Abstract
The annual number of human leukocyte antigen (HLA)-haploidentical allogeneic hematopoietic stem cell transplantation (haplo-HCT) is increasing steadily. Comparative studies about haplo-HCT versus HCT with HLA-matched sibling donors (MSD-HCT) have been tried in acute myeloid leukemia and B-cell acute lymphoblastic leukemia/lymphoma (ALL). Few studies were reported in adult T-cell ALL (T-ALL). In this retrospective study, a total of 88 consecutive patients with T-ALL were enrolled who underwent MSD-HCT (n = 24) and haplo-HCT (n = 64) with antithymocyte globulin (ATG)-based graft versus host disease (GVHD) prophylaxis between 2010 and 2022. Median follow-up for survivors was similar (43.5 [range: 7–88] months for MSD-HCT versus 43.5 (range: 6–144) months in the Haplo-HCT group). The 100-day cumulative incidence of grade II to IV acute GVHD (aGVHD) was similar, 33% (95% confidence interval [CI], 16%–52%) after MSD-HCT versus 44% (95% CI, 31%–55%) after haplo-HCT, P = 0.52. The cumulative incidences of grade III–IV aGVHD were 8% (95% CI, 1%–23%) in the MSD-HCT group and 5% (95% CI, 1%–12%) in the haplo-HCT group (P = 0.50). The 2-year cumulative incidence of chronic GVHD (limited and extensive) in the haplo-HCT, 11% (95% CI, 5%–20%) was significantly lower than that in the MSD-HCT group (42% [95% CI, 21%–62%], P = 0.002). The cumulative incidence of 4-year relapse rates (44% versus 37%, P = 0.56) and non-relapse mortality (7% versus 21%, P = 0.08) did not differ between these two groups. There were also no differences in 4-year overall survival (46% versus 47%, P = 0.44) and progression-free survival (49% versus 42%, P = 0.45) between these two groups. On multivariate analysis, using busulfan/fludarabine (BU/Flu) conditioning regimen was found to be associated with worse clinical outcome. Our results suggested that ATG-based haplo-HCT platform could work as an alternative to MSD-HCT for adult patients with T-ALL. Compared with MSD-HCT, haplo-HCT might carry a low risk for cGVHD.
Keywords: T-cell acute lymphoblastic leukemia, allogeneic stem cell transplantation, haploidentical, matched sibling donor, antithymocyte globulin (ATG)
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a relatively rare but highly aggressive lymphoid neoplasm of precursor T cells. T-cell lymphoblastic lymphoma (T-LBL), featuring with large mediastinal mass, has been grouped together with T-ALL since 2008 based on the World Health Organization (WHO) classification because of their shared features in morphology and immunophenotype 1 . It accounts for 20% to 25% of all cases of adult ALL 2 . Although there are distinct features in demographics, clinical presentation, biology, and genetic landscape for T-ALL, these patients are still treated with the similar pattern of B-ALL in the frontline setting3,4. Unlike the great progress in the management of B-ALL with blinatumomab, and CAR-T cells, little advances were made in the treatment of T-ALL. Given the relatively high risk of relapse, allogeneic hematopoietic cell transplantation (allo-HCT) is remaining an important consolidation therapy for adult T-ALL 5 .
A number of things should be considered before allo-HCT was recommended. These include age, the presence of high-risk features at diagnosis, remission and measurable residual disease (MRD) status upon frontline chemotherapies, patient’s physiologic status and comorbidities, and mostly importantly donor availability for allo-HCT. Currently, HLA-matched sibling donor (MSD) or unrelated donor was still consider to be the best donor for allo-HCT6,7. Unfortunately, the probability of finding an HLA-matched sibling or unrelated donor was largely depended on family size and ethnicity. To expand donor sources, the number of transplantation from alternative donors such as HLA-haploidentical related donor, or umbilical cord blood stem cell product, is increasing steadily. With the high probability that nearly everybody can find a donor from his or her family members and the advantage of immediate availability, the number of transplantation using HLA-haploindentical donor (haplo-HCT) is growing fast this decade7,8. Several studies from single centers or based registries indicated haplo-HCT either with post-cyclophosphamide or antithymocyte globulin (ATG)-based graft versus host disease (GHVD) prophylaxis could achieve comparable outcome when compared with transplantation using HLA-matched sibling donor (MSD-HCT)9–13. These data were initially reported in acute myeloid leukemia (AML) and then in ALL. But the number of patients with T-ALL was not specified in these studies due to the relative rarity of this disease. And there is yet no comparative study focusing on adult T-ALL about the relative efficacy of haplo-HCT versus MSD-HCT. Herein, in this study, we conducted a single-center retrospective study of 86 patients with T-ALL who underwent haplo-HCT or MSD-HCT in our medical center between 2010 and 2022.
Patients and Methods
Patients
This was a single-center, retrospective analysis that include all consecutive patients with a diagnosis of T-ALL who received their first allo-HSCT at the First Medical Center of Chinese PLA General Hospital, between January 2010 and December 2022. All patients were thoroughly examined with bone marrow aspirates, morphologic assessment, flow cytometry, core biopsy, immunohistochemistry and additional positron emission tomography/computed tomography (PET/CT) for patients with suspected extramedullary involvement. T-LBL was primarily defined as extramedullary mass with less than 25% bone marrow involvement 14 . All patients with T-LBL in our center were treated in the same way as T-ALL since 2009 and were included in this study. Patients who were aged <14 years or were diagnosed with bi-phenotypic leukemia, mixed-lineage ALL, or human T-cell lymphotrophic virus (HTLV) positive adult T-cell leukemia/lymphoma were excluded from this study.
The diagnosis early-thymic precursor (ETP) subtype and assessment of MRD were both evaluated with 8- to 10-color multi-parameter flow cytometry in bone marrow (BM) samples. The criteria of ETP were defined as previously 15 . The threshold for the positivity of MRD was >0.01%. Complete remission (CR) was defined by morphologic criteria with BM blast <5% but with no evidence of extramedullary mass. All patients signed written informed consent. This study was reviewed and approved by the Medical Ethics Review Board of Chinese PLA General Hospital.
HLA Matching, Donor Selection, and Stem Cell Collection
High-resolution DNA typing for HLA-A, -B, -C, -DRB1, and -DQB1 were performed for all patients and donors. Donor selection criteria were basically consistent with recommendations of the Chinese Society of Hematology 7 . Main considerations were based on HLA-matched loci, younger age, male sex and better performance status. MSD was the first choice for allo-HCT. HLA-haploidentical donor was considered as an alternative only when HLA-matched related or unrelated donors were unavailable. Donor was treated with recombinant human G-CSF (Filgrastim, Kyowa Kirin, Tokyo, Japan; 5–10 mg/kg/day) for 5 to 6 consecutive days. Stem cell collection was collected from the peripheral blood on the fifth day and continued until sufficient number of cells was achieved. G-CSF-primed peripheral blood stem cells was the sole source of graft for the majority of patients with the exception that rhG-CSF-mobilized BM were also used when their donors were older than 55 years. The target value was >5*108/kg of recipient weight for mononuclear cells and >2*106/kg of recipient weight for CD34+ cells.
Conditioning Regimen and GVHD Prophylaxis
Allo-HCT was recommended for all adult patients after diagnosis. For patients in CR1 but with high-risk features including high white blood cell (WBC) counts (WBC ≥ 100 × 109/L) or central nervous system (CNS) involvement at diagnosis, age ≥35 years, the need for more than one cycle of induction regimen to achieve CR1, the presence of MRD detected by flow cytometry after induction regimen, or ETP-ALL subtype, allo-HCT was strongly recommended. Allo-HCT was also recommended and proceeded for patients in active disease but also with sufficient organ function when they had a donor. The conditioning regimens used in this study were all myeloablative. Three conditioning regimens were used 16 : (1) TBI/Cy: total body irradiation (TBI, 8–10 Gy, days −7 to −6), cyclophosphamide (100 mg/kg, days, −4 to −3); (2) modified Bu/Cy regimen: cytarabine (8 g/m2 for haplo-HCT; 4 g/m2 for MSD-HCT, days −10 to −9), busulfan (9.6 mg/kg, intravenously, days −8 to −6), cyclophosphamide (100 mg/kg, days −4 to −3); (3) modified Flu/Bu regimen: substitution of cyclophosphamide in BuCy with fludarabine (150 mg/m2, days −7 to −3). The majority of patients in this study received ATG (Thymoglobulin, rabbit; Genzyme Europe B.V., Naarden, the Netherlands), cyclosporine A (CsA), short-term methotrexate (MTX) and mycophenolate mofetil (MMF) for GVHD prophylaxis. The dosage of ATG was 10 mg/kg (days −5 to −2) for patients undergoing haplo-HCT. For patients undergoing MSD-HCT, the total dosage of ATG was 5 mg/kg (day −5 to day −4). The usage of cyclosporine, mycophenolate, and short-term methotrexate was described in our previous study 16 . Non-ATG-based GVHD regimen was only used in a few patients who received MSD-HCT before 2016.
Supportive Care and Infection Prophylaxis and Treatment
All patients were supported with irradiated and filtered blood products and growth factors after stem cells infusion. Infection prophylaxis and treatment was provided as previously described 17 . Oral sulfamethoxazole and venous levofloxacin was routinely used for antibacterial prophylaxis. Ganciclovir was used for prophylaxis and treatment of cytomegalovirus (CMV) infection. Pre-emptive treatment with anti-CD20 antibody (rituximab, 375 mg/m2) was given for Epstein–Barr virus (EBV)-DNA viremia. Acyclovir or valacyclovir was used from day 1 until 1.5 years after transplantation for all patients. Antifungal agents were usually given for prophylaxis from 10 days before transplantation to +3 month post-transplantation.
Definitions and Statistical Analysis
Study endpoints include engraftment, acute GVHD (aGVHD), chronic GVHD (cGVHD), overall survival (OS), progression-free survival (PFS), non-relapse mortality (NRM), relapse. cGVHD was evaluated and classified as limited or extensive according to evaluated according to international criteria 18 . The day of transplantation was the start points for all. OS was calculated from start point to the date of death of any cause, or last follow-up for survivors. PFS was estimated from the day of transplantation to relapse, progressive disease, or death, or last follow-up for surviving patients without disease progression. NRM was defined as death from any cause related to transplantation without disease progression. Time to relapse and time to NRM were calculated from the date of transplantation. Statistical descriptive analyses (the chi-square test, Mann–Whitney U test and t-test.) were used to show demographic and transplantation-related characteristics. Kaplan–Meier analysis was used to calculate the probabilities of OS and PFS. For NRM, relapse, and GVHD, their cumulative incidences were estimated with competing-risk analysis and compared with Gray’s test. Cox proportional hazards regression was used on univariate and multivariate analysis to assess predictors of the outcomes of interest. Analyses were conducted with using SPSS 28.0 (IBM, Armonk, NY) R software, version 3.4.0. All P values are two sided, and a P value of <0.05 was considered significant.
Results
Patient, Transplant and Disease Characteristics
A total of 86 patients with T-ALL met the inclusion criteria and were analyzed in this study including MSD-HCT (n = 24) and haplo-HCT (n = 64). Patient demographic and transplant characteristics are summarized in Table 1. There were no significant differences in most variables. Median age was similar. And most patients (over 80%) were aged 35 or younger in both groups. Disease status was similar between these two groups. There were 54 (84%) patients in CR1 and 10 (16%) patients in active disease in the haplo-HCT group. While in the MSD-HCT group, 21 (87%) patients were in CR1 and 3 (13%) patients in active disease at the time of transplantation. However, the proportion of patients who received ATG-containing GVHD prophylaxis was significantly lower in the MSD-HCT cohort.
Table 1.
Patient, Disease, and Transplant Characteristics.
| Characteristic | Haploidentical (n = 64) | HLA-Identical (n = 24) | P value |
|---|---|---|---|
| Age at transplant, years | |||
| Median (range) | 23.5 (7–54) | 26.5 (19–49) | 1.00 |
| ≤35 | 56 (87%) | 20 (83%) | 0.612 |
| >35 | 8 (13%) | 4 (17%) | |
| Male, n (%) | |||
| Male | 48 (75%) | 18 (75%) | 1.00 |
| Female | 16 (25%) | 6 (25%) | |
| Diagnosis, n (%) | 0.643 | ||
| T-ALL | 42 (66%) | 17 (71%) | |
| T-LBL | 22 (34%) | 7 (21%) | |
| ALL subtype | |||
| ETP | 34 (53%) | 16 (67%) | 0.388 |
| Non-ETP | 22 (34%) | 7 (29%) | |
| Missing | 8 (13%) | 1 (4%) | |
| White blood cell count at diagnosis, ×109/L | 0.720 | ||
| <100 | 57 (89%) | 22 (92%) | |
| ≥100 | 7 (11%) | 2 (8%) | |
| LDH at diagnosis | 0.952 | ||
| Normal | 26 (41%) | 9 (38%) | |
| Elevated | 27 (42%) | 11 (45%) | |
| Missing | 11 (17%) | 4 (17%) | |
| Disease status at HCT | 0.713 | ||
| CR | 54 (84%) | 21 (87%) | |
| Advanced disease | 10 (16%) | 3 (13%) | |
| MRD status before HCT | 0.340 | ||
| MRD− | 44 (81%) | 19 (90%) | |
| MRD+ | 10 (19%) | 2 (10%) | |
| High-risk stratification | 0.396 | ||
| High risk | 41 (64%) | 13 (54%) | |
| Standard risk | 23 (36%) | 11 (46%) | |
| HCI-CI | 0.571 | ||
| 0 | 49 (76%) | 16 (66%) | |
| 1 | 12 (19%) | 7 (29%) | |
| ≥2 | 3 (5%) | 1 (5%) | |
| Cycles of chemotherapy before HCT | |||
| Median (range) | 5 (3–22) | 5.5 (3–9) | 0.056 |
| ≤4 | 24 (38%) | 4 (17%) | 0.062 |
| ≥5 | 40 (62%) | 20 (83%) | |
| Median time from diagnosis to HCT (months) | 7 (3–32) | 7 (4–18) | 0.165 |
| <8 | 27 (42%) | 11 (46%) | 0.758 |
| ≥8 | 37 (58%) | 13 (54%) | |
| Conditioning regimen | 0.353 | ||
| BU/CY | 48 (75%) | 18 (75%) | |
| TBI/CY | 13 (20%) | 6 (25%) | |
| BU/Flu | 3 (5%) | 0 | |
| GVHD prophylaxis | 0.0001 | ||
| CSA + MTX + MMF | 0 | 15 (62%) | |
| CSA + MTX + MMF + ATG | 64 (100%) | 9 (38%) | |
| PBSC graft, median (range) | |||
| MNCs × 108/kg | 8.9 (5.5–28.3) | 10.8 (5.5–21.3) | 0.335 |
| CD34+ cells × 106/kg | 5.0 (0.926–14.3) | 4.3 (1.4–23.5) | 0.238 |
| Engraftments | |||
| Neutrophil | 13 (9–20) | 11 (8–18) | 0.034 |
| platelet | 14 (9–31) | 14 (8–30) | 0.758 |
| Engraftment | 61 (95%) | 24 (100%) | 0.559 |
| Failure | 3 (5%) | 0 | |
| Last follow-up | 43.5 (6–144) | 43.5 (7–88) | 0.948 |
| Alive | 32 (50%) | 12 (50%) | |
| Dead | 32 (50%) | 10 (46%) | |
| Missing | 0 | 2 (4%) | |
T-ALL, T-cell acute lymphoblastic leukemia; T-LBL, T-cell lymphoblastic lymphoma; allo-HCT, allogeneic hematopoietic cell transplantation; ETP, early-thymic precursor; CR1, first complete remission; CR2, second complete remission; NR, non-remission; PIF/CR1, patients with primary induction failure who eventually achieved first CR; MRD, measurable residual disease; HCI-CI, hematopoietic cell transplantation comorbidity index; MSD, HLA-matched sibling donors; Haploidentical, HLA-haploidentical donors; MUD, HLA-matched unrelated donors; TBI/CY, total body irradiation/cyclophosphamide; BU/CY, busulfan/cyclophosphamide; GVHD, graft versus host disease; CSA, cyclosporine A; MMF, mycophenolate; MTX, methotrexate; ATG, antithymocyte globulinl; PBSC, peripheral blood stem cell; MNCs, mononuclear Cells.
Engraftment and GVHD
All patients achieved neutrophil engraftment by day 28 post transplantation. The median time of neutrophil engraftment was 13 (range: 9–20) days for the haplo-HCT group, which was longer than the 11 days in the MSD-HCT group (range: 8–18 days, P = 0.03). Platelet engraftment was successful for all patients in the MSD-HCT group. And three patients failed to achieve platelet engraftment by day 35 after transplantation in the haplo-HCT group. But no significant difference was observed in the median time of platelet engraftment, 14 days (range: 9–31 days) in the haplo-HCT group versus 14 days (range: 8–30 days) in the MSD-HCT group, P = 0.76. All patients in both groups had complete donor chimerism at day 28 evaluation after transplantation.
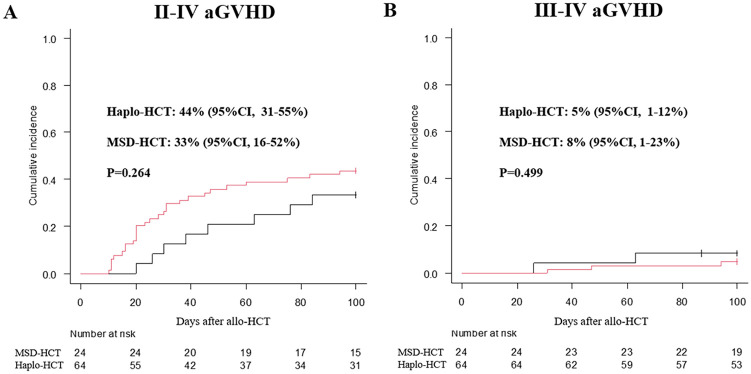
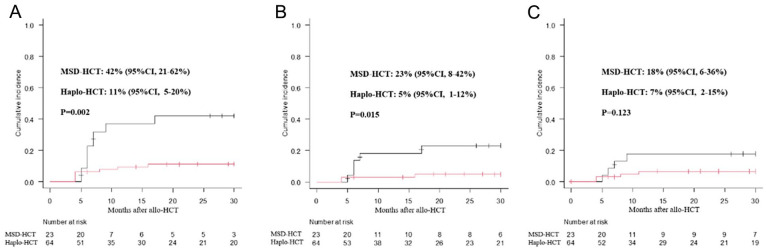
The cumulative incidence of II-IV aGVHD by day +100 post-transplantation was similar between these two groups, 44% (95% confidence interval [CI], 31%–55%) in the haplo-HCT group versus 33% [95% CI, 16%–52%] in the MSD-HCT group, P = 0.26, Fig. 1A. There was also no difference in the overall 100-day grade III–IV acute GVHD between these two groups (5% [95% CI, 1%–12%]) for the haplo-HCT group versus 8% [95% CI, 1%–23%] for the MSD-HCT group; P = 0.50, Fig. 1B). Of the 88 study patients, 83 patients survived longer than 100 days after transplant. The overall 3-year cumulative incidence of cGVHD (limited and extensive) post-transplantation in the MSD-HCT group (42% [95% CI, 21–62%]) was significantly higher than that in the haplo-HCT group (11% [95% CI, 5–20%]), P = 0.002, Fig. 2A). The incidence of limited cGVHD in the MSD-HCT group (23% [95% CI, 8–42%]) was also higher than that in the haplo-HCT group (5% [95% CI, 1–12%], P = 0.015, Fig. 2B).
Figure 1.
Cumulative incidence of 100-day grade II–Ⅳ aGVHD (A) and Ⅲ–Ⅳ aGVHD (B) after allo-HCT. allo-HCT, allogeneic hematopoietic cell transplantation; aGVHD, acute graft versus host disease.
Figure 2.
The overall 3-year cumulative incidence of cGVHD after allo-HCT: (A) limited and extensive cGVHD; (B) limited cGVHD; (C) extensive cGVHD. allo-HCT, allogeneic hematopoietic cell transplantation; cGVHD, chronic graft versus host disease.
Relapse and NRM
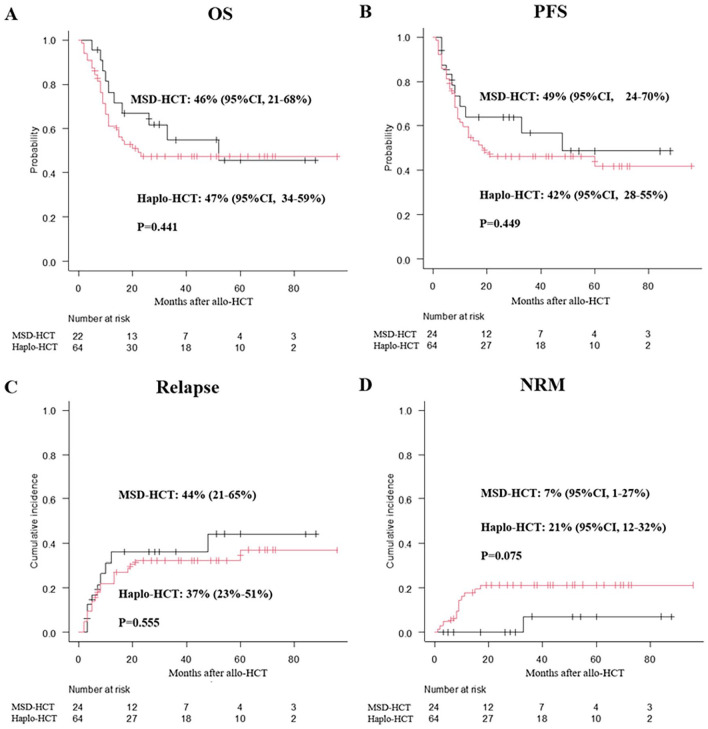
Thirty patients experienced relapse at a median of 6.5 (range: 2–60) months: 21 patients in the haplo-HCT group and 9 patients in the MSD-HCT group. The cumulative incidence of relapse/progression rate 3-years after transplantation was 37% [95% CI, 9%–48%] for patients in the Haplo-HCT group and 44% [95% CI, 21%–65%] in the MSD-HCT group (P = 0.55, Fig. 3A). Further univariate and multivariate analysis were performed for NRM and relapse. Using busulfan/fludarabine (BU/Flu) conditioning regimen was the only independent factor predicting higher risk of relapse (Table 2 and Supplemental Table 1). Although the 3-year cumulative incidence of NRM in the haplo-HCT group (21% [95% CI, 12%–32%]) seemed to be higher than that in the MSD-HCT group (7% [95% CI, 1%–27%]), no significantly difference was found (P = 0.075, Fig. 3B). Using BU/Flu conditioning regimen were identified to be associated with higher NRM (Table 2 and Supplemental Table 1).
Figure 3.
Overall transplant outcomes after allo-HCT. (A) 3-year cumulative incidence of relapse; (B) NRM; (C) OS; (D) PFS. allo-HCT, allogeneic hematopoietic cell transplantation; NRM, non-relapse mortality; OS, overall survival; PFS, progression-free survival.
Table 2.
Multivariable Analysis for Clinical Outcome.
| Variables | OS | Relapse | NRM | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex, female (ref. female) | 3 (1–7) | 0.02 | NA | NA | 8 (1–74) | 0.08 |
| Age, >35 years (ref. ≤35) | NA | NA | 1 (0.5–4) | 0.58 | NA | NA |
| T-ALL (ref. T-LBL) | 2 (1–3) | 0.25 | NA | NA | NA | NA |
| LDH Elevated (ref. Normal) | NA | NA | 2 (1–5) | 0.11 | NA | NA |
| Disease status at allo-HCT, Non-CR (ref. CR) | NA | NA | 2 (1–5) | 0.12 | NA | NA |
| HCI-CI | ||||||
| 0 (ref.) | 1 | 1 | 1 | |||
| 1 | 1 (1–3) | 0.64 | 2 (1–4) | 0.35 | 1 (0.2–3) | 0.85 |
| ≥2 | 4 (1–14) | 0.011 | 3 (0.5–18) | 0.20 | 6 (0.5–66) | 0.17 |
| Median months from diagnosis to HCT, ≥7 (ref. <7) | 3 (1–8) | 0.034 | NA | NA | NA | NA |
| Conditioning regimen | ||||||
| BU/CY(ref.) | 1 | 1 | 1 | |||
| TBI/CY | 1 (0.4–2) | 0.82 | 1 (0.34–3) | 0.99 | 0.3 (0.1–2) | 0.23 |
| BU/Flu | 35 (6–213) | 0.001 | 10 (1 -80) | 0.03 | 18 (1–253) | 0.03 |
OS, overall survival; NRM, non-relapse mortality; T-ALL, T-cell acute lymphoblastic leukemia; T-LBL, T-cell lymphoblastic lymphoma; allo-HCT, allogeneic hematopoietic cell transplantation; ETP, early-thymic precursor; MRD, measurable residual disease; HCI-CI, hematopoietic cell transplantation comorbidity index; MSD, HLA-matched sibling donors; Haploidentical, HLA-haploidentical donors; MUD, HLA-matched unrelated donors; TBI/CY, total body irradiation/cyclophosphamide; BU/CY, busulfan/cyclophosphamide.
Causes of dead were specifically listed in Supplemental Table 2. And the cumulative incidence of NRM at different time points after allo-HCT is shown in Supplemental Table 3. Besides disease progression (19/32, 60%), 12 patients died of NRM in the haplo-HCT group. On the contrary, only one patient died of cGVHD, and 9 patients died of disease progression in the MSD-HCT group. More patients (7/62, 11%) were found to die of infections which included invasive pulmonary fungal infection (n = 3), bacterial pneumonia (n = 2), Pneumocystis carnii pneumonia (n = 1) and CMV disease (n = 1) in the haplo-HCT group.
Survival
Forty-two patients died during follow-up: 32 patients in the haplo-HCT group and 10 patients in the MSD-HCT group. Causes of dead were listed in Supplemental Table 2. Disease progression was the common cause of death in both groups, especially in the MSD-HCT group (Supplemental Table 2).
Median follow-up time for survivors was similar between these two groups (43.5 [range: 6–144] months for the haplo-HCT group versus 43.5 [range: 7–88] months for the MSD-HCT group, P = 0.95). There was no significant difference in 3-year OS between these two groups (47% [95% CI, 34%–59%] for the Haplo-HCT group versus 46% [95% CI, 21%–68%] for the MSD-HCT group, P = 0.44, Fig. 3C). The 3-year PFS was not significantly different between these two groups (42% [95% CI, 28%–55%] for the Haplo-HCT group versus 49% [95% CI, 24%–70%] for the MSD-HCT group, P = 0.44, Fig. 3D). Female patients, higher HCI-CI (≥2), and using BU/Flu conditioning regimen were associated with inferior survival in multivariate analysis (Supplemental Tables 1 and 2).
Discussion
Given the relatively high relapse rate for adult T-ALL and the amazing results of CD19-targeted immunotherapy in B-ALL, allo-HCT continues to be an important curative option for adult T-ALL. The number of haplo-HCT increases steadily this decade and comparative studies about haplo-HCT versus MSD-HCT have been conducted in MDS, AML, and ALL9–13,19–21. However, few studies focused on the relative efficacy of haplo-HCT versus MSD-HCT in the specific set of adult patients with T-ALL. Our retrospective study compared the outcome of myeloablative haplo-HCT in platforms of ATG-based GVHD prophylaxis versus MSD-HCT in adult T-ALL. Our results indicated that compared with MSD-HCT, ATG-based haplo-HCT can achieve similar survival, but carry a low risk for cGVHD.
Generally, T-ALL is more aggressive than B-ALL. And allo-HCT was more strongly recommended in the real world. Currently, HLA-MSDs are still universally considered to be the best donor. With the great advantage that almost every patient can find a donor in their family members, and it usually takes a shorter time for the preparation for stem cell collection, the number of haplo-HCT using T-cell replete grafts either in ATG-based or post-transplant cyclophosphamide platforms is growing rapidly both in China and western countries7,22. Consistent with the comparative studies in AML and ALL, our study further extended the fact that OS after ATG-based haplo-HCT is similar to that after MSD-HCT in adult T-ALL. Although the number of patients in the MSD-HCT group was small in our study, the distribution of baseline variables like age, sex, and disease status, were almost equally comparable to those in the Haplo-HCT group. All patients achieved neutrophil engraftment in this study. In line with comparative studies that using ATG-based haplo-HCT platforms in AML and ALL9,12, neutrophil recovery was also faster in the MSD-HCT cohort (11 versus 13 days) in our study. Although more studies suggested that MSD-HCT recipients had a somewhat faster platelet recovery, median time of platelet engraftment appeared to be similar (14 days) between these two groups in our study. It may be due to the small number of patients in the MSD-HCT group. Even though 3 patients developed primary poor platelet engraftment in the haplo-HCT group, all patients had complete full donor chimerism by 1-month post-transplantation. All these indicated that engraftment was no longer a problem and was reasonably acceptable in ATG-based haplo-HCT platforms.
Many factors like age, underlying disease, disease status before transplantation, intensity of conditioning regimen, the choice of donor and the way of GVHD prophylaxis, all contribute to the clinical outcome after allo-HCT. In our study, demographic feature, the indication for allo-HCT, transplantation procedure include conditioning regimen were all comparable or similar between these two groups. Main differences were the source of donor and the use of ATG in haplo-HCT group. It is much meaningful to compare the clinical outcomes of haplo-HCT versus MSD-HCT in this specific set of adult patients with T-ALL. The major concern for the use of HLA-haploidentical donors is the potential high risk of GVHD. But everything has its two sides. Owing to the inherent broader HLA disparity for haplo-HCT, a stronger GVL effect was both reported in ATG-based or post-transplant cyclophosphamide platforms23–27.
Previous studies from ATG-based haplo-HCT platform indicated that the incidence of grade II–IV GVHD was similar to that in MSD-HCT28,29. While other studies also reported a higher incidence of II–IV aGVHD for haplo-SCT12,27. And in our study, the incidence of II–IV aGVHD after haplo-HCT tended to be higher than that (44% versus 33%) in the MSD-HCT group. It may be due to the small number of patients. The incidence of grade III–IV aGVHD for haplo-HCT (5%) was also similar to that after MSD-HCT (8%) in our study. This was consistent with the relative low incidence of III to IV aGVHD after haplo-HCT with ATG-based platform reported in retrospective studies30,31. One recent randomized prospective study also confirmed that the incidence of grade III–IV aGVHD was only 6% in ATG-based haplo-HCT and was similar to that after MSD-HCT 28 . While large registry-based retrospective study, which include large number of patients, tended to be concluded that the incidence of II–IV (36% versus 29%) and III–IV aGVHD (15% versus 11%) after haplo-HCT was higher than those in MSD-HCT 13 . Regarding the incidence of cGVHD, similar to our previous study that overall incidence of cGVHD after haplo-HCT was found to be lower than that after MSD-HCT 32 . In line with the relative low incidence of cGVHD in our study, Di Bartolomeo et al 33 also reported that overall and extensive cGVHD were 17 and 6% for haplo-HCT recipients. But other studies also reported that the incidence of cGVHD after ATG-based myeloablative haplo-HCT was similar to that in MSD-HCT12,25,34. Major difference was graft sources of stem cells and the dosing of ATG. The role of these two variables in the development of GVHD was not well determined and needed to be evaluated in large prospective studies.
Relapse was another important obstacle affecting the success of allo-HCT. And it was more closely associated with the primary underlying diseases and remission status before transplantation. In this study, the relapse rate was similar (44% versus 37%) between these two groups. The OS and relative higher relapse rate after allo-HCT in this study was somewhat similar to those in previous studies that focusing on adult T-ALL patients35–37. It was higher than those of patients with ALL and AML undergoing allo-HCT38,39, suggesting a more aggressive nature for T-ALL. Previous studies reported that relapse rate after haplo-HCT was lower than that in MSD-HCT13,27. However, there was no difference in relapse rate between these two groups in our study. It may be due to the small number of patients. non-relapse mortality (NRM) was another important concern for haplo-HCT. Although, there was no significant difference, the 4-year NRM rate in the haplo-HCT group tended to be higher (21%) than that (7%) in the MSD-HCT group in our study. In accordance with this, more studies concluded that a relatively higher NRM rate was associated haplo-HCT13,26,40. Possible reason was thought to be related with a relatively higher intensity of immunosuppression either by ATG or post-cyclophosphamide regimen in haplo-HCT in comparison with MSD-HCT. And haplo-HCT were found to associated with more death secondary to infections and thus have a high risk of NRM41–43. Supporting this, more patients also were found to die of infections in the haplo-HCT group in our study. Theoretically, due to a stronger GVL effect and thus a relatively lower incidence of relapse after haplo-HCT, it has the potential to improve OS by decreasing the rate of NRM. Unfortunately, the risk for GVHD and NRM was still seen to be higher in haplo-HCT than those in MSD-HCT. These two effects in haplo-HCT led to a similar survival to that in MDS-HCT. Considering the risk of GVHD, NRM, OS, and MSD-HCT was still universally held to be the standard of care.
Some cautions are needed during the interpretation of our study. First, it was a single-center retrospective study. Second, the number of patients in this study is relatively small. Third, patients who were unable to receive all-HCT were not included in this study. And a whole picture of adult T-ALL cannot be perceived in this study. Nevertheless, our study further extended that ATG-based haplo-HCT platform could achieve similar survival with that after MSD-HCT in the specific set of adult T-ALL patients. And our results also indicated that haplo-HCT may carry a low risk for cGVHD. So it is feasible and plausible to choose halpo-HCT for adult T-ALL patients who lack an MSD. Further prospective studies are urgently needed to validate our finding.
Supplemental Material
Supplemental material, sj-docx-1-cll-10.1177_09636897241270401 for Similar Survival But Less Chronic GVHD in Antithymocyte Globulin–Based Myeloablative Haploidentical Transplant Compared With Matched Sibling Transplant for Adult T-cell Acute Lymphoblastic Leukemia/Lymphoma by Zhenyang Gu, Fei Li, Meng Li, Linlin Zhang, Sai Xu, Lu Wang, Lili Wang, Yu Jing, Jian Bo, Chunji Gao, Liping Dou and Daihong Liu in Cell Transplantation
Acknowledgments
The authors thank all the faculty members who participated in this study.
Footnotes
Authors’ Note: All authors agree that this manuscript can be published in Annals of Hematology.
Author Contributions: D.L. and L.D. designed the study and initiated this work; Data were obtained by Z.G., F.L., M.L., L.W., L.Z., S.X., L.W., Y.J., J.B., and C.C. All statistical analyses were performed by Z.G., F.L., and M.L. Z.G. wrote the paper; all the authors were involved in the interpretation of the results; read, gave comments, and approved the final version of the manuscript; had full access to the data in the study; and take responsibility for the accuracy of the data analysis.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Medical Ethics Committee of PLA General Hospital reviewed and approved this study. This study was approved by the Medical Ethics Committee of PLA General Hospital (approval no. 2023-DYYXZX-0065).
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study. Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Data Availability: The data set supporting the conclusions of this article are available in the clinical data repository of the First Medical Center of Chinese PLA General Hospital, Beijing 100853, China; Tel: +86-010-66937232. The data sets are available from the corresponding author on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (grant no. 82200232).
ORCID iD: Zhenyang Gu  https://orcid.org/0000-0003-0463-5065
https://orcid.org/0000-0003-0463-5065
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ribera JM, Oriol A, Sanz MA, Tormo M, Fernández-Abellán P, del Potro E, Abella E, Bueno J, Parody R, Bastida P, Grande C, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the programa espanol de tratamiento en hematologia pediatric-based protocol ALL-96. J Clin Oncol. 2008;26:1843–49. [DOI] [PubMed] [Google Scholar]
- 2. Marks DI, Rowntree C. Management of adults with T-cell lymphoblastic leukemia. Blood. 2017;129(9):1134–42. [DOI] [PubMed] [Google Scholar]
- 3. Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, Ha CS, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547–61. [DOI] [PubMed] [Google Scholar]
- 4. Rytting ME, Thomas DA, O’Brien SM, Ravandi-Kashani F, Jabbour EJ, Franklin AR, Kadia TM, Pemmaraju N, Daver NG, Ferrajoli A, Garcia-Manero G, et al. Augmented Berlin-Frankfurt-Munster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer. 2014;120:3660–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43. [DOI] [PubMed] [Google Scholar]
- 6. Kekre N, Antin JH. Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist. Blood. 2014;124:334–43. [DOI] [PubMed] [Google Scholar]
- 7. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, Lai YR, Liu DH, Liu QF, Liu T, Ren HY, et al. The consensus from the Chinese society of hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, Gennery A, Kröger N, Kuball J, Lanza F, Montoto S, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52(6):811–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, Fan ZP, Wu DP, Huang XJ. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62. [DOI] [PubMed] [Google Scholar]
- 10. Shouval R, Fein JA, Labopin M, Kröger N, Duarte RF, Bader P, Chabannon C, Kuball J, Basak GW, Dufour C, Galimard JE, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019;6(11):e573–84. [DOI] [PubMed] [Google Scholar]
- 11. Salvatore D, Labopin M, Ruggeri A, Battipaglia G, Ghavamzadeh A, Ciceri F, Blaise D, Arcese W, Sociè G, Bourhis JH, Van Lint MT, et al. Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical versus matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2018;103(8):1317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han LJ, Wang Y, Fan ZP, Huang F, Zhou J, Fu YW, Qu H, Xuan L, Xu N, Ye JY, Bian ZL, et al. Haploidentical transplantation compared with matched sibling and unrelated donor transplantation for adults with standard-risk acute lymphoblastic leukaemia in first complete remission. Br J Haematol. 2017;179(1):120–30. [DOI] [PubMed] [Google Scholar]
- 13. Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM, Al Zahrani M, Bondarenko S, Arat M, Angelucci E, Koc Y, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. J Hematol Oncol. 2021;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cortelazzo S, Ferreri A, Hoelzer D, Ponzoni M. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2017;113:304–17. [DOI] [PubMed] [Google Scholar]
- 15. Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, Ferrando A, Fielding AK, Goldstone AH, Ketterling RP, Litzow MR, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood. 2009;114:5136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu Z, Wang L, Wang Q, Li H, Bo J, Wang S, Zhao Y, Li F, Gao C, Liu D, Huang W. Outcomes of myeloablative peripheral blood stem cell transplantation for non-complete remission patients with relapsed/refractory peripheral T cell lymphomas. Ann Hematol. 2019;98(5):1237–47. [DOI] [PubMed] [Google Scholar]
- 17. Gu Z, Xiong Q, Wang L, Wang L, Li F, Hou C, Dou L, Zhu B, Liu D. The impact of intestinal microbiota in antithymocyte globulin-based myeloablative allogeneic hematopoietic cell transplantation. Cancer. 2022;128:1402–10. [DOI] [PubMed] [Google Scholar]
- 18. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santoro N, Ruggeri A, Labopin M, Bacigalupo A, Ciceri F, Gülbaş Z, Huang H, Afanasyev B, Arcese W, Wu D, Koc Y, et al. Unmanipulated haploidentical stem cell transplantation in adults with acute lymphoblastic leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michel C, Robin M, Morisset S, Blaise D, Maertens J, Chevalier P, Castilla-Llorente C, Forcade E, Ceballos P, Yakoug-Agha I, Poire X, et al. Outcome after allogeneic stem cell transplantation with haploidentical versus HLA-matched donors in patients with higher-risk MDS. Bone Marrow Transplant. 2023;58(5):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raj K, Eikema DJ, Sheth V, Koster L, de Wreede LC, Blaise D, Di Grazia C, Koc Y, Potter V, Chevallier P, Lopez-Corral L, et al. Comparison of outcomes for HLA-matched sibling and haplo-identical donors in Myelodysplastic syndromes: report from the chronic malignancies working party of EBMT. Blood Cancer J. 2022;12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li T, He Q, Yang J, Cai Y, Huang C, Xu X, Qiu H, Niu J, Zhou K, Zhang Y, Xia X, et al. Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as an effective regimen for prophylaxis of graft versus host disease after haploidentical peripheral blood stem cell transplantation with maternal/collateral related donors. Cell Transplant. 2022;31:9636897221139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo H, Chang YJ, Hong Y, Xu LP, Wang Y, Zhang XH, Wang M, Chen H, Chen YH, Wang FR, Sun YQ, et al. Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation. Cell Mol Immunol. 2021;18(5):1172–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, Han W, Shi HX, Huang XJ. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant. 2011;17(6):821–30. [DOI] [PubMed] [Google Scholar]
- 25. Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, Xie W, Zheng W, Zhu Y, Ye X, Yu X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124:2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu S, Fan Q, Sun J, Fan Z, Zhang Y, Jiang Q, Huang F, Xuan L, Dai M, Zhou H, Liu H, et al. Haploidentical transplantation without in vitro T-cell depletion results in outcomes equivalent to those of contemporaneous matched sibling and unrelated donor transplantation for acute leukemia. Medicine (Baltimore). 2016;95(11):e2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, Wu MQ, Wu DP, Huang XJ. Haploidentical versus matched-sibling transplant in adults with Philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res. 2016;22:3467–76. [DOI] [PubMed] [Google Scholar]
- 28. Chang YJ, Wang Y, Xu LP, Zhang XH, Chen H, Chen YH, Wang FR, Wei-Han Sun YQ, Yan CH, Tang FF, et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. 2020;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li SQ, Fan QZ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, et al. Different effects of pre-transplantation measurable residual disease on outcomes according to transplant modality in patients with Philadelphia chromosome positive ALL. Front Oncol. 2020;10:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, Chen H, Liu DH, Gao ZY, Chen YH, Xu LP, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, Chen H, Chen YH, Wang FR, Wang JZ, Sun YQ, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119:978–85. [DOI] [PubMed] [Google Scholar]
- 32. Li HH, Li F, Gao CJ, Huang WR, Bo J, Dou LP, Wang LL, Jing Y, Wang L, Li WJ, Yu L, et al. Similar incidence of severe acute GVHD and less severe chronic GVHD in PBSCT from unmanipulated, haploidentical donors compared with that from matched sibling donors for patients with haematological malignancies. Br J Haematol. 2017;176(1):92–100. [DOI] [PubMed] [Google Scholar]
- 33. Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, Adorno G, Angelini S, Andreani M, De Felice L, Rapanotti MC, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121:849–57. [DOI] [PubMed] [Google Scholar]
- 34. Lai YR, Chen YH, Hu DM, Jiang M, Liu QF, Liu L, Hou J, Schwarzenberger P, Li QC, Zhang ZM, Liu KY, et al. Multicenter phase II study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG). J Hematol Oncol. 2014;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bakr M, Rasheed W, Mohamed SY, Al-Mohareb F, Chaudhri N, Al-Sharif F, Al-Zahrani H, Al-Dawsari G, Saleh AJ, Nassar A, Ahmed S, et al. Allogeneic hematopoietic stem cell transplantation in adolescent and adult patients with high-risk T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2012;18(12):1897–904. [DOI] [PubMed] [Google Scholar]
- 36. Hamilton BK, Rybicki L, Abounader D, Adekola K, Advani A, Aldoss I, Bachanova V, Bashey A, Brown S, DeLima M, Devine S, et al. Allogeneic hematopoietic cell transplantation for adult T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2017;23:1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bazarbachi A, Labopin M, Angelucci E, Gülbas Z, Ozdogu H, Arat M, de Rosa L, Pastano R, Pioltelli P, Montserrat R, Martino M, et al. Haploidentical transplantation with post-transplantation cyclophosphamide for T cell acute lymphoblastic leukemia: a report from the European society for blood and marrow transplantation acute leukemia working party. Biol Blood Marrow Transplant. 2020;26(5):936–42. [DOI] [PubMed] [Google Scholar]
- 38. Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, Nagler A, Di Bartolomeo P, Lacerda JF, Lupo Stanghellini MT, Polge E, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112:3574–81. [DOI] [PubMed] [Google Scholar]
- 39. Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, Han W, Wang Y, Qin YZ, Huang XJ. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:3256–62. [DOI] [PubMed] [Google Scholar]
- 40. Owattanapanich W, Leelakanok N, Sanpakit K, Buaboonnam J. A comparison of the clinical outcomes of haploidentical transplantation and other graft sources in acute lymphoblastic leukemia: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2022;22(3):174–91. [DOI] [PubMed] [Google Scholar]
- 41. Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, Wang SA, Konopleva M, Fernandez-Vina M, Montes N, Bosque D, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Battipaglia G, Boumendil A, Labopin M, Ciceri F, Tischer J, Stelljes M, Ehninger G, Beelen D, Finke J, Van Lint MT, Eder M, et al. Unmanipulated haploidentical versus HLA-matched sibling allogeneic hematopoietic stem cell transplantation in relapsed/refractory acute myeloid leukemia: a retrospective study on behalf of the ALWP of the EBMT. Bone Marrow Transplant. 2019;54(9):1499–510. [DOI] [PubMed] [Google Scholar]
- 43. Robinson TM, Fuchs EJ, Zhang MJ, St Martin A, Labopin M, Keesler DA, Blaise D, Bashey A, Bourhis JH, Ciceri F, Ciurea SO, et al. Related donor transplants: has posttransplantation cyclophosphamide nullified the detrimental effect of HLA mismatch? Blood Adv. 2018;2:1180–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cll-10.1177_09636897241270401 for Similar Survival But Less Chronic GVHD in Antithymocyte Globulin–Based Myeloablative Haploidentical Transplant Compared With Matched Sibling Transplant for Adult T-cell Acute Lymphoblastic Leukemia/Lymphoma by Zhenyang Gu, Fei Li, Meng Li, Linlin Zhang, Sai Xu, Lu Wang, Lili Wang, Yu Jing, Jian Bo, Chunji Gao, Liping Dou and Daihong Liu in Cell Transplantation