Abstract
Background
To establish and validate the criterion-referenced standards of functional fitness in predicting physical independence in 80 + years.
Methods
A group of 2,749 older community dwellers (60–84 years) were recruited, and 2,050 were identified with moderate-to-high independent living ability according to the proposed minimum composite physical function score. The Senior Fitness Test battery was applied to measure functional fitness at five-year intervals. The declining rate for each fitness dimension was calculated based on the differences between any two adjacent age groups and was adjusted according to the reported degradation rate differences between the cross-sectional and longitudinal studies.
Results
The age-and-sex-specific criterion-referenced standards were identified for muscle strength, cardiovascular endurance, and dynamic balance that older adults should possess at 60–79 to maintain independent living abilities. Moderate to high consistency (k = 0.622–0.650) and associations (φ = 0.641–0.694) were found between the predicted physical independence by criterion-referenced standards of functional fitness and the results from the composite physical function scale. Moreover, the predicted independent living abilities in later years from the criterion-referenced standards of functional fitness showed high test-retest reliability (Pa = 0.90–0.96).
Conclusion
The criterion-referenced standards for functional fitness are valid and reliable to predict independent living abilities in later years, and provide the threshold to identify the limitations in physical fitness and detect the risks of functional disabilities among older adults in an early stage.
Keywords: Functional fitness, Independence, Older adult, Validity, Reliability
Background
The global population is ageing rapidly, resulting in unprecedented set of challenges to society, families, and individuals [1]. In line with the increasing older population, the number of disabled older adults is continuously increasing [2]. Functional disability is a critical risk factor for healthy ageing, inhibiting people from living independently as they wish [3]. As the mean life expectancy increases, whether older adults, especially old older adults, can enjoy an independent and high-quality life is heavily dependent on their ability to perform daily activities independently, healthily, and actively [4]. Functional fitness (FF) was then proposed and referred explicitly to the physical capability of conducting daily activities safely and independently without extreme fatigue [5]. Generally, the FF covers five physical components: aerobic endurance, muscle strength and endurance, flexibility, dynamic balance, and body composition [5]. Numerous studies have acknowledged the value of FF in evaluating physical health and predicting life quality in later years [6].
To date, different regions and countries have established FF norms for evaluating physical fitness and age-matched individual or group comparisons [6–8]. A potential problem in normative data would be the loss of information indicating any specific clinical symptoms, resulting in limited guidance for clinical practice [9]. However, the criterion-referenced standards (CRS) are established with a clear orientation and can provide concrete and targeted evaluation information for determining whether a specific state or behaviour is met [10]. Furthermore, the essence of the FF concept depicts and expresses the essential physical capacity older adults have to engage in daily activities safely and independently. A question raised hereby is the minimum FF level that older adults should maintain to ensure an independent life, especially in their later years. To be specific, young-old and middle-old adults must demonstrate a certain FF level to cope with age-related physical degenerations and ensure independent living abilities when they reach old-old age.
The FF CRS have been established in America, Portugal, and Chile, and significant differences were found in their established FF CRS. Given the potential influence of cultural diversities on physical capacities between Westerners and Easterners [11], it is warranted to understand the minimum FF levels that older Easterners should maintain to ensure an independent life in later years. Therefore, the purpose of this study was to establish the CRS of FF for older Chinese adults of different ages to help maintain independent lives at 80 + years. The results of this study would help in a comprehensive understanding towards the FF CRS of different regions and would be beneficial for identifying older adults with a high risk of functional decline.
Methods
Study design
By taking the cross-sectional design, this study applied a typical three-step procedure to establish the CRS of FF for older adults of different age groups, including (1) identification of independent living abilities, (2) establishment of the cut-off value for each FF dimension, and (3) the verification of the reliability and validity of the FF CRS [10].
Participants
Two rounds of participant recruitment were conducted in 2019, one (n = 2,549) for the establishment of FF CRS and the other (n = 200) for the validation of FF CRS. They were reached through flyers and activities in urban community senior centres in Nanjing, China. Nanjing is a well-known historic city located in eastern China. It is the capital of Jiangsu province and one of China’s fifteen sub-provincial cities. Unlike the most commonly studied super-cities such as Shanghai, Beijing and Guangzhou, Nanjing is a well-representative city of China with the typical characteristics associated with the rapidly ageing population and urbanizing areas. By the end of 2021, the permanent population of Nanjing was about 9.49 million, and nearly 87% were urban populations. Individuals aged 60 and above were 1.83 million, accounting for 19.36% of the whole population.
Older adults aged between 60 and 84 years were recruited from 108 senior service community centres in Nanjing’s six districts (18 centres in each district). Those who needed walking assistance, had cognitive impairment (Chinese Mini-mental State Examination score < 24) or any physical problems (e.g., dizziness, congestive heart failure, and uncontrolled hypertension) advised by clinical doctors that may prohibit them from participating in exercise or fitness tests were excluded from this study [12]. Participants who cannot read or write were provided with one-by-one verbal guidance.
Measurements
Independent living ability
The independent living ability is embodied in daily activities, including but not limited to shopping, cooking, dressing, climbing stairs, lifting heavy objects, and showering. The composite physical function (CPF) scale was applied to assess the level of independent living abilities [13]. The CPF scale consists of 12 items, each scoring from 0 to 2. Participants who can complete the task independently score 2, those who require assistance score 1, and those who cannot score 0. Participants who can complete all 12 items (score 24) indicate a favourable ability to live independently, those who can complete at least seven items (score = 14 ~ 23) would have a moderate ability to live independently, and those who cannot complete seven items (< 14) indicate lack of independent living ability.
Given the side effects of ageing-related decline in physical fitness, independent living ability would decrease with advancing age. To ensure that older adults aged 80 + can complete at least seven items (i.e., moderate ability to live independently), older adults aged 60–79 years must have the ability to complete more than seven items. Thus, the cut-off score of 14 on the CPF scale does not apply to young and middle-aged older adults. According to the related studies, the independent-living ability-related physical function declines by approximately 10 -15% per decade among older adults [14, 15]. Therefore, the CPF score for older adults aged 70–79 years to be able to live independently is (14–24) × [1+ (10–15%)] = 16–24; in other words, they must be able to complete at least eight CPF items independently. Likewise, the CPF score for older adults aged 60–69 is 18–24; that is, they must be able to complete at least nine CPF items independently.
Functional fitness
The FF was tested using the Senior Fitness Test battery (SFT), including cardiovascular endurance, muscle strength, flexibility, dynamic balance, and body mass index (BMI) [16]. Since there is no evidence suggesting the direct effects of flexibility and BMI on the capability to live independently [17–19], this study only incorporated the 30s arm curl (AC) test (reps.) and the 30s chair stand (CS) test (reps.) to measure muscle strength of the upper and lower extremities, the 8ft up-and-go (UG) test (s) for dynamic balance, and the 2-min step (Step) test (reps.) for cardiovascular endurance. Each test was conducted in strict adherence to the SFT manual [16].
It should be noted that the degeneration rate derived from cross-sectional research might differ from that of longitudinal research. Results from cross-sectional studies often underestimate the speed at which physical functions decline [20]. The present study consisted of participants from the 1930s to 1950s. In this case, participants were born in different eras and experienced different socio-economic development, which might have caused the declining fitness rate inconsistencies. Hughes et al. [21] compared the results from cross-sectional and longitudinal studies in a group of participants aged 46–78 years and discovered that the actual rate of leg muscle strength decline was 60% more than that estimated using cross-sectional data. Hollenberg et al. [22] conducted a longitudinal study on people aged 53–96 years to follow the changes in maximum oxygen consumption rate (VO2max) in 6 years and found the decline rate was 1.7 times that of the cross-sectional study results. Rikli (2012) [15] summarized the related study results and suggested that the actual rate of physical function decline was approximately 1.25 times the estimation from cross-sectional studies across people of different ages. Moreover, this rate did not differ between people of varying physical functions or sexes [19].
Test protocol
All participants shall complete a series of questionnaires at their first arrival, including demographic surveys and assessments for independent living abilities. Participants with moderate-to-high independent living abilities were requested to attend formal tests, wear proper clothes and shoes, and be in good physical condition without discomfort or fatigue. The resting blood pressure and heart rate were recorded after 10 min of seated rest. Afterwards, each participant shall finish a warm-up, including 3 to 5 min of stretching. There were no special arrangements on the test order, except that the aerobic endurance test was arranged at the end to avoid any potential physical fatigue to other test items. Participants can stop or end the test at any time during the test process. After tests, participants were suggested to stay until the blood pressure and heart rate returned to the pre-test level.
Data analysis
Descriptive statistics were used to summarize the characteristics of participants and the performance in each FF parameter and CPF scores. The validity of FF CRS means that the predicted living condition (i.e., possess moderate-to-high abilities or not) based on FF CRS is consistent with the results of the CPF scale. The Cohen’s Kappa (k) was adopted to test the consistency between the predicted and actually measured data of living ability (dependence or independence). Additionally, the Phi coefficient (φ) test was conducted to examine the correlation coefficients of the results from the two evaluation methods. Regarding the reliability test, the same group of participants performed the FF test again after seven days, and the FF CRS were employed to assess their ability to live independently. When the results of the two times tests were identical (i.e., they both showed that the participants possessed a moderate-to-high ability or could not live independently), it signified a high probability of test-retest agreement (Pa). A two-sided p < 0.05 was considered significant. All the statistical analyses were performed using SPSS 26.0 software.
Results
Participant flow
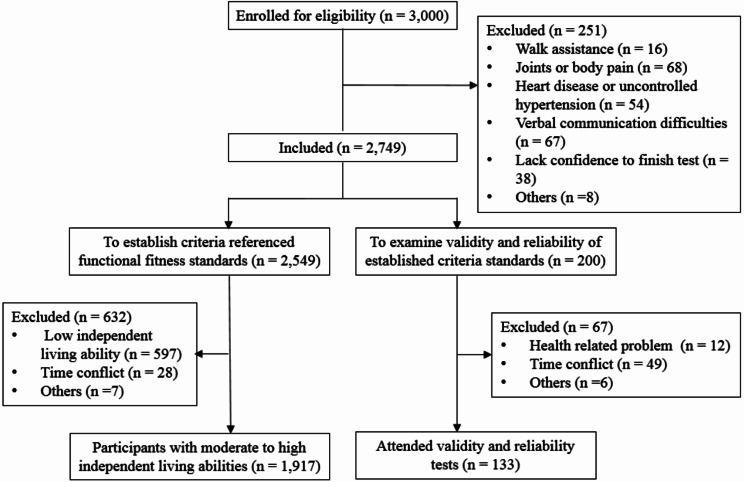
A total of 3,000 participants were reached at the beginning of this study, and 251 were excluded mainly because of physical problems. Among the 2,749 qualified participants, 2,549 attended fitness tests and independent living ability assessments, and the FF CRS was established among 1,917 participants who were identified with moderate to high independent living abilities. A group of 200 participants from the 2,749 sample pool was kept to examine the validity and reliability of the established FF CRS. Since validity and reliability tests were arranged at the end of data collection, 67 participants had to withdraw from this study due to the changes in health conditions and time conflicts. Finally, 133 participants attended the validity and reliability tests of the FF CRS (Fig. 1).
Fig. 1.
Participants flow
Demographic characteristics of participants with moderate-to-high independent living ability
According to the proposed minimum CPF score for each age group, 1,917 were identified with moderate-to-high independent living ability. They were then divided into five age groups (AG): AG1 = 60–64 years, AG2 = 65–69 years, AG3 = 70–74 years, AG4 = 75–79 years, and AG5 = 80–84 years. 86.2% exercised at least five days a week for at least 30 min a day. The education level of older men was higher than that of older women (Table 1).
Table 1.
Demographic information of participants
| Items | All (n = 1,917) | Men (n = 892) | Women (n = 1,025) |
|---|---|---|---|
| Height (cm) | 159.0 (8.79) | 166.0 (6.63) | 153.3 (5.98) |
| Weight (Kg) | 62.5 (11.0) | 68.0 (10.1) | 57.7 (9.44) |
| Body Mass Index (kg/m2) | 24.6 (3.32) | 24.7 (3.05) | 24.5 (3.54) |
| Systolic blood pressure (mmHg) | 137.6 (17.3) | 138.8 (18.2) | 136.9 (16.5) |
| Diastolic blood pressure (mmHg) | 76.1 (10.8) | 78.6 (11.6) | 73.9 (9.55) |
| Resting heart rate (b/min) | 74.2 (11.2) | 74.4 (11.3) | 74.0 (10.1) |
| a Active lifestyles | 1653 (86.2%) | 780 (87.5%) | 873 (85.3%) |
| Monthly fixed income | 1539 (80.3%) | 797 (89.4%) | 742 (72.4%) |
| High school and above experience | 613 (32.0%) | 376 (42.2%) | 237 (23.1%) |
| Primary or middle school experience | 932 (48.6%) | 467 (52.4%) | 465 (45.4%) |
| No education experience | 372 (19.4%) | 49 (5.50%) | 323 (31.5%) |
| Need assistance | 744 (38.8%) | 302 (33.9%) | 442 (43.1%) |
| b Falling history | 520 (27.1%) | 175 (19.6%) | 345 (33.7%) |
Number(Standardized Deviation) or Number (Percentage)
a three times per week and over 30 min per day
b Falling history in the past three years
Establishing the age-and-sex-specific CRS for each fitness dimension
As shown in Table 2, the performance in each FF test decreased with advancing age. The 20-year mean degeneration rate of FF in this cross-sectional study was 29.8% for men and 28.0% for women. As mentioned above, the longitudinal fitness data declined approximately 1.25 times greater than those measured cross-sectionally. The FF values of older adults aged 60–64 were then adjusted by increasing the decline rate by 1.25 times based on the measured fitness data of older adults aged 80–84. For example, the older men aged 80–84 had a mean of 13.6 reps. in the 30-s AC test, given a 20-year degeneration rate of 40.6%, the AC test of male older adults aged 60–64 is 13.6 / (1 − 1.25 × 40.6%) = 27.6 reps.
Table 2.
Gender-and-age specific functional fitness scores
| Tests | Age (Men/Women) | #Decline% | ||||
|---|---|---|---|---|---|---|
| 60–64 years (198/240) |
65–69 years (198/275) |
70–74 years (184/216) |
75–79 years (174/168) |
80–84 years (138/126) |
||
| 30-s Arm curl test (reps.) | ||||||
| Men | 22.9(4.60) | 18.2(5.69) | 17.4(6.26) | 16.0(5.46) | 13.6(4.63) | 40.6 |
| Women | 20.4(3.95) | 16.9(5.34) | 16.0(4.82) | 15.2(4.82) | 13.6(5.01) | 33.3 |
| 30-s Chair stand test (reps.) | ||||||
| Men | 16.9(3.76) | 17.2(4.54) | 15.7(3.85) | 14.9(3.99) | 12.7(3.52) | 24.9 |
| Women | 17.1(3.40) | 16.2(4.20) | 16.1(3.89) | 14.4(4.11) | 13.0(3.67) | 24.0 |
| 8-ft up-and-go test (s) | ||||||
| Men | 5.53(1.14) | 5.53(1.53) | 5.87(1.44) | 6.50(1.70) | 7.75(2.40) | 28.7 |
| Women | 5.24(1.14) | 5.91(1.44) | 6.19(1.45) | 7.07(1.87) | 8.22(2.33) | 36.3 |
| 2-min Step test (reps.) | ||||||
| Men | 104.3(16.8) | 96.4(18.8) | 93.7(17.1) | 86.6(18.6) | 78.4(20.0) | 24.8 |
| Women | 95.9(15.9) | 93.8(19.7) | 93.7(18.4) | 86.3(19.0) | 78.4(21.1) | 18.3 |
Data presented as means (standard deviations)
#The absolute difference between the 60–64 years group and the 80–84 years group divided by the value of the 60–64 years group (or the 80–84 years group for the 8-ft up-and-go test)
After clarifying the CRS of each FF dimension for AG1 and AG5, the decline rate of each FF dimension of the other three age groups (i.e., AG2, AG3, and AG4) should take the consideration of age and sex effects. According to empirical studies, the decline rate with advancing age shall be increased [23, 24] and the decline rate of men in each physical fitness dimension has been significantly higher than those of women of the same age group [17, 20, 22, 25]. Therefore, this study made suitable adjustments for the decline rates of each FF dimension for AG2, AG3, and AG4 by approximately 1.25 times the decline rate for each FF dimension. Consequently, all data were then rounded down or up to the nearest integer (except for UG test keep one decimal place) to facilitate data interpretation and application (Table 3).
Table 3.
Criterion-referenced functional fitness standards in predicting physical independence in later years
| Tests | 60–64 years | 65–69 years | 70–74 years | 75–79 years | 80–84 years | #Decline% |
|---|---|---|---|---|---|---|
| 30-s Arm curl test (reps.) | ||||||
| Men | 28 | 22 | 19 | 17 | 14 | 50.0 |
| Women | 23 | 20 | 18 | 17 | 14 | 39.1 |
| 30-s Chair stand test (reps.) | ||||||
| Men | 19 | 18 | 16 | 15 | 13 | 31.6 |
| Women | 18 | 17 | 16 | 14 | 13 | 27.8 |
| 8-ft up-and-go test (s) | ||||||
| Men | 5.0 | 5.6 | 6.0 | 6.8 | 7.8 | 37.5 |
| Women | 4.5 | 5.7 | 6.2 | 7.2 | 8.2 | 43.8 |
| 2-min Step test (reps.) | ||||||
| Men | 114 | 104 | 98 | 90 | 78 | 31.6 |
| Women | 102 | 98 | 97 | 88 | 78 | 23.5 |
#The absolute difference between the 60–64 years group and the 80–84 years group divided by the value of the 60–64 years group (or the 80–84 years group for the 8-ft up-and-go test)
Validity and reliability assessment
To assess the validity and reliability of the established FF CRS, a group of 133 participants (men = 31; mean age = 71.8 ± 7.15 years) received the SFT for assessing the FF level and the CPF scale for independent living ability. As shown in Table 4, all k values were over 0.6 (k = 0.622–0.650), indicating that the independent living abilities estimated based on FF CRS were highly consistent with those tested from the CPF scale [26]. Also, results revealed high correlations (φ = 0.641–0.694) between the inferences from the FF CRS and the CPF scores [27]. Additionally, a high probability of agreement (Pa = 0.90–0.96) was found in the independent living abilities as reflected by the FF CRS at two times FF tests with a 7-day interval, indicating that the CRS for each FF dimension had high test-retest reliability.
Table 4.
Validity and reliability of criterion-referenced functional fitness standards
| Tests | Cohen’s Kappa (k) | Phi coefficient (φ) | Test-retest reliability (Pa) | Classification accuracy |
|---|---|---|---|---|
| 30-s Arm curl test (reps.) | 0.622 | 0.672 | 0.91 | 80.5% |
| 30-s Chair stand test (reps.) | 0.650 | 0.694 | 0.95 | 82.0% |
| 8-ft up-and-go test (s) | 0.622 | 0.672 | 0.90 | 80.5% |
| 2-min Step test (reps.)a | 0.630 | 0.641 | 0.96 | 81.6% |
a114 participants completed the test
Discussion
Results from the present study confirmed the ageing-related degradations in each FF dimension, and the 20-year mean degeneration rate of fitness (from 60 to 64 to 80–84 years) was about 28.9%. Given the well-evidenced differences in fitness declining rate between cross-sectional and longitudinal data, this study integrated empirical evidence and inductive reasoning to adjust the degeneration rate for each age group. This study finally clarifies and refines the required physical capacity to ensure older adults of different age groups can live independently in later years. The present results would be of great value in identifying older adults at risk of physical dysfunction and provide the cornerstone for extending independent lifespans. Based upon the present cut-off values for each FF dimension, it is also easy to identify physical shortages in specific fitness dimensions and improve clinical prevention strategies.
Overall, the FF CRS established among older Chinese adults was relatively higher than that in older Americans [15]. The differences in independent living abilities of included participants can mainly explain this. The 2,140 American participants had moderate abilities to live independently, and the 1,917 participants in this study possessed moderate-to-high independent living abilities. Given the close relationships between physical fitness and living abilities, a higher fitness performance shall be expected in the present study [28, 29]. Also, approximately 86% of the present participants had regular outdoor exercise habits (e.g., walking, Taichi, and stretching) and could be more physically active. With the improvements in living standards and increases in life expectancy, a higher FF CRS would be preferred to encourage older adults to maintain an active lifestyle and retain sufficient physical capabilities to have a high (rather than only moderate) level of living independence.
Reviewing the related studies, the FF CRS established in Portugal and Chile were lower than those in the present study [30, 31]. Except for the variations in demographic characteristics of included participants, study and statistical methods applied to deduce FF CRS may help to explain the CRS differences. To date, three commonly employed methods for developing fitness CRS were inferences based on expert experiences or norm data, comparisons and classifications based on different groups (such as the receiver-operating characteristic [ROC] curves method), and inferences based on the validity criteria and empirical data [15, 30, 32]. The first method is not often used in recent studies since it is mainly based on expert experiences, and huge variations exist in study results given the high subjective attributes. The second method (i.e., ROC curve) is relatively objective and mainly applied when a dataset is big enough. The studies conducted in Chile and Portugal established the FF CRS using the ROC method, but they found that the areas under the curve of the FF CRS were between 0.7 and 0.8, and the sensitivities and specificities of the CRS for different FF dimensions varied considerably (53–86%) [30, 31]. Therefore, the reliability and validity of the FF CRS that they established using the ROC method merit further investigations. Additionally, studies applying the ROC method mainly depend on one-time data collection without considering that the cross-sectional fitness declining rate is lower than those from longitudinal follow-ups, resulting in a high risk of underestimation.
By taking the third method, the present study adjusted the FF declining rate according to the difference reported in cross-sectional and longitudinal studies, clarified the criterion tool (i.e., CPF scale) for living abilities initially and adjusted its cut-off scores for different age groups. One thing should put forward here is that the present study made suitable adjustments for the decline rates of each FF dimension for AG2, AG3, and AG4 by approximately 1.25 times the decline rate for each FF dimension by considering age and sex effects. It has been well evidenced that the rate of physical function decline differs between age groups. Take the VO2max as an example. When people reach 30 years old, their VO2max starts to decline. The 10-year rate of decline is approximately 3–6%. After they reach 70 years, the 10-year rate of decline exceeds 20% [23]. The decline rate of muscle strength among people aged 50–60 years is 1.5% per year and 3% per year thereafter [26]. Additionally, the decline rates of men and women differed in each fitness dimension. According to empirical studies, the decline rate of men in each physical fitness dimension has been significantly higher than those of women of the same age group [17, 20, 22, 25]. All these considerations were applied when setting the cut-off values for AG2, AG3, and AG4. To an extent, the method applied in the present study does compensate for the design shortcomings of a cross-sectional study, and the results shall be more clinically convincing.
In establishing the FF CRS, this study considered age 80 or older as old or later years, while some studies considered old as 90 + years [15, 30]. The span of the later years would have tremendous effects on the established FF CRS since it provides the target and initial points for FF CRS calculation. Although the average life expectancy of human beings has increased a lot because of advanced medical technology and increased material living standards, the average lifespan in China was 77.3 years by the end of 2020, while healthy life expectancy was only 69 years [32]. Most Chinese older adults were found to be functionally disabled or partially disabled for 3 to 5 years before death [33]. Based on the average life expectancy and the functional disability characteristics of the older Chinese population, it is preferred to set 80 + years as the target age segment for defining later years in the present study. Future studies are suggested to clarify the potential differences by setting different target age segments on the FF CRS of 60–79 years Chinese adults.
This study has several limitations that should be fully considered in its application. First, all the participants were recruited from local community senior centres and reported regular outdoor activity behaviour. Therefore, it warrants further caution when applying the results to those community dwellers who prefer or have to stay home. Second, the fitness degradation rates were estimated from the related studies, and the present FF CRS may not be suitable to give absolute predictions towards independent living abilities in later years. Last, although previous studies have indicated that degradation rates from longitudinal studies would be 1.25 times the degradation rate from cross-sectional studies, variances would exist in different FF dimensions. Future studies are recommended to explore the detailed differences in degradation rates of different fitness dimensions between cross-sectional and longitudinal studies.
Conclusion
This study applied the SFT to assess the FF performance and established the CRS for muscle strength, cardiovascular endurance, and dynamic balance that older adults should possess at 60–79 years to live independently at 80 or older. The CRS for each FF dimension demonstrated acceptable reliability and validity.
Acknowledgements
This study would sincerely thank all participants and staff from the local communities and student helpers for helping with data collection.
Author contributions
YZ: Conceptualization, Investigation, Data curation, Writing - original draft, Supervision, Funding acquisition, Writing - review & editing. PC & YG: Participants recruitment, Investigation, Data curation. ZG: Conceptualization, Data curation, editing. WZ: Participants recruitment and screening, Test implementation.
Funding
This work was supported by the Humanities and Social Science Foundation of Ministry of Education of China (No.23YJAZH213) and National Natural Science Foundation of China (No. 81801387).
Data availability
Data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All participants provided written informed consent before the formal tests. This study was conducted following the Declaration of Helsinki and relevant clinical research regulations and has received approval from the Ethical Committee of Nanjing Normal University (Number: 202012026).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bloom DE, Canning D, Lubet A. Global population aging: facts, challenges, solutions & perspectives. Daedalus. 2015;144(2):80–92. 10.1162/DAED_a_00332 [DOI] [Google Scholar]
- 2.Ansah JP, Chiu CT, Wei-Yan AC, Min TLS, Matchar DB. Trends in functional disability and cognitive impairment among the older adult in China up to 2060: estimates from a dynamic multi-state population model. BMC Geriatr. 2021;21(1):1–10. 10.1186/s12877-021-02309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tchalla A, Laubarie-Mouret C, Cardinaud N, Gayot C, Rebiere M, Dumoitier N, et al. Risk factors of frailty and functional disability in community-dwelling older adults: a cross-sectional analysis of the FREEDOM-LNA cohort study. BMC Geriatr. 2022;22(1):1–9. 10.1186/s12877-022-03447-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halaweh H, Dahlin-Ivanoff S, Svantesson U, Willén C. Perspectives of older adults on aging well: a focus group study. J Aging Res. 2018;7(2):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129–61. 10.1123/japa.7.2.129 [DOI] [Google Scholar]
- 6.Chung PK, Zhao YN, Liu JD, Quach B. Functional fitness norms for community-dwelling older adults in Hong Kong. Arch Gerontol Geriatr. 2016;65:54–62. 10.1016/j.archger.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Chen HT, Lin CH, Yu LH. Normative physical fitness scores for community-dwelling older adults. J Nurs Res. 2009;17(1):30–41. 10.1097/JNR.0b013e3181999d4c [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Wang Z, Chung PK, et al. Functional fitness norms and trends of community-dwelling older adults in urban China. Sci Rep. 2021;11:17745. 10.1038/s41598-021-97320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu W, Mahar MT, Welk GJ, Going SB, Cureton KJ. Approaches for development of criterion-referenced standards in health-related youth fitness tests. Am J Prev Med. 2011;41(4):68–76. 10.1016/j.amepre.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Cureton KJ, Warren GL. Criterion-referenced standards for youth health-related fitness tests: a tutorial. Res Q Exerc Sport. 1990;61(1):7–19. 10.1080/02701367.1990.10607473 [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay CC, Henze R, Moses YT. How real is race? A sourcebook on race, culture, and biology. Rowman & Littlefield; 2013.
- 12.Tan TK, Feng Q. Validity and reliability of Mini-mental State examination in older adults in China: Inline Mini-mental State examination with cognitive functions. Int J Popul Stud. 2022;8(1):1–16. 10.18063/ijps.v8i1.1285 [DOI] [Google Scholar]
- 13.Rikli RE, Jones CJ. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act. 1998;6(4):363–75. 10.1123/japa.6.4.363 [DOI] [Google Scholar]
- 14.Paterson DH, Jones GR, Rice CL. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Appl Physiol Nutr Metab. 2007;32(S2):69–108. 10.1139/H07-111 [DOI] [PubMed] [Google Scholar]
- 15.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2012;53(2):255–67. 10.1093/geront/gns071 [DOI] [PubMed] [Google Scholar]
- 16.Rikli RE, Jones CJ. Senior Fitness Test Manual. England: Human Kinetics; 2013. [Google Scholar]
- 17.Stathokostas L, Little R, Vandervoort A et al. Flexibility training and functional ability in older adults: a systematic review. J Aging Res. 2012: 306818. [DOI] [PMC free article] [PubMed]
- 18.Borda MG, Venegas-Sanabria LC, Garcia-Cifuentes E, Gomez RC, Cano-Gutierrez CA, Tovar-Rios DA, Soennesyn H. Body mass index, performance on activities of daily living and cognition: analysis in two different populations. BMC Geriatr. 2021;21:1–11. 10.1186/s12877-021-02127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simoes EJ, Kobau R, Kapp J, Waterman B, Mokdad A, Anderson L. Associations of physical activity and body mass index with activities of daily living in older adults. J Community Health. 2006;31:453–67. 10.1007/s10900-006-9024-6 [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers J, Hebert R, Bravo G, Rochette A. Comparison of cross-sectional and longitudinal designs in the study of aging of upper extremity performance. J Gerontol Biol Sci Med Sci. 1998;53(5):362–8. 10.1093/gerona/53A.5.B362 [DOI] [PubMed] [Google Scholar]
- 21.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol Biol Sci Med Sci. 2001;56(5):209–17. 10.1093/gerona/56.5.B209 [DOI] [PubMed] [Google Scholar]
- 22.Hollenberg M, Yang J, Haight TJ, Tager IB. Longitudinal changes in aerobic capacity: implications for concepts of aging. J Gerontol Biol Sci Med Sci. 2006;61(8):851–8. 10.1093/gerona/61.8.851 [DOI] [PubMed] [Google Scholar]
- 23.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–82. 10.1161/CIRCULATIONAHA.105.545459 [DOI] [PubMed] [Google Scholar]
- 24.von Haehling S, Morley JE, Anker SD. An overview of Sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33. 10.1007/s13539-010-0014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol Biol Sci Med Sci. 2006;61(10):1059–64. 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 26.Mchugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–82. 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–3. 10.1016/j.tjem.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology. 2016;17:567–80. 10.1007/s10522-016-9641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oppewal A, Hilgenkamp TI, van Wijck R, Schoufour JD, Evenhuis HM. Physical fitness is predictive for a decline in the ability to perform instrumental activities of daily living in older adults with intellectual disabilities: results of the HA-ID study. Res Dev Disabil. 2015;41:76–85. 10.1016/j.ridd.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 30.Merellano-Navarro E, Collado-Mateo D, García-Rubio J, Gusi N, Olivares PR. Criterion-referenced fitness standards associated with maintaining functional capacity in Chilean older adults. Rejuvenation Res. 2017;20(6):484–91. 10.1089/rej.2016.1913 [DOI] [PubMed] [Google Scholar]
- 31.Sardinha LB, Santos DA, Marques EA, Mota J. Criterion-referenced fitness standards for predicting physical independence into later life. Exp Gerontol. 2015;61:142–6. 10.1016/j.exger.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. The global health observatory. http://www.who.int/date/gho/date/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy. [2022-02-22].
- 33.Liu Z, Han L, Wang X, Feng Q, Gill TM. Disability prior to death among the oldest-old in China. J Gerontol Biol Sci Med Sci. 2018;73(12):1701–7. 10.1093/gerona/gly010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.