Abstract
The surgical management of posterior cruciate ligament (PCL) injuries can be challenging. As most PCL injuries occur in a flexed knee position, the anterolateral bundle is thought to be more commonly injured than the posteromedial bundle (PMB); however, in hyperextension, the PMB plays a more significant role. The smaller size of the PMB compared with the anterolateral bundle and its lower strength may explain why isolated hyperextension PMB injuries can be easily overlooked. In this Technical Note, a surgical technique to perform a nonanatomic PMB augmentation of the PCL using a gracilis tendon autograft or allograft is reported. These technical features aim to overcome current limitations in existing techniques to address the symptoms after partial PCL hyperextension injuries.
Technique Video
This is a case of a 17-year-old male patient who experienced a hyperextension injury of his left knee while playing basketball after a direct trauma to the front of the knee. The diagnosis was delayed until referral. A previous attempt to return to sports failed because of chronic AL pain. As most PCL injuries occur in a flexed knee position, the ALB is thought to be more commonly injured than the PMB; however, in hyperextension, the PMB plays a more significant role. Clinical examination revealed a negative posterior drawer sign at 90° of knee flexion but a positive pseudo-Lachman sign at 15°, a greater hyperextension compared with the contralateral side, and a positive step off at 15° of knee flexion. The rest of the examination was normal, as well as the MRI scan. This clinical presentation led to recommend a PM PCL bundle augmentation. The patient is placed lying supine on the operating table with the operated limb being positioned in an electric leg-holder at 90° of knee flexion. Standard high AL and low anteromedial portals are created. During the initial arthroscopic evaluation, no associated intra-articular injuries were detected. With the arthroscope positioned in the high AL portal, the insertion point of the PMB on the femur is identified and marked using radiofrequency to ensure accuracy when the guide pin is passed. Using the transnotch view, the entry point of the PM viewing portal is identified with the help of a needle, and a no. 11 blade scalpel is used for skin, subcutaneous, and capsular incisions under arthroscopic control. The arthroscope is switched to the PM portal, and the tibial PMB footprint is identified using a radiofrequency ablation device. The PCL tibial tunnel drill-guide is inserted through the anteromedial portal and placed close to the native PMB footprint. Its position is nonanatomic because the PMB is posterior to the anterolateral bundle in the native knee. Reproducing its anatomy would thus lead to an undesired injury of the ALB. Therefore, the authors choose to place it 5 mm more medially to the native PCL. A 2.4-mm guide pin is inserted and an outside-in tibial tunnel of 6 or 7 mm that matches the graft diameter is drilled with a cannulated reamer. Then, a looped nonabsorbable suture is inserted into the tibial tunnel using a suture passer and retrieved from the anteromedial portal with a KingFisher grasper. The arthroscope is switched to the high AL portal and the femoral 2.4-mm guide pin is introduced through an accessory inferolateral portal and drilled, with a free-hand technique, across the medial femoral condyle and the skin. A 6- to 7-mm inside-out femoral socket is reamed and a shuttle suture is passed and retrieved from the anteromedial portal with a KingFisher grasper. The graft is inserted from the tibia to the femur and first fixed on the femoral side with a bioabsorbable interference screw. Before fixing the graft on the tibial side, normalization of posterior drawer close to knee extension can be seen when applying tension on the tibial side. The graft is then secured on the tibial side with a bioabsorbable interference screw at 30° of knee flexion and neutral rotation, after applying an anterior drawer. During the clinical follow-up at 6 weeks, the patient did not experience any more symptoms and the knee was stabilized. (AL, anterolateral; ALB, anterolateral bundle; MRI, magnetic resonance imaging; PCL, posterior cruciate ligament; PM, posteromedial; PMB, posteromedial bundle.)
Isolated posterior cruciate ligament (PCL) tears are uncommon, with an estimated prevalence of 3% of all knee injuries.1, 2, 3 PCL injuries do generally occur in the context of multiligament injuries, which are usually caused by vehicle or sports accidents.1,2 The most common isolated PCL injury mechanism is a direct trauma to the anterior aspect of the proximal tibia with the knee flexed, resulting in an excessive posterior tibial translation (PTT).3, 4, 5 In other injury mechanisms (hyperextension, hyperflexion, or rotational injuries), other structures often are damaged as well,3, 4, 5, 6, 7 with hyperextension injuries being more frequent in athletes, most often without contact, on a fully extended leg during landing, or a forward fall on one foot fixed to the ground.5, 6, 7
The main function of the PCL is to control PTT and tibial external rotation.8,9 Both the anterolateral (ALB) and posteromedial (PMB) bundles of the PCL have been reported to play a significant role in resisting PTT at all knee flexion angles.8,10,11 As most PCL injuries occur with a flexed knee and because the ALB is known to be the primary restraint of PTT from 30° to 120° of knee flexion,10 this bundle is considered to be more commonly injured than the PMB. In hyperextension, however, the PMB plays a more significant role than the ALB. Its smaller size10 and less strength may explain why isolated PMB injuries may easily be overlooked after a hyperextension trauma. Furthermore, magnetic resonance imaging (MRI) is also reported to be limited in determining the extent of a PCL injury12,13 and, commonly, the PCL regains continuity over time.14
In patients presenting with knee pain of unknown origin after a hyperextension injury and normal imaging findings, a partial PCL injury may be suspected even if the standard clinical examination of the PCL at 90° of knee flexion is normal. Indeed, a clinical trial comprising knee hyperextension as well as a grade 1+ pseudo-Lachman sign and a grade 1+ tibiofemoral step-off sign at 20° of knee flexion may be indicative of a partial PCL injury concerning the PMB. In such cases, isolated augmentations of the PMB could be a therapeutic strategy.
In this Technical Note, the authors report a surgical technique to perform a nonanatomic PMB augmentation of the PCL using a gracilis autograft or allograft. These technical features aim to overcome current limitations in existing techniques to address the symptoms after hyperextension injuries of the knee with partial tears of the PCL.
Surgical Technique
Informed consent from the patient was required before performing the surgery.
Patient Positioning
The patient is placed lying supine on the operating table with the operated limb being positioned in an electric leg-holder (Maquet, Rastatt, Germany) at 90° of knee flexion. A tourniquet is placed on the proximal thigh. The fluoroscopy system is present in the room if necessary.
During the initial arthroscopic evaluation, the tourniquet is generally not inflated except at the time of graft harvesting. The operative extremity is prepared and draped in standard sterile fashion.
Portal Placement and Knee Arthroscopy
A standard high anterolateral (AL) portal is first created adjacent to the patellar tendon and a standard superomedial outflow portal is established, followed by a low anteromedial (AM) portal, which is performed under direct visualization. Then, a comprehensive arthroscopic examination of the knee is performed using a 30° arthroscope (Arthrex, Munich, Germany). Inspection in this case revealed a normal anterior cruciate ligament (ACL) as well as normal collateral ligament tensioning. An elongation of the PCL could be identified near knee extension, as expressed by a posterior drawer and a pseudo-ACL insufficiency. At 90° of flexion, however, PCL function was normal. The PMB was distended between 0° and 20° of knee flexion, and the decision to perform a nonanatomic partial PMB PCL augmentation was confirmed.
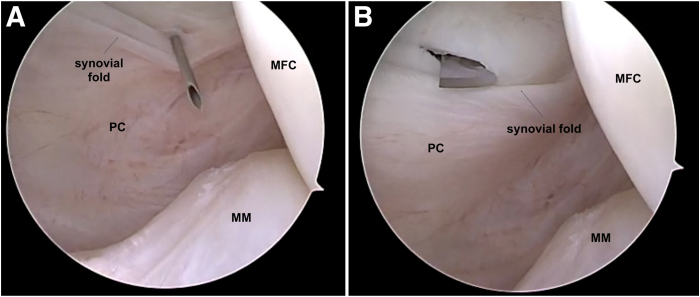
After addressing any associated intra-articular injuries including meniscus tears or cartilage lesions, a standard posteromedial (PM) viewing portal is created. The PM viewing portal is established with transillumination to visualize and avoid iatrogenic injuries to the saphenous vein and nerve. Using the transnotch view, the entry point of the viewing portal is identified with the help of a needle. Its position should be cranial to the easily identifiable PM synovial fold (Fig 1A). Once the entry point and direction have been validated, a no. 11 blade scalpel is used for skin, subcutaneous, and capsular incisions (Fig 1B) under arthroscopic control. A switching stick is then introduced into the PM portal. The camera can be introduced into the PM viewing portal over the switching rod. It allows for complete visualization of the posteromedial compartment of the knee, the PCL footprint and to facilitate graft passage.
Fig 1.
Right knee. Posteromedial viewing portal approach. Using the transnotch view, the entry point of the viewing portal is identified with a needle. Its position should be cranial to the synovial fold (A). Once the entry point and direction have been validated, a no. 11 blade scalpel is used for skin, subcutaneous, and capsular incisions (B) under arthroscopic control. (MFC, medial femoral condyle; MM, medial meniscus; PC, posterior capsule.)
Graft Harvesting and Preparation
To perform the PMB augmentation technique, either an autograft or an allograft can be used. In those cases in which an autograft is chosen, a 2-cm vertical skin incision is made medially to the anterior tibial tuberosity to harvest the gracilis tendon and to create the tibial tunnel. The tendon is harvested with a standard open tendon stripper (ACL Instrumentation System; Arthrex). The isolated gracilis graft is prepared on an auxiliary table by the assistant surgeon.
The tendon, either autograft or allograft, is then prepared in a dedicated station. The tendon is folded on itself in the middle, over 2 strong nonabsorbable sutures (TigerWire No. 2; Arthrex, Naples, FL). Two additional high-strength sutures are placed on each end of the graft and circumferential compression stiches are passed all over the tendon, which is then soaked in a vancomycin solution.15 The sutures from both ends can be further used as traction sutures to position the graft. A graft thickness of 6 to 7 mm is considered adequate to avoid overstuffing and allow smooth passage.
Arthroscopic Approach and PMB Augmentation
With the knee in 90° flexion, the arthroscope is positioned in the high AL portal and the insertion point of the PMB on the femur is then identified and marked using radiofrequency to ensure accuracy when the guide pin is passed. The arthroscope is switched to the PM portal, and the tibial PMB footprint is identified using a radiofrequency ablation device and shaver placed through the AL portal. According to Amis et al.,16 the PMB tibial attachment is distal and lateral to the ALB attachment.
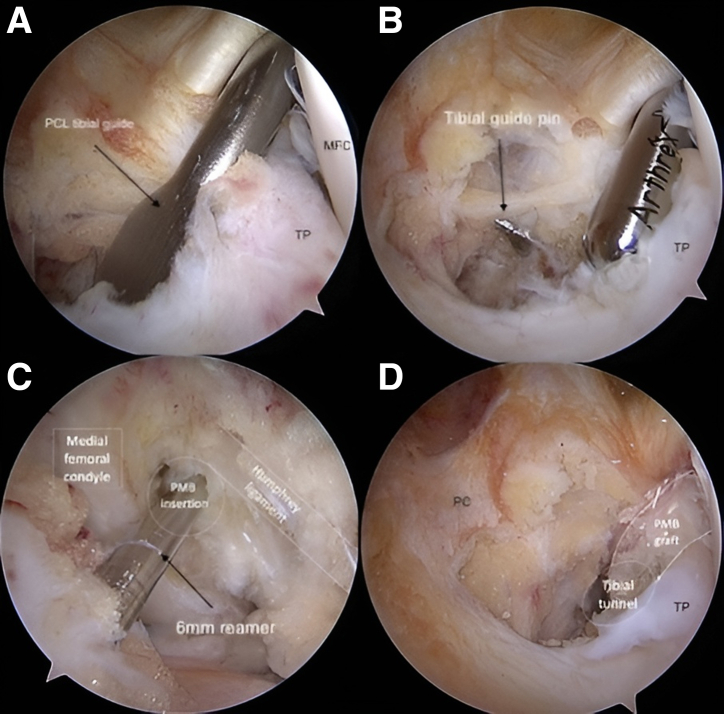
The PCL tibial tunnel drill-guide is inserted through the AM portal and placed close to the native PMB footprint (Fig 2A), approximately 5 mm more medially to the native PCL. The drill sleeve is placed at the medial proximal tibia and an incision of approximately 2 cm is created if it has not been previously made to obtain the gracilis autograft. The angulation of the drill guide can be adjusted by the surgeon.
Fig 2.
Arthroscopic view from the posteromedial portal of the posterior compartment of a left knee. A PCL tibial guide pin was placed close to the native PMB footprint (A) and a tibial tunnel of 6 mm was drilled under posteromedial visualization (B). A 6-mm in-out femoral tunnel was subsequently drilled next to the attachment site of the PMB, which is outlined on the wall of the intercondylar notch and distal to the medial arch point (C, arthroscopic view from the high anterolateral portal). The graft was routed from the tibia to the femur and first fixed on the femoral side with a bioabsorbable interference screw (D). (MFC, medial femoral condyle; PC, posterior capsule; PCL, posterior cruciate ligament; PMB, posteromedial bundle; TP, tibial plateau.)
A 2.4-mm guide pin is inserted carefully to avoid damaging the posterior neurovascular structures, and an outside-in tibial tunnel of 6 or 7 mm that matches the graft diameter is drilled with a cannulated reamer under direct visualization from the PM portal (Fig 2B). When drilling the tunnel, a curette could be placed over the guide pin inserted from the AM portal to protect the posterior neurovascular structures, if needed. Then, a looped nonabsorbable suture (FiberWire; Arthrex), is inserted into the tibial tunnel using a suture passer. With an arthroscopic KingFisher grasper (Arthrex), this suture is retrieved from the AM portal through the intercondylar notch.
The arthroscope is switched to the high AL portal and the femoral 2.4-mm guide pin is introduced through an accessory inferolateral portal and positioned at the PMB footprint near the articular surface of the femoral condyle. Then, the guide pin is drilled, with a free-hand technique, across the medial femoral condyle and the skin.
A 6- to 7-mm inside-out femoral socket is subsequently reamed next to the attachment site of the PMB, which is outlined on the wall of the intercondylar notch, just underneath the insertion of the anterior meniscofemoral ligament and distal to the medial arch point (Fig 2C). The depth of the socket is 30 mm. A shuttle looped nonabsorbable suture (FiberWire; Arthrex) is passed out of the thigh using the guide passing pin and retrieved from the AM portal with a KingFisher grasper. The tibial and femoral shuttle suture are tied together and pulled through the tibial tunnel. The tie is opened and the tibial suture is removed, leaving only the femoral shuttle suture through both femoral and tibial tunnel.
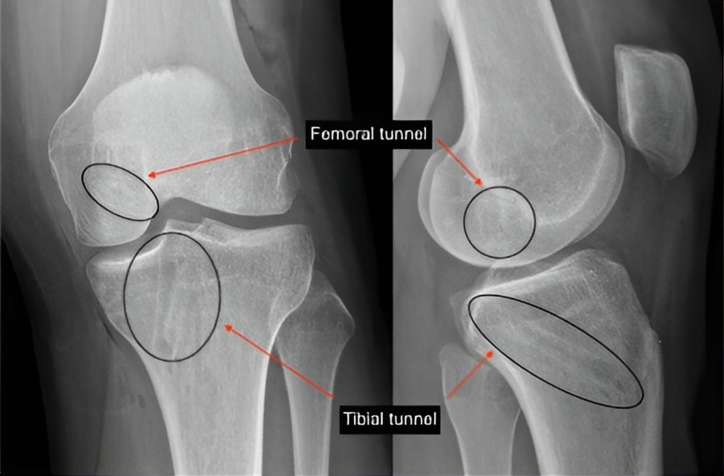
The graft is inserted from the tibia to the femur (Fig 2D), and first fixed on the femoral side with a bioabsorbable interference screw (6 or 7 × 20 mm, BioRCI; Smith & Nephew, Andover, MA). Before fixing the graft, normalization of posterior drawer close to knee extension can be seen when applying tension on the tibial side. The graft is then secured on the tibial side with a bioabsorbable interference screw (6 or 7 × 25 mm, BioRCI; Smith & Nephew) at 30° of knee flexion and neutral rotation after applying an anterior drawer (Fig 3).
Fig 3.
Postoperative anteroposterior and lateral-view radiographs of a reconstructed left knee, showing the position of the tunnels and screws
Because of the relatively small thickness of the graft, the authors found no difficulties related to the killer turn angle at the posterior exit of the tibial tunnel during graft passage. However, if so, a useful maneuver can be to place the probe in the PM portal and use it as a pulley for the graft, or use the KingFisher through the AM portal, to pull up the sutures, thus reducing the tibial killer angle.
Postoperatively, all patients remain in a PCL brace for 120 days with partial weight-bearing (10 kg) but immediately begin a standard PCL rehabilitation protocol, focusing on prevention of posterior subluxation and strengthening the quadriceps muscle. A step-by-step summary of this technique is provided in Table 1. Pearls and pitfalls of the surgical procedure are listed in Table 2.
Table 1.
Step-by-Step Nonanatomic PMB Augmentation of the PCL After Hyperextension Trauma
| Step | Description |
|---|---|
| 1 | The patient is placed supine on the operating table with the operated limb positioned in a leg-holder at 90° of knee flexion. A tourniquet is placed on the proximal thigh. |
| 2 | A standard AL arthroscopic portal is created to explore the joint. Through direct visualization, the best position for the AM is decided. |
| 3 | A standard PM viewing portal is established with transillumination to visualize and avoid iatrogenic injuries to the saphenous vein and nerve. A switching stick is then introduced into the PM portal. The camera can be introduced into the PM viewing portal over the switching rod. |
| 4 | With the arthroscope positioned in the high AL portal, the insertion point of the PMB on the femur is identified and marked using radiofrequency to ensure accuracy when the guide pin is passed. |
| 5 | The arthroscope is switched to the PM portal, and the tibial PMB footprint is identified using a radiofrequency ablation device. The PCL tibial tunnel drill-guide is inserted through the AM portal and placed close to the native PMB footprint. |
| 6 | A 2.4-mm guide pin is inserted carefully to avoid damaging the posterior neurovascular structures, and an outside-in tibial tunnel of 6 or 7 mm that matches the graft diameter is drilled with a cannulated reamer under direct visualization. |
| 7 | A looped nonabsorbable suture is inserted into the tibial tunnel using a suture passer and retrieved from the AM portal with a KingFisher grasper. |
| 8 | The arthroscope is switched to the high AL portal and a femoral 2.4-mm guide pin is introduced through an accessory inferolateral portal and drilled, with a free-hand technique, across the medial femoral condyle and the skin. |
| 9 | A 6- to 7-mm inside-out femoral socket is reamed and a shuttle looped nonabsorbable suture is passed out of the thigh and retrieved from the AM portal with a KingFisher grasper. |
| 10 | The tibial and femoral shuttle suture are tied together and pulled through the tibial tunnel. The tie is opened and the tibial suture is removed, leaving only the femoral shuttle suture through both femoral and tibial tunnel. |
| 11 | The graft is inserted from the tibia to the femur and first fixed on the femoral side with a bioabsorbable interference screw. The graft is then secured on the tibial side with a bioabsorbable interference screw in full extension and neutral rotation, after applying an anterior drawer. |
AL, anterolateral; AM, anteromedial; PCL, posterior cruciate ligament; PM, posteromedial; PMB, posteromedial bundle.
Table 2.
Pearls, Pitfalls, and Risks
Pearls
|
Pitfalls and Risks
|
ALB, anterolateral bundle; AM, anteromedial; PCL, posterior cruciate ligament; PM, posteromedial; PMB, posteromedial bundle.
Discussion
This Technical Note presents a surgical technique using a nonanatomic PMB augmentation of the PCL with a hamstring tendon autograft or allograft in patients with near-extension posterior knee laxity after a hyperextension injury. In comparison with previously published PCL augmentation techniques,17, 18, 19 the current procedure may allow for a proper knee laxity restoration and pain relief in patients with an often-overlooked isolated PMB injury.
The literature on PCL injuries after a knee hyperextension injury is scarce, making it difficult to recognize the existence of PCL/PMB tears after such a traumatic event. In 12 pairs of cadaver knees, Bizot et al.20 reproduced a passive hyperextension of the knee until rupture. The authors found that the posterior capsule was the first structure to be damaged at an average of 23° and that the PCL was the last structure to be injured before the knee dislocated. In a similar setting, Meyer et al.21 reported that both the ACL and PCL were damaged at the time of knee dislocation, which occurred at an average of 33.6° of hyperextension. After a forced hyperextension of 15° and 30°, Fornalski et al.22 observed no gross PCL injury but only posterolateral corner lesions associated with ACL ruptures. As previous authors mainly looked for gross injuries, it remains unknown whether hyperextension trauma may have caused an isolated partial damage of the PCL. These findings are largely limited by their cadaveric nature, where both the bone quality and the viscoelastic properties of the examined soft tissues may not be representative of young and active patients.
After being neglected for many years, the debate regarding the PMB has been recently revitalized. Paschos,23 in an editorial commentary, insisted on the importance of the PMB in the double-bundle PCL reconstructions to provide additional resistance to PTT at lower degrees of knee flexion. This provided the rationale for the isolated PMB augmentation presented in this report. Several biomechanical studies support this hypothesis and show that double-bundle PCL reconstructions do better to restore native graft forces and knee kinematics in terms of posterior translation and internal rotation than single-bundle PCL reconstructions, which are solely based on restoring the ALB.11,24, 25, 26, 27 Likewise, in the absence of a possibility to diagnose a posterior capsular injury after forced knee hyperextension and of a surgical strategy to eventually reconstruct it, PMB augmentations may provide a valuable alternative for these patients, who are often presenting with chronic knee pain.
The diagnostic strategy still remains challenging. It is known that MRI has a limited capacity to directly identify an isolated or partial PCL injury with certainty,12,13 but some indirect signs such as bone marrow edema may provide important information for these injuries. In a retrospective diagnostic study of 25 patients with an MRI of the knee within 1 year after a hyperextension injury, Ali et al.28 indeed reported that the presence of an edema at the AL tibial plateau after knee hyperextension injuries was strongly and significantly associated with a PCL injury as observed on MRI (odds ratio 26.0, P = .003). The authors speculated that this observation could be caused by a reverse pivot shift mechanism (i.e., internal rotation and varus hyperextension), leading to an impact on the AL tibial plateau. Interestingly, PCL injuries were all partial-thickness tears. Unfortunately, the authors did not further investigate whether the ALB and/or the PMB were involved, nor did they confirm the structural tissue damage under clinical examination or arthroscopy. Further investigations on whether MRI may be able to help orientating the diagnosis of PMB lesions after hyperextension injuries are needed.
Biomechanical studies have shown that the native PMB is tight in full knee extension and slack in the midrange, acting as a hyperextension restrictor but without contributing to resist the PTT at 90° of knee flexion, because its fibers are aligned in a proximal distal direction.8,10,11 It could be said that, with the knee in extension, the PMB serves to restrict posterior translation, whereas in knee flexion, the PMB restricts internal rotation.8,24 Those biomechanical properties could explain why in those patients present with a normal clinical examination of the PCL at 90° of knee flexion, only the clinical triad (hyperextension, 1+ pseudo-Lachman and 1+ tibiofemoral step off signs) at 20° of knee flexion revealing some unusual clinical findings.
In cases in which a partial PCL/PMB injury may be suspected, the proposed technique for an isolated bundle augmentation is relatively simple. On the femur, the native PMB footprint is easy to identify. The main relative difficulty lies in the positioning of the tibial tunnel which requires a posteromedial viewing portal. Its position is slightly nonanatomic because the PMB is posterior to the ALB in the native knee. Reproducing its anatomy would thus lead to an undesired injury of the ALB. Therefore, the authors choose to place it at the posterior border of the tibial champagne glass deformity, but approximately 5 mm more medially to the native PCL. Until now, this did not result in any clinical restrictions in the operated knees. However, further clinical and experimental studies will need to confirm these preliminary clinical observations. A list of advantages and limitations of the procedure can be found in Table 3.
Table 3.
Advantages and Limitations
| Advantages Effective therapeutic solution for PCL/PMB augmentation in patients with symptoms after hyperextension knee trauma. May allow for a proper knee laxity restoration and pain relief in patients with an often-overlooked isolated PMB injury. Because it is a nonanatomic technique, it avoids a possible iatrogenic injury to the ALB. It may be performed either with a hamstring autograft or allograft. |
| Limitations Requires advanced skills in arthroscopic surgery and a non-negligible learning curve. Requires small incisions besides the arthroscopic portals to perform graft harvesting. Challenging diagnosis of the isolated PMB injury after a hyperextension trauma. |
ALB, anterolateral bundle; PCL, posterior cruciate ligament; PMB, posteromedial bundle.
This nonanatomic posteromedial bundle augmentation of the PCL after hyperextension trauma may be an effective therapeutic solution for these infrequent and difficult injuries. However, future biomechanical and clinical studies are essential to broaden knowledge about this subject and definitely validate the presented findings.
Disclosures
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: C.M. reports being Chairwoman of the Basic Committe of the European Society for Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA) and part of the editorial board for Knee Surgery, Sports Traumatology, Arthroscopy (KSSTA) and Journal of Experimental Orthopaedics. J.C.M. reports consulting or advisory for Smith & Nephew and Conmed; Vice-President of the ESSKA; past president of the Spanish Arthroscopy Association; and editorial board member of Arthroscopy. Ro.S. reports consulting or advisory for Smith & Nephew, Olympus Corporation, and Amplitude Ventures; President of Luxembourg Institute of Research in Orthopedics, Sports Medicine and Science; past president of the Society for Orthopaedic Traumatologic Sports Medicine (GOTS; Gesellschaft für Orthopädisch-Traumatologische Sportmedizin)/ESSKA, Chairman of THE MENISCUS 2022; Vice Chairman of ESSKA meniscus certification module; and editorial board member of KSSTA, Arthroskopie, and Sports Orthopaedics and Traumatology (SOT). All other authors (M.I., JV., F.H., C.P., R.S.) declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Data
This is a case of a 17-year-old male patient who experienced a hyperextension injury of his left knee while playing basketball after a direct trauma to the front of the knee. The diagnosis was delayed until referral. A previous attempt to return to sports failed because of chronic AL pain. As most PCL injuries occur in a flexed knee position, the ALB is thought to be more commonly injured than the PMB; however, in hyperextension, the PMB plays a more significant role. Clinical examination revealed a negative posterior drawer sign at 90° of knee flexion but a positive pseudo-Lachman sign at 15°, a greater hyperextension compared with the contralateral side, and a positive step off at 15° of knee flexion. The rest of the examination was normal, as well as the MRI scan. This clinical presentation led to recommend a PM PCL bundle augmentation. The patient is placed lying supine on the operating table with the operated limb being positioned in an electric leg-holder at 90° of knee flexion. Standard high AL and low anteromedial portals are created. During the initial arthroscopic evaluation, no associated intra-articular injuries were detected. With the arthroscope positioned in the high AL portal, the insertion point of the PMB on the femur is identified and marked using radiofrequency to ensure accuracy when the guide pin is passed. Using the transnotch view, the entry point of the PM viewing portal is identified with the help of a needle, and a no. 11 blade scalpel is used for skin, subcutaneous, and capsular incisions under arthroscopic control. The arthroscope is switched to the PM portal, and the tibial PMB footprint is identified using a radiofrequency ablation device. The PCL tibial tunnel drill-guide is inserted through the anteromedial portal and placed close to the native PMB footprint. Its position is nonanatomic because the PMB is posterior to the anterolateral bundle in the native knee. Reproducing its anatomy would thus lead to an undesired injury of the ALB. Therefore, the authors choose to place it 5 mm more medially to the native PCL. A 2.4-mm guide pin is inserted and an outside-in tibial tunnel of 6 or 7 mm that matches the graft diameter is drilled with a cannulated reamer. Then, a looped nonabsorbable suture is inserted into the tibial tunnel using a suture passer and retrieved from the anteromedial portal with a KingFisher grasper. The arthroscope is switched to the high AL portal and the femoral 2.4-mm guide pin is introduced through an accessory inferolateral portal and drilled, with a free-hand technique, across the medial femoral condyle and the skin. A 6- to 7-mm inside-out femoral socket is reamed and a shuttle suture is passed and retrieved from the anteromedial portal with a KingFisher grasper. The graft is inserted from the tibia to the femur and first fixed on the femoral side with a bioabsorbable interference screw. Before fixing the graft on the tibial side, normalization of posterior drawer close to knee extension can be seen when applying tension on the tibial side. The graft is then secured on the tibial side with a bioabsorbable interference screw at 30° of knee flexion and neutral rotation, after applying an anterior drawer. During the clinical follow-up at 6 weeks, the patient did not experience any more symptoms and the knee was stabilized. (AL, anterolateral; ALB, anterolateral bundle; MRI, magnetic resonance imaging; PCL, posterior cruciate ligament; PM, posteromedial; PMB, posteromedial bundle.)
References
- 1.Hassebrock J.D., Gulbrandsen M.T., Asprey W.L., Makovicka J.L., Chhabra A. Knee ligament anatomy and biomechanics. Sports Med Arthrosc Rev. 2020;28:80–86. doi: 10.1097/JSA.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 2.LaPrade R., Floyd E., Falaas K., et al. The posterior cruciate ligament: Anatomy, biomechanics, and double-bundle reconstruction. J Arthrosc Surg Sports Med. 2021;2:1–14. [Google Scholar]
- 3.Winkler P.W., Zsidai B., Wagala N.N., et al. Evolving evidence in the treatment of primary and recurrent posterior cruciate ligament injuries, part 1: Anatomy, biomechanics and diagnostics. Knee Surg Sports Traumatol Arthrosc. 2021;29:672–681. doi: 10.1007/s00167-020-06357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee B.K., Nam S.W. Rupture of posterior cruciate ligament: Diagnosis and treatment principles. Knee Surg Relat Res. 2011;23:135–141. doi: 10.5792/ksrr.2011.23.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zsidai B., Horvath A., Winkler P.W., et al. Different injury patterns exist among patients undergoing operative treatment of isolated PCL, combined PCL/ACL, and isolated ACL injuries: A study from the Swedish National Knee Ligament Registry. Knee Surg Sports Traumatol Arthrosc. 2022;30:3451–3460. doi: 10.1007/s00167-022-06948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler P.J., Messieh S.S. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15:553–557. doi: 10.1177/036354658701500606. [DOI] [PubMed] [Google Scholar]
- 7.Lundblad M., Hägglund M., Thomeé C., et al. Epidemiological data on LCL and PCL injuries over 17 seasons in men’s professional soccer: The UEFA Elite Club Injury Study. Open Access J Sports Med. 2020;11:105–112. doi: 10.2147/OAJSM.S237997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy N.I., Wijdicks C.A., Goldsmith M.T., et al. Kinematic analysis of the posterior cruciate ligament, part 1: The individual and collective function of the anterolateral and posteromedial bundles. Am J Sports Med. 2013;41:2828–2838. doi: 10.1177/0363546513504287. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A., Sinha S., Arora R., Gaba S., Khan R., Kumar M. The 50 top-cited articles on the posterior cruciate ligament: A bibliometric analysis and review. Orthop J Sports Med. 2021;9 doi: 10.1177/23259671211057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amis A.A., Bull A.M.J., Gupte C.M., Hijazi I., Race A., Robinson J.R. Biomechanics of the PCL and related structures: Posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11:271–281. doi: 10.1007/s00167-003-0410-7. [DOI] [PubMed] [Google Scholar]
- 11.Harner C.D., Janaushek M.A., Kanamori A., Yagi M., Vogrin T.M., Woo S.L. Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med. 2000;28:144–151. doi: 10.1177/03635465000280020201. [DOI] [PubMed] [Google Scholar]
- 12.Park H.J., Lee S.Y., Choi Y.J., et al. The usefulness of the oblique coronal plane of three-dimensional isotropic T2-weighted fast spin-echo (VISTA) knee MRI in the evaluation of posterior cruciate ligament reconstruction with allograft: Comparison with the oblique coronal plane of two-dimensional fast spin-echo T2-weighted sequences. Eur J Radiol. 2019;114:105–110. doi: 10.1016/j.ejrad.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Winters K., Tregonning R. Reliability of magnetic resonance imaging of the traumatic knee as determined by arthroscopy. N Z Med J. 2005;118 [PubMed] [Google Scholar]
- 14.Boks S.S., Vroegindeweij D., Koes B.W., Hunink M.G.M., Bierma-Zeinstra S.M.A. Follow-up of posttraumatic ligamentous and meniscal knee lesions detected at MR imaging: Systematic review. Radiology. 2006;238:863–871. doi: 10.1148/radiol.2382050063. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Prieto D., Totlis T., Madjarevic T., et al. ESSKA and EBJIS recommendations for the management of infections after anterior cruciate ligament reconstruction (ACL-R): Prevention, surgical treatment and rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2023;31:4204–4212. doi: 10.1007/s00167-023-07463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amis A.A., Gupte C.M., Bull A.M.J., Edwards A. Anatomy of the posterior cruciate ligament and the meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2006;14:257–263. doi: 10.1007/s00167-005-0686-x. [DOI] [PubMed] [Google Scholar]
- 17.Wang C.J., Chan Y.S., Weng L.H. Posterior cruciate ligament reconstruction using hamstring tendon graft with remnant augmentation. Arthroscopy. 2005;21:1401. doi: 10.1016/j.arthro.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Panigrahi R., Sahoo U.K. All-inside posterior cruciate ligament reconstruction with remnant preservation: Anteromedial portal technique. Arthrosc Tech. 2023;12:e1695–e1700. doi: 10.1016/j.eats.2023.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermanowicz K., Mrozek T., Góralczyk A., et al. Arthroscopic remnant preserving 3-portal posterior cruciate ligament reconstruction with reverse passage of the graft. Arthrosc Tech. 2023;12:e621–e627. doi: 10.1016/j.eats.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bizot P., Meunier A., Christel P., Witvoët J. Experimental capsulo-ligamentar lesions of the knee during passive hyperextension. Biomechanical aspects. A lesional evaluation and consequences. Rev Chir Orthop Reparatrice Appar Mot. 1995;81:211–220. [in French] [PubMed] [Google Scholar]
- 21.Meyer E.G., Baumer T.G., Haut R.C. Pure passive hyperextension of the human cadaver knee generates simultaneous bicruciate ligament rupture. J Biomech Eng. 2011;133 doi: 10.1115/1.4003135. [DOI] [PubMed] [Google Scholar]
- 22.Fornalski S., McGarry M.H., Csintalan R.P., Fithian D.C., Lee T.Q. Biomechanical and anatomical assessment after knee hyperextension injury. Am J Sports Med. 2008;36:80–84. doi: 10.1177/0363546507308189. [DOI] [PubMed] [Google Scholar]
- 23.Paschos N.K. Editorial Commentary: The posterior cruciate ligament posteromedial bundle is small but vital to posterior cruciate ligament biomechanics: Don’t ignore the underdog. Arthroscopy. 2020;36:2885–2887. doi: 10.1016/j.arthro.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Chahla J., Williams B.T., LaPrade R.F. Posterior cruciate ligament. Arthroscopy. 2020;36:333–335. doi: 10.1016/j.arthro.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Forsythe B., Patel B.H., Lansdown D.A., et al. Dynamic three-dimensional computed tomography mapping of isometric posterior cruciate ligament attachment sites on the tibia and femur: Single- versus double-bundle analysis. Arthroscopy. 2020;36:2875–2884. doi: 10.1016/j.arthro.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Wijdicks C.A., Kennedy N.I., Goldsmith M.T., et al. Kinematic analysis of the posterior cruciate ligament, part 2: A comparison of anatomic single- versus double-bundle reconstruction. Am J Sports Med. 2013;41:2839–2848. doi: 10.1177/0363546513504384. [DOI] [PubMed] [Google Scholar]
- 27.Wright J.O., Skelley N.W., Schur R.P., Castile R.M., Lake S.P., Brophy R.H. Microstructural and mechanical properties of the posterior cruciate ligament: A comparison of the anterolateral and posteromedial bundles. J Bone Joint Surg Am. 2016;98:1656–1664. doi: 10.2106/JBJS.16.00032. [DOI] [PubMed] [Google Scholar]
- 28.Ali A.M., Pillai J.K., Gulati V., Gibbons C.E.R., Roberton B.J. Hyperextension injuries of the knee: Do patterns of bone bruising predict soft tissue injury? Skeletal Radiol. 2018;47:173–179. doi: 10.1007/s00256-017-2754-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This is a case of a 17-year-old male patient who experienced a hyperextension injury of his left knee while playing basketball after a direct trauma to the front of the knee. The diagnosis was delayed until referral. A previous attempt to return to sports failed because of chronic AL pain. As most PCL injuries occur in a flexed knee position, the ALB is thought to be more commonly injured than the PMB; however, in hyperextension, the PMB plays a more significant role. Clinical examination revealed a negative posterior drawer sign at 90° of knee flexion but a positive pseudo-Lachman sign at 15°, a greater hyperextension compared with the contralateral side, and a positive step off at 15° of knee flexion. The rest of the examination was normal, as well as the MRI scan. This clinical presentation led to recommend a PM PCL bundle augmentation. The patient is placed lying supine on the operating table with the operated limb being positioned in an electric leg-holder at 90° of knee flexion. Standard high AL and low anteromedial portals are created. During the initial arthroscopic evaluation, no associated intra-articular injuries were detected. With the arthroscope positioned in the high AL portal, the insertion point of the PMB on the femur is identified and marked using radiofrequency to ensure accuracy when the guide pin is passed. Using the transnotch view, the entry point of the PM viewing portal is identified with the help of a needle, and a no. 11 blade scalpel is used for skin, subcutaneous, and capsular incisions under arthroscopic control. The arthroscope is switched to the PM portal, and the tibial PMB footprint is identified using a radiofrequency ablation device. The PCL tibial tunnel drill-guide is inserted through the anteromedial portal and placed close to the native PMB footprint. Its position is nonanatomic because the PMB is posterior to the anterolateral bundle in the native knee. Reproducing its anatomy would thus lead to an undesired injury of the ALB. Therefore, the authors choose to place it 5 mm more medially to the native PCL. A 2.4-mm guide pin is inserted and an outside-in tibial tunnel of 6 or 7 mm that matches the graft diameter is drilled with a cannulated reamer. Then, a looped nonabsorbable suture is inserted into the tibial tunnel using a suture passer and retrieved from the anteromedial portal with a KingFisher grasper. The arthroscope is switched to the high AL portal and the femoral 2.4-mm guide pin is introduced through an accessory inferolateral portal and drilled, with a free-hand technique, across the medial femoral condyle and the skin. A 6- to 7-mm inside-out femoral socket is reamed and a shuttle suture is passed and retrieved from the anteromedial portal with a KingFisher grasper. The graft is inserted from the tibia to the femur and first fixed on the femoral side with a bioabsorbable interference screw. Before fixing the graft on the tibial side, normalization of posterior drawer close to knee extension can be seen when applying tension on the tibial side. The graft is then secured on the tibial side with a bioabsorbable interference screw at 30° of knee flexion and neutral rotation, after applying an anterior drawer. During the clinical follow-up at 6 weeks, the patient did not experience any more symptoms and the knee was stabilized. (AL, anterolateral; ALB, anterolateral bundle; MRI, magnetic resonance imaging; PCL, posterior cruciate ligament; PM, posteromedial; PMB, posteromedial bundle.)
This is a case of a 17-year-old male patient who experienced a hyperextension injury of his left knee while playing basketball after a direct trauma to the front of the knee. The diagnosis was delayed until referral. A previous attempt to return to sports failed because of chronic AL pain. As most PCL injuries occur in a flexed knee position, the ALB is thought to be more commonly injured than the PMB; however, in hyperextension, the PMB plays a more significant role. Clinical examination revealed a negative posterior drawer sign at 90° of knee flexion but a positive pseudo-Lachman sign at 15°, a greater hyperextension compared with the contralateral side, and a positive step off at 15° of knee flexion. The rest of the examination was normal, as well as the MRI scan. This clinical presentation led to recommend a PM PCL bundle augmentation. The patient is placed lying supine on the operating table with the operated limb being positioned in an electric leg-holder at 90° of knee flexion. Standard high AL and low anteromedial portals are created. During the initial arthroscopic evaluation, no associated intra-articular injuries were detected. With the arthroscope positioned in the high AL portal, the insertion point of the PMB on the femur is identified and marked using radiofrequency to ensure accuracy when the guide pin is passed. Using the transnotch view, the entry point of the PM viewing portal is identified with the help of a needle, and a no. 11 blade scalpel is used for skin, subcutaneous, and capsular incisions under arthroscopic control. The arthroscope is switched to the PM portal, and the tibial PMB footprint is identified using a radiofrequency ablation device. The PCL tibial tunnel drill-guide is inserted through the anteromedial portal and placed close to the native PMB footprint. Its position is nonanatomic because the PMB is posterior to the anterolateral bundle in the native knee. Reproducing its anatomy would thus lead to an undesired injury of the ALB. Therefore, the authors choose to place it 5 mm more medially to the native PCL. A 2.4-mm guide pin is inserted and an outside-in tibial tunnel of 6 or 7 mm that matches the graft diameter is drilled with a cannulated reamer. Then, a looped nonabsorbable suture is inserted into the tibial tunnel using a suture passer and retrieved from the anteromedial portal with a KingFisher grasper. The arthroscope is switched to the high AL portal and the femoral 2.4-mm guide pin is introduced through an accessory inferolateral portal and drilled, with a free-hand technique, across the medial femoral condyle and the skin. A 6- to 7-mm inside-out femoral socket is reamed and a shuttle suture is passed and retrieved from the anteromedial portal with a KingFisher grasper. The graft is inserted from the tibia to the femur and first fixed on the femoral side with a bioabsorbable interference screw. Before fixing the graft on the tibial side, normalization of posterior drawer close to knee extension can be seen when applying tension on the tibial side. The graft is then secured on the tibial side with a bioabsorbable interference screw at 30° of knee flexion and neutral rotation, after applying an anterior drawer. During the clinical follow-up at 6 weeks, the patient did not experience any more symptoms and the knee was stabilized. (AL, anterolateral; ALB, anterolateral bundle; MRI, magnetic resonance imaging; PCL, posterior cruciate ligament; PM, posteromedial; PMB, posteromedial bundle.)