.Shapiro AE, Ross JM, Yao M, Schiller I, Kohli M, Dendukuri N, et al. Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms. Cochrane Database Syst Rev. 2021;3:CD013694. doi: 10.1002/14651858.CD013694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021;2:CD009593. doi: 10.1002/14651858.CD009593.pub5. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y, Yu G, Zhao W, Lang Y. Efficacy of Xpert MTB/RIF Ultra in diagnosing tuberculosis meningitis. Medicine (Baltimore) 2021;100:e26778. doi: 10.1097/MD.0000000000026778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadesse M, Abebe G, Bekele A, Bezabih M, Yilma D, Apers L, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a diagnostic evaluation study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2019;25:1000–1005. doi: 10.1016/j.cmi.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:CD012768. doi: 10.1002/14651858.CD012768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donovan J, Cresswell FV, Thuong NTT, Boulware DR, Thwaites GE, Bahr NC, et al. Xpert MTB/RIF Ultra for the diagnosis of tuberculous meningitis: A small step Forward. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71:2002–2005. doi: 10.1093/cid/ciaa473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO standard: universal access to rapid tuberculosis diagnostics n.d https://www.who.int/publications-detail-redirect/9789240071315 Available from: (Accessed May 21, 2024) [DOI] [PMC free article] [PubMed]
- 51.Penn-Nicholson A, Georghiou SB, Ciobanu N, Kazi M, Bhalla M, David A, et al. Detection of isoniazid, fluoroquinolone, ethionamide, amikacin, kanamycin, and capreomycin resistance by the Xpert MTB/XDR assay: a cross-sectional multicentre diagnostic accuracy study. Lancet Infect Dis. 2022;22:242–9. doi: 10.1016/S1473-3099(21)00452-7. [DOI] [PubMed] [Google Scholar]
- 52.Madhuri K, Deshpande S, Dharmashale S, Bharadwaj R. Utility of Line Probe Assay for the Early Detection of Multidrug-Resistant Pulmonary Tuberculosis. J Glob Infect Dis. 2015;7:60–65. doi: 10.4103/0974-777X.157237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pillay S, de Vos M, Derendinger B, Streicher EM, Dolby T, Scott LA, et al. Non-actionable results, accuracy, and effect of first- and second-line line probe assays for diagnosing drug-resistant tuberculosis, including on smear-negative specimens, in a high-volume laboratory. Clin Infect Dis. 2023;76:e920–e929. doi: 10.1093/cid/ciac556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horne DJ, Kohli M, Zifodya JS, Schiller I, Dendukuri N, Tollefson D, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2019;2019:CD009593. doi: 10.1002/14651858.CD009593.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ssengooba W, Katamba A, Sserubiri J, Semugenze D, Nyombi A, Byaruhanga R, et al. Performance evaluation of Truenat MTB and Truenat MTB-RIF DX assays in comparison to gene XPERT MTB/RIF ultra for the diagnosis of pulmonary tuberculosis in Uganda. BMC Infect Dis. 2024;24:190. doi: 10.1186/s12879-024-09063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pillay S, Steingart KR, Davies GR, Chaplin M, De Vos M, Schumacher SG, et al. Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin. Cochrane Database Syst Rev. 2022;5:CD014841. doi: 10.1002/14651858.CD014841.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Li R, Ge S, Li Y, Cai C, Weng T, et al. Rapid detection of extensive drug resistance by Xpert MTB/XDR optimizes therapeutic decision-making in rifampin-resistant tuberculosis patients. J Clin Microbiol. 2023;61:e0183222. doi: 10.1128/jcm.01832-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: Ameta-analysis. Eur Respir J. 2008;32:1165–1174. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 59.Bai Y, Wang Y, Shao C, Hao Y, Jin Y. GenoType MTBDRplus assay for rapid detection of multidrug resistance in mycobacterium tuberculosis: A meta-analysis. PLOS ONE. 2016;11:e0150321. doi: 10.1371/journal.pone.0150321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilpin C, Korobitsyn A, Weyer K. Current tools available for the diagnosis of drug-resistant tuberculosis. Ther Adv Infect Dis. 2016;3:145–151. doi: 10.1177/2049936116673553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Technical manual for drug susceptibility testing of medicines used in the treatment of tuberculosis n.d https://www.who.int/publications-detail-redirect/9789241514842 Available from: (Accessed May 21, 2024)
- 62.Cabibbe AM, Walker TM, Niemann S, Cirillo DM. Whole genome sequencing of mycobacterium tuberculosis. Eur Respir J. 2018;52:1801163. doi: 10.1183/13993003.01163-2018. [DOI] [PubMed] [Google Scholar]
- 63.Rageade F, Picot N, Blanc-Michaud A, Chatellier S, Mirande C, Fortin E, et al. Performance of solid and liquid culture media for the detection of mycobacterium tuberculosis in clinical materials: meta-analysis of recent studies. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2014;33:867–870. doi: 10.1007/s10096-014-2105-z. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues C, Shenai S, Sadani M, Sukhadia N, Jani M, Ajbani K, et al. Evaluation of the bactec MGIT 960 TB system for recovery and identification of Mycobacterium tuberculosis complex in a high through put tertiary care centre. Indian J Med Microbiol. 2009;27:217–221. doi: 10.4103/0255-0857.53203. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PloS One. 2019;14:e0213728. doi: 10.1371/journal.pone.0213728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekermans P, Dusé A, George J. The dubious value of cerebrospinal fluid adenosine deaminase measurement for the diagnosis of tuberculous meningitis. BMC Infect Dis. 2017;17:104. doi: 10.1186/s12879-017-2221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pannu AK, Selvam S, Rahman N, Kumar D, Saroch A, Sharma AK, et al. Cerebrospinal fluid adenosine deaminase for the diagnosis of tuberculous meningitis. Biomark Med. 2023;17:209–218. doi: 10.2217/bmm-2022-0838. [DOI] [PubMed] [Google Scholar]
- 68.Zhou R, Qiu X, Ying J, Yue Y, Ruan T, Yu L, et al. Diagnostic performance of adenosine deaminase for abdominal tuberculosis: A systematic review and meta-analysis. Front Public Health. 2022;10:938544. doi: 10.3389/fpubh.2022.938544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu X, Xing B, Wang W, Yang P, Sun Y, Zheng X, et al. Diagnostic values of Xpert MTB/RIF, T-SPOT.TB and adenosine deaminase for HIV-negative tuberculous pericarditis in a high burden setting: A prospective observational study. Sci Rep. 2020;10:16325. doi: 10.1038/s41598-020-73220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhowmik A, Herth FJF. In: Migliori GB, Bothamley G, Duarte R, Rendon A, editors. Tuberculosis, Sheffield, United Kingdom: European Respiratory Society; 2018. Bronchoscopy and other invasive procedures for tuberculosis diagnosis. In: pp. 137–151. [DOI] [Google Scholar]
- 71.JoR Free full-text Extrapulmonary tuberculosis—An update on the diagnosis, treatment and drug resistance n.d https://www.mdpi.com/2673-527X/1/2/15 Available from: (Accessed May 21, 2024)
- 72.Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2015;32:87–93. doi: 10.1016/j.ijid.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 73.Malherbe ST, Chen RY, Dupont P, Kant I, Kriel M, Loxton AG, et al. Quantitative 18F-FDG PET-CT scan characteristics correlate with tuberculosis treatment response. EJNMMI Res. 2020;10:8. doi: 10.1186/s13550-020-0591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kethireddy S, Light RB, Mirzanejad Y, Maki D, Arabi Y, Lapinsky S, et al. Mycobacterium tuberculosis septic shock. Chest. 2013;144:474–482. doi: 10.1378/chest.12-1286. [DOI] [PubMed] [Google Scholar]
- 75.Rao PS, Moore CC, Mbonde AA, Nuwagira E, Orikiriza P, Nyehangane D, et al. Population Pharmacokinetics and Significant Under-Dosing of Anti-Tuberculosis Medications in People with HIV and Critical Illness. Antibiotics. 2021;10:739. doi: 10.3390/antibiotics10060739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mer M, Zumla A, Dünser MW. Limiting consumption in tuberculosis: current concepts in anti-tuberculosis treatment in the critically ill patient. Intensive Care Med. 2018;44:2229–31. doi: 10.1007/s00134-018-5161-5. [DOI] [PubMed] [Google Scholar]
- 77.WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment n.d https://www.who.int/publications-detail-redirect/9789240048126 Available from: (Accessed May 21, 2024) [PubMed]
- 78.Index-TB guidelines: guidelines on extra-pulmonary tuberculosis in India n.d https://iris.who.int/handle/10665/278953 Available from: (Accessed July 18, 2024)
- 79.Anton C, Lemos CX, Machado FD, Bernardi RM, Freitas AA, Silva DR. Tuberculosis in the intensive care unit: alternative treatment regimens and association with mortality. Trop Med Int Health TM IH. 2021;26:111–114. doi: 10.1111/tmi.13511. [DOI] [PubMed] [Google Scholar]
- 80.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: A multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis. 2017;17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, van der Ven AJ, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13:27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 82.Koegelenberg CFN, Nortje A, Lalla U, Enslin A, Irusen EM, Rosenkranz B, et al. The pharmacokinetics of enteral antituberculosis drugs in patients requiring intensive care. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2013;103:394–398. doi: 10.7196/samj.6344. [DOI] [PubMed] [Google Scholar]
- 83.Hernandez-Cardenas C, Lugo-Goytia G. Pharmacokinetics of antituberculosis drugs in critically ill patients with tuberculosis and acute respiratory failure. Crit Care. 2014;18:354. doi: 10.1186/cc13544. p. [DOI] [Google Scholar]
- 84.Perumal R, Naidoo K, Naidoo A, Letsoalo MP, Esmail A, Joubert I, et al. The impact of enteral feeding and therapeutic monitoring of rifampicin with dose escalation in critically ill patients with tuberculosis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2023;126:174–180. doi: 10.1016/j.ijid.2022.11.033. [DOI] [PubMed] [Google Scholar]
- 85.Peloquin CA, Bulpitt AE, Jaresko GS, Jelliffe RW, James GT, Nix DE. Pharmacokinetics of pyrazinamide under fasting conditions, with food, and with antacids. Pharmacotherapy. 1998;18:1205–1211. 9855317 [PubMed] [Google Scholar]
- 86.Yunivita V, Dian S, Ganiem AR, Hayati E, Achmad TH, Dewi AP, et al. Pharmacokinetics and safety/tolerability of higher oral and intravenous doses of rifampicin in adult tuberculous meningitis patients. Int J Antimicrob Agents. 2016;48:415–421. doi: 10.1016/j.ijantimicag.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 87.Cresswell FV, Meya DB, Kagimu E, Grint D, Te Brake L, Kasibante J, et al. High-dose oral and intravenous rifampicin for the treatment of tuberculous meningitis in predominantly Human immunodeficiency virus (HIV)-positive Ugandan adults: A phase II open-label randomized controlled trial. Clin Infect Dis. 2021;73:876–884. doi: 10.1093/cid/ciab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wasserman S, Davis A, Stek C, Chirehwa M, Botha S, Daroowala R, et al. Plasma pharmacokinetics of high-dose oral versus intravenous Rrifampicin in patients with tuberculous meningitis: A randomized controlled trial. Antimicrob Agents Chemother. 65:e00140–21. doi: 10.1128/AAC.00140-21. n.d.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butov D, Feshchenko Y, Kuzhko M, Gumenuik M, Yurko K, Grygorova A, et al. Effectiveness of intravenous isoniazid and ethambutol administration in patients with tuberculosis meningoencephalitis and HIV infection. Tuberc Respir Dis. 2020;83:96–103. doi: 10.4046/trd.2019.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuzhko M, Gumeniuk M, Butov D, Tlustova T, Denysov O, Sprynsian T. Features of intravenous anti TB therapy in patients with first diagnosed pulmonary TB in the intensive phase of treatment. Eur Respir J. 2017:50. doi: 10.1183/1393003.congress-2017.PA3496. [DOI] [Google Scholar]
- 91.Kwon BS, Kim Y, Lee SH, Lim SY, Lee YJ, Park JS, et al. The high incidence of severe adverse events due to pyrazinamide in elderly patients with tuberculosis. PloS One. 2020;15:e0236109. doi: 10.1371/journal.pone.0236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Modongo C, Sobota RS, Kesenogile B, Ncube R, Sirugo G, Williams SM, et al. Successful MDR-TB treatment regimens including amikacin are associated with high rates of hearing loss. BMC Infect Dis. 2014;14:542. doi: 10.1186/1471-2334-14-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pranger AD, van der Werf TS, Kosterink JGW, Alffenaar JWC. The Role of fluoroquinolones in the treatment of tuberculosis in 2019. Drugs. 2019;79:161–171. doi: 10.1007/s40265-018-1043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alffenaar JWC, van Altena R, Bökkerink HJ, Luijckx GJ, van Soolingen D, Aarnoutse RE, et al. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;49:1080–1082. doi: 10.1086/605576. [DOI] [PubMed] [Google Scholar]
- 95.Sotgiu G, D'Ambrosio L, Centis R, Tiberi S, Esposito S, Dore S, et al. Carbapenems to treat multidrug and extensively drug-resistant tuberculosis: A systematic review. Int J Mol Sci. 2016;17:373. doi: 10.3390/ijms17030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Jager V, Gupte N, Nunes S, Barnes GL, van Wijk RC, Mostert J, et al. Early bactericidal activity of meropenem plus clavulanate (with or without ifampin) for tuberculosis: The COMRADE randomized, phase 2A clinical trial. Am J Respir Crit Care Med. 2022;205:1228–1235. doi: 10.1164/rccm.202108-1976oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Rijn SP, Zuur MA, Anthony R, Wilffert B, van Altena R, Akkerman OW, et al. Evaluation of Carbapenems for treatment of multi- and extensively drug-resistant mycobacterium tuberculosis. Antimicrob Agents Chemother. 2019;63:e01489–14918. doi: 10.1128/AAC.01489-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maranchick NF, Alshaer MH, Smith AGC, Avaliani T, Gujabidze M, Bakuradze T, et al. Cerebrospinal fluid concentrations of fluoroquinolones and carbapenems in tuberculosis meningitis. Front Pharmacol. 2022;13:1048653. doi: 10.3389/fphar.2022.1048653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conradie F, Bagdasaryan TR, Borisov S, Howell P, Mikiashvili L, Ngubane N, et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med. 2022;387:810–823. doi: 10.1056/NEJMoa2119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mase A, Lowenthal P, True L, Henry L, Barry P, Flood J. Low-dose linezolid for treatment of patients with multidrug-resistant tuberculosis. Open Forum Infect Dis. 2022;9:ofac500. doi: 10.1093/ofid/ofac500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kempker RR, Smith AGC, Avaliani T, Gujabidze M, Bakuradze T, Sabanadze S, et al. Cycloserine and linezolid for tuberculosis meningitis: Ppharmacokinetic evidence of potential Uusefulness. Clin Infect Dis Off Publ Infect Dis Soc Am. 2022;75:682–629. doi: 10.1093/cid/ciab992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdelgawad N, Wasserman S, Abdelwahab MT, Davis A, Stek C, Wiesner L, et al. Linezolid population pharmacokinetic model in plasma and cerebrospinal fluid among patients with tuberculosis Meningitis. J Infect Dis. 2024;229:1200–8. doi: 10.1093/infdis/jiad413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Francesca C, Andreas HD, Nosipho N, PaulineDaniel F, Angela MC, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bern-Thomas N, Catherine B, Emil K, Ilaria M, Nargiza P, Zinaida T, et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N Engl J Med. 2022;387:2331–2343. doi: 10.1056/NEJMoa2117166. [DOI] [PubMed] [Google Scholar]
- 105.Riccardi N, Canetti D, Rodari P, Besozzi G, Saderi L, Dettori M, et al. Tuberculosis and pharmacological interactions: A narrative review. Curr Res Pharmacol Drug Discov. 2021;2:100007. doi: 10.1016/j.crphar.2020.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, et al. The risk of tuberculosis in transplant candidates and recipients: A TBNET consensus statement. Eur Respir J. 2012;40:990–1013. doi: 10.1183/09031936.00000712. [DOI] [PubMed] [Google Scholar]
- 107.Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain Spanish Transplantation Infection Study Group, GESITRA - PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/9158022/ Available from: (Accessed April 20, 2024) [DOI] [PubMed]
- 108.Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis Off Publ Infect Dis Soc Am. 1998;27:1266–1277. doi: 10.1086/514993. [DOI] [PubMed] [Google Scholar]
- 109.al-Sulaiman MH, Dhar JM, al-Khader AA. Successful use of rifampicin in the treatment of tuberculosis in renal transplant patients immunosuppressed with cyclosporine. Transplantation. 1990;50:597–598. doi: 10.1097/00007890-199010000-00014. [DOI] [PubMed] [Google Scholar]
- 110.Körner MM, Hirata N, Tenderich G, Minami K, Mannebach H, Kleesiek K, et al. Tuberculosis in heart transplant recipients. Chest. 1997;111:365–369. doi: 10.1378/chest.111.2.365. [DOI] [PubMed] [Google Scholar]
- 111.Muñoz P, Palomo J, Muñoz R, Rodríguez-Creixéms M, Pelaez T, Bouza E. Tuberculosis in heart transplant recipients. Clin Infect Dis Off Publ Infect Dis Soc Am. 1995;21:398–402. doi: 10.1093/clinids/21.2.398. [DOI] [PubMed] [Google Scholar]
- 112.Ryan H, Yoo J, Darsini P. Corticosteroids for tuberculous pleurisy. Cochrane Database Syst Rev. 2017;3:CD001876. doi: 10.1002/14651858.CD001876.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;2016:CD002244. doi: 10.1002/14651858.CD002244.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wakamatsu K, Nagata N, Kumazoe H, Honjo S, Hamada M, Katsuki K, et al. Efficacy of steroid pulse therapy for miliary tuberculosis complicated by acute respiratory distress syndrome. J Clin Tuberc Mycobact Dis. 2022;29:100341. doi: 10.1016/j.jctube.2022.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Critchley JA, Young F, Orton L, Garner P. Corticosteroids for prevention of mortality in people with tuberculosis: A systematic review and meta-analysis. Lancet Infect Dis. 2013;13:223–237. doi: 10.1016/S1473-3099(12)70321-3. [DOI] [PubMed] [Google Scholar]
- 116.Donald PR, Van Toorn R. Use of corticosteroids in tuberculous meningitis. Lancet Lond Engl. 2016;387:2585–2587. doi: 10.1016/S0140-6736(16)30770-X. [DOI] [PubMed] [Google Scholar]
- 117.Yang JY, Han M, Koh Y, Kim WS, Song JW, Oh YM, et al. Effects of corticosteroids on cCritically ill pPulmonary tuberculosis patients with acute rRespiratory failure: A propensity analysis of mortality. Clin Infect Dis. 2016;63:1449–1455. doi: 10.1093/cid/ciw616. [DOI] [PubMed] [Google Scholar]
- 118.Wiysonge CS, Ntsekhe M, Thabane L, Volmink J, Majombozi D, Gumedze F, et al. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev. 2017;9:CD000526. doi: 10.1002/14651858.CD000526.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boeree MJ, Diacon AH, Dawson R, Narunsky K, du Bois J, Venter A, et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med. 2015;191:1058–1065. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
- 120.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 121.Galvin J, Tiberi S, Akkerman O, Kerstjens HAM, Kunst H, Kurhasani X, et al. Pulmonary tuberculosis in intensive care setting, with a focus on the use of severity scores, a multinational collaborative systematic review. Pulmonology. 2022;28:297–309. doi: 10.1016/j.pulmoe.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Azoulay E, Russell L, Van de Louw A, Metaxa V, Bauer P, Povoa P, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 2020;46:298–314. doi: 10.1007/s00134-019-05906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loh WJ, Yu Y, Loo CM, Low SY. Factors associated with mortality among patients with active pulmonary tuberculosis requiring intensive care. Singapore Med J. 2017;58:656–659. doi: 10.11622/smedj.2016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gras J, De Castro N, Montlahuc C, Champion L, Scemla A, Matignon M, et al. Clinical characteristics, risk factors, and outcome of tuberculosis in kidney transplant recipients: A multicentric case-control study in a low-endemic area. Transpl Infect Dis. 2018;20:e12943. doi: 10.1111/tid.12943. [DOI] [PubMed] [Google Scholar]
- 125.Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and cChildren. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017;64:111–115. doi: 10.1093/cid/ciw778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ai JW, Ruan QL, Liu QH, Zhang WH. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect. 2016;5:e10. doi: 10.1038/emi.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang CT, Ruan SY, Tsai YJ, Kuo PH, Ku SC, Lee PL, et al. Effects of acute critical illnesses on the performance of interferon-gamma release assay. Sci Rep. 2016;6:19972. doi: 10.1038/srep19972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ward JD, Cornaby C, Schmitz JL. Indeterminate QuantiFERON Gold Plus results reveal deficient Interferon Gamma responses in severely ill COVID-19 patients. J Clin Microbiol. 2021;59:e0081121. doi: 10.1128/JCM.00811-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de la Cámara R, Martino R, Granados E, Rodriguez-Salvanés FJ, Rovira M, Cabrera R, et al. Tuberculosis after hematopoietic stem cell transplantation: Incidence, clinical characteristics and outcome. Spanish Group on Infectious Complications in Hematopoietic Transplantation. Bone Marrow Transplant. 2000;26:291–298. doi: 10.1038/sj.bmt.1702506. [DOI] [PubMed] [Google Scholar]
- 130.Torre-Cisneros J, Doblas A, Aguado JM, San Juan R, Blanes M, Montejo M, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;48:1657–1665. doi: 10.1086/599035. [DOI] [PubMed] [Google Scholar]
- 131.Ramos JF, Batista MV, Costa SF. Tuberculosis in hematopoietic stem cell transplant recipients. Mediterr J Hematol Infect Dis. 2013;5:e2013061. doi: 10.4084/MJHID.2013.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ip MS, Yuen KY, Woo PC, Luk WK, Tsang KW, Lam WK, et al. Risk factors for pulmonary tuberculosis in bone marrow transplant recipients. Am J Respir Crit Care Med. 1998;158:1173–1177. doi: 10.1164/ajrccm.158.4.9712072. [DOI] [PubMed] [Google Scholar]
- 133.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 134.Fiske CT, Griffin MR, Erin H, Warkentin J, Lisa K, Arbogast PG, et al. Black race, sex, and extrapulmonary tuberculosis risk: an observational study. BMC Infect Dis. 2010;10:16. doi: 10.1186/1471-2334-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marques IDB, Azevedo LS, Pierrotti LC, Caires RA, Sato VAH, Carmo LPF, et al. Clinical features and outcomes of tuberculosis in kidney transplant recipients in Brazil: A report of the last decade. Clin Transplant. 2013;27:E169–E176. doi: 10.1111/ctr.12077. [DOI] [PubMed] [Google Scholar]
- 136.Pereira M, Gazzoni FF, Marchiori E, Irion K, Moreira J, Giacomelli IL, et al. High-resolution CT findings of pulmonary Mycobacterium tuberculosis infection in renal transplant recipients. Br J Radiol. 2016;89:20150686. doi: 10.1259/bjr.20150686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pecego AC, Amancio RT, Ribeiro C, Mesquita EC, Medeiros DM, Cerbino J, et al. Six-month survival of critically ill patients with HIV-related disease and tuberculosis: A retrospective study. BMC Infect Dis. 2016;16:270. doi: 10.1186/s12879-016-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mittal S, Madan K, Aggarwal N, Dhar A. Tuberculosis and short bowel: A tTherapeutic challenge. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2019;23:199. doi: 10.5005/jp-journals-10071-23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Erdstein AA, Daas P, Bradstock KF, Robinson T, Hertzberg MS. Tuberculosis in allogeneic stem cell transplant recipients: still a problem in the 21st century. Transpl Infect Dis Off J Transplant Soc. 2004;6:142–146. doi: 10.1111/j.1399-3062.2004.00068.x. [DOI] [PubMed] [Google Scholar]
- 140.Tseng YT, Chuang YC, Shu CC, Hung CC, Hsu CF, Wang JY. Empirical use of fluoroquinolones improves the survival of critically ill patients with tuberculosis mimicking severe pneumonia. Crit Care. 2012;16:R207. doi: 10.1186/cc11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meintjes G, Stek C, Blumenthal L, Thienemann F, Schutz C, Buyze J, et al. Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med. 2018;379:1915–1925. doi: 10.1056/NEJMoa1800762. [DOI] [PubMed] [Google Scholar]
- 142.Iglesias J, Ledesma KJ, Couto PJ, Liu J. Immune Reconstitution Inflammatory Syndrome occurring in a kidney transplant pPatient with extrapulmonary tTuberculosis. Case Rep Transplant. 2017;2017:6290987. doi: 10.1155/2017/6290987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee S, Yi NJ, Kwak N, Kim H, Young HS, Lee JM, et al. Immune Reconstitution Inflammatory Syndrome and drug-induced liver injury during treatment of disseminated tTuberculosis in a liver transplant recipient: A case report. Transplant Proc. 2023;55:1972–1974. doi: 10.1016/j.transproceed.2023.06.007. [DOI] [PubMed] [Google Scholar]
- 144.Liu S, Huo F, Dai G, Wu J, Qin M, Mao H, et al. Case report: Immune reconstitution inflammatory syndrome after hematopoietic stem cell transplantation for severe combined immunodeficiency. Front Immunol. 2022;13:960749. doi: 10.3389/fimmu.2022.960749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levy H, Kallenbach JM, Feldman C, Thorburn JR, Abramowitz JA. Acute respiratory failure in active tuberculosis. Crit Care Med. 1987;15:221–225. doi: 10.1097/00003246-198703000-00008. [DOI] [PubMed] [Google Scholar]
- 146.Bhurayanontachai R, Maneenil K. Factors influencing development and mortality of acute respiratory failure in hospitalized patient with active pulmonary tuberculosis: A 10-year retrospective review. J Thorac Dis. 2016;8:1721–30. doi: 10.21037/jtd.2016.06.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49:727–759. doi: 10.1007/s00134-023-07050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hui DS, Chow BK, Lo T, Tsang OTY, Ko FW, Ng SS, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53:1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 149.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PloS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Agarwal R, Gupta D, Handa A, Aggarwal AN. Noninvasive ventilation in ARDS caused by Mycobacterium tuberculosis: report of three cases and review of literature. Intensive Care Med. 2005;31:1723–1724. doi: 10.1007/s00134-005-2823-x. [DOI] [PubMed] [Google Scholar]
- 151.Aso H, Kondoh Y, Taniguchi H, Kimura T, Nishiyama O, Kato K, et al. Noninvasive ventilation in patients with acute exacerbation of pulmonary tuberculosis sequelae. Intern Med Tokyo Jpn. 2010;49:2077–2083. doi: 10.2169/internalmedicine.49.3749. [DOI] [PubMed] [Google Scholar]
- 152.Kang NM, Zhang N, Luo BJ, Wu ED, Shi JQ, Li L, et al. Sequential non-invasive following short-term invasive mechanical ventilation in the treatment of tuberculosis with respiratory failure: A randomized controlled study. BMC Pulm Med. 2021;21:203. doi: 10.1186/s12890-021-01563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Idris R, Zielbauer AS, Koepsell J, Kloka J, Wetzstein N. Extracorporeal membrane oxygenation (ECMO) in patients with tuberculosis: systematic review and meta-analysis of 43 cases. BMC Pulm Med. 2024;24:47. doi: 10.1186/s12890-023-02715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. AJR Am J Roentgenol. 2001;177:861–867. doi: 10.2214/ajr.177.4.1770861. [DOI] [PubMed] [Google Scholar]
- 155.Revel MP, Fournier LS, Hennebicque AS, Cuenod CA, Meyer G, Reynaud P, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? AJR Am J Roentgenol. 2002;179:1217–1224. doi: 10.2214/ajr.179.5.1791217. [DOI] [PubMed] [Google Scholar]
- 156.Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis. 2017;9:S1069–1086. doi: 10.21037/jtd.2017.06.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wand O, Guber E, Guber A, Shochet GE, Israeli-Shani L, Shitrit D. Inhaled tranexamic acid for hHemoptysis treatment: A rRandomized controlled trial. Chest. 2018;154:1379–1384. doi: 10.1016/j.chest.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 158.Gopinath B, Mishra PR, Aggarwal P, Nayaka R, Naik SR, Kappagantu V, et al. Nebulized vs IV Tranexamic acid for hemoptysis: A pilot randomized controlled trial. Chest. 2023;163:1176–1184. doi: 10.1016/j.chest.2022.11.021. [DOI] [PubMed] [Google Scholar]
- 159.Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: A systematic review. Diagn Interv Radiol Ank Turk. 2017;23:307–317. doi: 10.5152/dir.2017.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng H, Fang Q, Chen K, Zhang X. Early versus late tracheotomy in ICU patients: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2021;100:e24329. doi: 10.1097/MD.0000000000024329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically ill patients: A meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147:450–459. doi: 10.1001/jamaoto.2021.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Angoulvant D, Mohammedi I, Duperret S, Bouletreau P. Septic shock caused by Mycobacterium tuberculosis in a non-HIV patient. Intensive Care Med. 1999;25:238. doi: 10.1007/s001340050825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dasarathan LV, Gaikwad P, Telugu RB. Disseminated mycobacterial septicemia presented as acute abdomen: a surgeon's perspective on Landouzy's sepsis. BMJ Case Rep CP. 2020;13:e237574. doi: 10.1136/bcr-2020-237574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Michel P, Barbier C, Loubière Y, Hayon JH, Ricôme JL. Three cases of septic shock due to tuberculosis without HIV pathology. Intensive Care Med. 2002;28:1827–1828. doi: 10.1007/s00134-002-1526-9. [DOI] [PubMed] [Google Scholar]
- 165.British Thoracic Society Standards of Care Committee and Joint Tuberculosis Committee. Milburn H, Ashman N, Davies P, Doffman S, Drobniewski F, et al. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax. 2010;65:557–570. doi: 10.1136/thx.2009.133173. [DOI] [PubMed] [Google Scholar]
- 166.Hickey AJ, Gounder L, Moosa MYS, Drain PK. A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect Dis. 2015;15:209. doi: 10.1186/s12879-015-0944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohamed MMG, Osman A, El-Halawany H. Plasma exchange for patients with acute or acute on chronic liver failure; meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2022;46:102014. doi: 10.1016/j.clinre.2022.102014. [DOI] [PubMed] [Google Scholar]
- 168.Goel A, Zachariah U, Daniel D, Eapen CE. Growing evidence for survival benefit with plasma exchange to treat liver failure. J Clin Exp Hepatol. 2023;13:1061–1073. doi: 10.1016/j.jceh.2023.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singh KA, Kumar SE, Zachariah UG, Daniel D, David V, Subramani K, et al. Single-centre eExperience with low-volume plasma exchange and low-dDose steroid to treat patients with idiosyncratic drug-induced acute liver failure. J Clin Exp Hepatol. 2024;14:101303. doi: 10.1016/j.jceh.2023.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jothimani D, Sachan D, Saha S, Kalyamoorthy I, Rela M. 4. Role of therapeutic plasma exchange (TPE) in patients with drug induced liver injury. J Clin Exp Hepatol. 2018;8:S83. doi: 10.1016/j.jceh.2018.06.425. [DOI] [Google Scholar]
- 171.Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among Health Care Workers. Emerg Infect Dis. 2011;17:488–494. doi: 10.3201/eid1703.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.WHO consolidated guidelines on tuberculosis: Module 1: Prevention – infection prevention and control n.d https://www.who.int/publications-detail-redirect/9789240055889 Available from: (Accessed May 21, 2024) [PubMed]
- 173.Karat AS, Gregg M, Barton HE, Calderon M, Ellis J, Falconer J, et al. Evidence for the use of triage, Rrespiratory isolation, and effective Ttreatment to reduce the transmission of Mycobacterium Tuberculosis in healthcare settings: A systematic review. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;72:155–1572. doi: 10.1093/cid/ciaa720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Azeredo ACV, Holler SR, de Almeida EGC, Cionek OAGD, Loureiro MM, Freitas AA, et al. Tuberculosis in health care workers and the impact of implementation of hospital infection-control measures. Workplace Health Saf. 2020;68:519–525. doi: 10.1177/2165079920919133. [DOI] [PubMed] [Google Scholar]
- 175.Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005 n.d https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5417a1.htm Available from: (Accessed May 21, 2024) [PubMed]
- 176.Mphaphlele M, Dharmadhikari AS, Jensen PA, Rudnick SN, van Reenen TH, Pagano MA, et al. Institutional Tuberculosis Transmission. Controlled trial of upper room ultraviolet air disinfection: A basis for new dosing guidelines. Am J Respir Crit Care Med. 2015;192:477–484. doi: 10.1164/rccm.201501-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu P, Kujundzic E, Peccia J, Schafer MP, Moss G, Hernandez M, et al. Impact of environmental factors on efficacy of upper-room air ultraviolet germicidal irradiation for inactivating airborne mycobacteria. Environ Sci Technol. 2005;39:9656–9664. doi: 10.1021/es0504892. [DOI] [PubMed] [Google Scholar]
- 178.Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2007;11:593–605. [PubMed] [Google Scholar]
- 179.Australian Guidelines for the Prevention and Control of Infection in Healthcare (2019) NHMRC n.d. https://www.nhmrc.gov.au/about-us/publications/australian-guidelines-prevention-and-control-infection-healthcare-2019 Available from: (Accessed May 21, 2024)
- 180.Beck-Sagué C, Dooley SW, Hutton MD, Otten J, Breeden A, Crawford JT, et al. Hospital outbreak of multidrug-resistant Mycobacterium tuberculosis infections. Factors in transmission to staff and HIV-infected patients. JAMA. 1992;268:1280–1286. doi: 10.1001/jama.1992.03490100078031. [DOI] [PubMed] [Google Scholar]
- 181.Pizzichini E, Pizzichini MMM, Leigh R, Djukanović R, Sterk PJ. Safety of sputum induction. Eur Respir J Suppl. 2002;37:9s–18s. doi: 10.1183/09031936.02.00000902. [DOI] [PubMed] [Google Scholar]
- 182.Culver DA, Gordon SM, Mehta AC. Infection control in the bronchoscopy suite: A review of outbreaks and guidelines for prevention. Am J Respir Crit Care Med. 2003;167:1050–1056. doi: 10.1164/rccm.200208-797CC. [DOI] [PubMed] [Google Scholar]
- 183.Petersen E, Khamis F, Migliori GB, Bay JG, Marais B, Wejse C, et al. De-isolation of patients with pulmonary tuberculosis after start of treatment — clear, unequivocal guidelines are missing. Int J Infect Dis. 2017;56:34–38. doi: 10.1016/j.ijid.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 184.Rouillon A, Perdrizet S, Parrot R. Transmission of tubercle bacilli: The effects of chemotherapy. Tubercle. 1976;57:275–299. doi: 10.1016/s0041-3879(76)80006-2. [DOI] [PubMed] [Google Scholar]
- 185.Dharmadhikari AS, Mphahlele M, Venter K, Stoltz A, Mathebula R, Masotla T, et al. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2014;18:1019–1025. doi: 10.5588/ijtld.13.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet Lond Engl. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 187.Christopher DJ, James P, Daley P, Armstrong L, Isaac BTJ, Thangakunam B, et al. High annual risk of tuberculosis infection among nursing students in South India: A Cohort Study. PLOS ONE. 2011;6:e26199. doi: 10.1371/journal.pone.0026199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hamada Y, Gupta RK, Quartagno M, Izzard A, Acuna-Villaorduna C, Altet N, et al. Predictive performance of interferon-gamma release assays and the tuberculin skin test for incident tuberculosis: an individual participant data meta-analysis. eClinicalMedicine. 2023;56:101815. doi: 10.1016/j.eclinm.2022.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Islam MS, Gurley ES, Banu S, Hossain K, Heffelfinger JD, Chowdhury KIA, et al. Prevalence and incidence of tuberculosis infection among healthcare workers in chest diseases hospitals, Bangladesh: Putting infection control into context. PLOS ONE. 2023;18:e0291484. doi: 10.1371/journal.pone.0291484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nightingale R, Carlin F, Meghji J, McMullen K, Evans D, van der Zalm MM, et al. Post-TB health and wellbeing. Int J Tuberc Lung Dis. 2023;27:248–283. doi: 10.5588/ijtld.22.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Allwood BW, van der Zalm MM, Amaral AFS, Byrne A, Datta S, Egere U, et al. Post-tuberculosis lung health: Perspectives from the First International Symposium. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2020;24:820–828. doi: 10.5588/ijtld.20.0067. [DOI] [PubMed] [Google Scholar]
- 192.Visca D, Centis R, Munoz-Torrico M, Pontali E. Post-tuberculosis sequelae: The need to look beyond treatment outcome. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2020;24:761–762. doi: 10.5588/ijtld.20.0488. [DOI] [PubMed] [Google Scholar]
- 193.Meghji J, Simpson H, Squire SB, Mortimer K. A Systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLOS ONE. 2016;11:e0161176. doi: 10.1371/journal.pone.0161176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kwan CK, Ernst JD. HIV and tuberculosis: A deadly human syndemic. Clin Microbiol Rev. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ivanova O, Hoffmann VS, Lange C, Hoelscher M, Rachow A. Post-tuberculosis lung impairment: systematic review and meta-analysis of spirometry data from 14,621 people. Eur Respir Rev. 2023;32:220221. doi: 10.1183/16000617.0221-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Amaral AFS, Coton S, Kato B, Tan WC, Studnicka M, Janson C, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J. 2015;46:1104–1112. doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 198.Menezes AMB, Hallal PC, Perez-Padilla R, Jardim JRB, Muiño A, Lopez MV, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30:1180–1185. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 199.Gupte AN, Paradkar M, Selvaraju S, Thiruvengadam K, Shivakumar SVBY, Sekar K, et al. Assessment of lung function in successfully treated tuberculosis reveals high burden of ventilatory defects and COPD. PLoS ONE. 2019;14:e0217289. doi: 10.1371/journal.pone.0217289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculous destroyed lung. Respir Med. 2003;97:1237–1242. doi: 10.1016/s0954-6111(03)00255-5. [DOI] [PubMed] [Google Scholar]
- 201.Gai X, Allwood B, Sun Y. Post-tuberculosis lung disease and chronic obstructive pulmonary disease. Chin Med J (Engl) 2023;136:1923–1928. doi: 10.1097/CM9.0000000000002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Louw E, Baines N, Maarman G, Osman M, Sigwadhi L, Irusen E, et al. The prevalence of pulmonary hypertension after successful tuberculosis treatment in a community sample of adult patients. Pulm Circ. 2023;13:e12184. doi: 10.1002/pul2.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.van Heerden JK, Louw EH, Thienemann F, Engel ME, Allwood BW. The prevalence of pulmonary hypertension in post-tuberculosis and active tuberculosis populations: a systematic review and meta-analysis. Eur Respir Rev. 2024;33:230154. doi: 10.1183/16000617.0154-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Page ID, Byanyima R, Hosmane S, Onyachi N, Opira C, Richardson M, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53:1801184. doi: 10.1183/13993003.01184-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]