Abstract
Background
The pathogenic causes of primary gout include urate overproduction and/or renal or extra-renal urate underexcretion. The aim of this study was to evaluate the association of gout subtypes with the response to low-purine diet (LPD).
Methods
This is a single-center prospective clinical study. Gout patients visiting from 2019 to 2022, from Shandong Gout Clinic Center at the Affiliated Hospital of Qingdao University, China, assigned to three groups according to clinical subtypes, were enrolled and all treated with 2-week low-purine diet. General characteristics, serum uric acid (sUA) and other clinical biochemical variables before and after the diet were evaluated.
Results
A total of 626 gout patients (age 41.20 ± 13.41 years, male 98.0%) were included. Of these, 69 (11.0%) were overproduction type, 428 (68.37%) were underexcretion type, and 129 (20.61%) were combined type. Overall, there was a substantial decrease in sUA after a 2-week LPD (p < 0.001). In addition, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), serum triglycerides (TG), serum total cholesterol (TC), blood urea nitrogen (BUN) and serum creatinine (Scr) levels were lower than those at baseline (p < 0.05). On the other hand, there were significant differences in the reduction of sUA among different types, the rank order being overproduction type (− 88.81 ± 63.01 μmol/L) > combined type (− 65.22 ± 44.13 μmol/L) > underexcretion type (− 57.32 ± 61.19 μmol/L). After adjusting for age, BMI and baseline sUA and eGFR, there were still significant differences in the decline of serum uric acid among different types. Higher baseline sUA (95%CI − 0.285, − 0.191; p < 0.001) and BUN (95%CI − 6.751, − 0.602; p < 0.001) were correlated with greater decrease of sUA.
Conclusions
Our findings support the protective role of low-purine diet on sUA levels in gout patients, especially overproduction type. Furthermore, LPD could exert a beneficial effect on gout patients’ blood pressure, BMI, blood lipid, BUN and Scr levels.
Trial registration Registered with ChiCTR, No. ChiCTR1900022981 at 06/05/2019.
Keywords: Low-purine diet, Gout, Uric acid, Clinical subtypes
Introduction
Gout is a heterogeneous disease caused by deposition of monosodium urate crystals, characterized as hyperuricemia (HUA), recurrent acute arthritis, gouty tophus sedimentation, chronic arthritis and joint deformity [1]. Many evidences showed that hyperuricemia and gout are independently risk factors for chronic kidney disease, hypertension, cardiovascular and cerebrovascular diseases and diabetes [2]. Recently, due to the change of living condition and peoples’ food structure, especially with the increasing consumption of high-purine food, such as seafood, broth and animal viscera, the prevalence of hyperuricemia and gout is significantly increasing [3–5]. Several European and North American studies reported gout prevalence in the range of 1.9 and 3.9% [4], and prevalence of HUA and gout in China was 13.3% and 1.1%, respectively [6].
It is commonly accepted that two-thirds of the urate is excreted from the kidney into urine via the ‘renal excretion’ pathway, and the remaining one-third via the ‘extra-renal excretion’ pathway [7]. Taking extra-renal urate excretion into account, the current classification of hyperuricemia and gout is based on patients’ urinary urate excretion (UUE) and fractional excretion of urate clearance (urate clearance/creatinine clearance ratio, FEUA). Previous studies have shown that the type of hyperuricemia classification might affect the curative effect of urate-lowering therapy [8–10]. Our latest study also indicated for patients of renal uric acid underexcretion type, benzbromarone had superior urate-lowering compared to Febuxostat [11].
Gout is related to heredity, environment, race, dietary and lifestyle factors. Long-established dietary risk factors, including meat, seafood, alcohol, sweet food, fructose beverage, is known to play an important role in HUA and gout [1, 12–15]. Diet adjustment has become a supplement to drug treatment of gout patients. It was reported that simple dietary regimens may be beneficial to gout patients [16]. A cohort study convincingly demonstrated that vegetarians, who avoiding purine-rich meat and seafood, while consuming more vegetables, whole grains, seeds and nuts [17, 18], experienced a lower risk of gout (without adjustment for hyperuricemia: HR: 0.33; 95%CI 0.14, 0.79; with adjustment for hyperuricemia: HR: 0.40; 95%CI 0.17, 0.97) [19]. Previous investigations indicate that sUA level of HUA patients decreased by 60–120 μmol/L on average after the intervention of purine-free diet for 7–10 days [20]. So far, it was unclear that effect of low-purine loading test on sUA of gout patients based on different clinical types. Thus, the present study aims to evaluate the effect of low-purine diet on sUA and other biochemical indexes levels of gout patients in different clinical types and provide evidence-based solutions for doctors and patients.
Materials and methods
Participants
Adult primary gout patients were recruited from 2019 to 2022. The diagnostic criteria of gout refer to the classification criteria of gout jointly developed by ACR/EULAR in 2015 [3]. Any patients with severe malnutrition, tumors, serious kidney, liver, or heart diseases, history of standardized urate-lowering therapy (ULT), or an acute gout attack within two weeks were excluded. The patients were divided into three clinical types according to the FEUA and UUE: (1) urate ‘overproduction’ type: UUE > 600 mg/d/1.73 m2 and FEUA ≥ 5.5%; (2) ‘underexcretion’ type: UUE ≤ 600 mg/d/1.73 m2 and FEUA < 5.5%; (3) ‘combined’ type: UUE > 600 mg/d/1.73 m2 and FEUA < 5.5% [3, 20, 21]. Written informed consent was obtained from all participants.
Study design
The single-center prospective cohort study was conducted in Shandong Gout Clinic Center in the Affiliated Hospital of Qingdao University. The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY WZLL 26607) and registered at the China Clinical Trial Registration Center (No. ChiCTR1900022981) at 06/05/2019.
Before the study participants attended group and individual meetings for a detailed explanation of the study design and diet requirements. Each patient was given a low-purine diet tailored by a nutritionist. The low-purine diet in this experiment aims to limit the purine content in food to below 200 mg per day. The recommended diet is low in fat (up to 30% of total caloric value), high in carbohydrates, low in protein (0.8 g/kg/day), and limited in the consumption of foods high in purines (100–1000 mg/100 g). These foods include viscera, consommé, meat extract, fish (like herring, mackerel, and sardines), mussels, anchovies, alcoholic beverages, yeast, foods containing eggs and yeast, and partridge. It is also advisable to limit consumption of foods with moderate purine content (9 to 100 mg per 100 g), including meats, seafood, and certain vegetables. Additionally, it is recommended to avoid foods high in oxalates, such as dark green vegetables, cauliflower, beet/chard, beetroot, eggplant, okra, sweet potatoes, chestnuts, coconuts, wheat germ, tomatoes, asparagus, mushrooms, strawberries, whole wheat cereals, quince marmalade, and chocolate. The recommended daily liquid intake is 2.5 to 3.5 L [22]. All patients were expected to under low-purine diet for 2 weeks and underwent a follow-up visit to evaluate the effects of the low-purine loading on biochemical tests. The compliance to the diet intervention was assessed during the follow-up visit by asking the patients and questionnaire. Patients were also advised to do regular moderate-intensity exercise (≥ 30 min a day) for both normal-weight and overweight. Patients were required not to take urate-lowering drugs and/or drugs affecting serum urate for 2 weeks. It should be specifically noted that although the population of the current study overlaps with that of other cohort studies conducted by the same group, there was no noninterference between interventions because other research interventions, such as oral urate-lowering drugs, were conducted carried out after the completion of the current study.
Data collection
The general information including gender, age, occupation, smoking and drinking habits, onset age, attack frequency, comorbidity, family history of gout, medication history were collected at the baseline visit (PRE).
Before and after LPD, height, weight, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured with unified tools and standard measurement methods. The biochemical parameters, such as sUA, ALT, AST, FPG, TG, CH, BUN, Scr, creatinine clearance rate (Ccr), urine creation (Cr-U) and urine uric acid (UA-U) were collected by automatic biochemical analyzer (Roche Cobas C501). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. The estimating glomerular filtration rate (eGFR) was calculated followed the modified MDRD equation. The creatinine clearance rate (Ccr) were used to assess the renal function [Cockcroft–Gault (CG) equation, Ccr = (140-age) × body weight (kg)/0.818 × Scr (μmol/L)]. The FEUA (uU/uCr × sCr/sUA × 100%) and UUE (uU × 24 h urinary volume/(0.0061 × height (cm) + 0.0128 × weight (kg) − 0.1529) × 1.73 (mg/d/1.73 m2)) were tested using 24-h urine samples.
Outcomes
The main outcomes were the change of sUA level in subgroups after 2 weeks low-purine diet. The physical and chemical indicators (SBP, DBP, BMI, TG, TC, liver and kidney function, etc.) were the secondary endpoints. Associations of change of sUA and clinical variables were analyzed.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software (SPSS, Inc., Chicago, IL, USA). Data were tested for normal distribution using the Shapiro–Wilk test and for equality of variances when needed (Levene’s test). For continuous variables, the results were described as the mean ± standard or median (25–75th percentile), while the categorical variables were described as percentages (%). A one-way analysis of variance (ANOVA) or Kruskal–Wallis ANOVA was used for continuous variables and a Chi-square test was used to assess the distribution of categorical variables. Differences of measurement data inside group were compared with paired samples t-test or Wilcoxon rank-sum test, a one-way analysis of variance (ANOVA) or Kruskal–Wallis ANOVA was used to compare among groups. The baseline sUA level was corrected by covariance analysis to obtain the uric acid level after the test. Multiple stepwise linear regression was used to analyze the independent risk factors of uric acid change (end-of-period minus baseline) between baseline and follow-up with BMI, family history, average weekly exercise, daily water quantity, tophi, sUA and other biochemical indexes levels at baseline being independent variables and variation of sUA level (ΔsUA) being dependent variables, respectively. A p value < 0.05 was defined as statistically significant.
Results
Baseline population characteristics
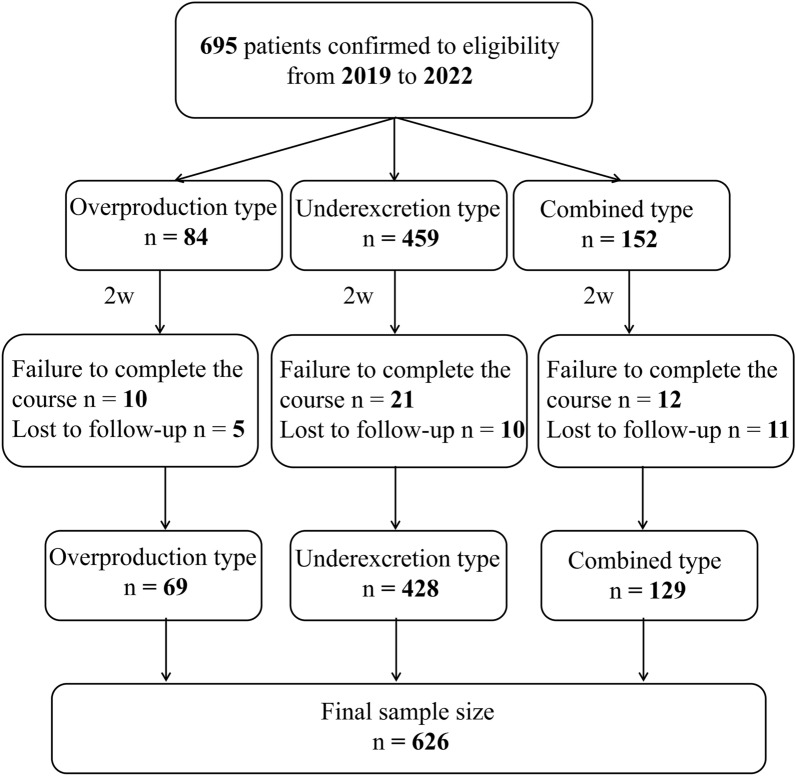
During the low-purine diet, 43 patients failed to complete the course and 26 patients were lost to follow-up. Ultimately, 626 participants (69 in the overproduction type, 428 in underexcretion type and 129 in the combined type) completed the study and were included in the outcome analyses (Fig. 1).
Fig. 1.
Flow diagram of the participants included in the study. The types of gout patients are based on patients’ urinary urate excretion (UUE) and fractional excretion of urate clearance (urate clearance/creatinine clearance ratio, FEUA)
Table 1 shows clinical characteristics of the total gout patients. Mean age and BMI were 41.20 (± 13.41) year and 27.53 (± 3.63) kg/m2. Most of the total 626 subjects were males (98%). 41.5% of the patients with gout reported a history of smoking, 65.8% of them reported a history of drinking alcohol and 17.6% had tophi. The combined type has been characterized by a higher baseline BMI, sUA and eGFR than other two types. We also observed younger age in combined type than overproduction type but did not differ from underexcretion type. TC was observed to be highest in overproduction type, which was significantly higher than underexcretion type, but did not reach statistical significance from combined type.
Table 1.
Baseline characteristics of all the subjects
| Total | Type | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Overproduction type 1 | Underexcretion type 2 | Combined type 3 | 1 vs 2 | 1 vs 3 | 2 vs 3 | |||
| General characteristics | ||||||||
| N | 626 | 69 | 428 | 129 | ||||
| Age (year) | 41.20 ± 13.41 | 45.80 ± 13.55 | 41.09 ± 13.51 | 39.12 ± 12.44 | 0.003 | 0.020* | 0.002* | 0.421* |
| Body mass index (kg/m2) | 27.53 ± 3.63 | 27.75 ± 3.67 | 27.13 ± 3.52 | 28.71 ± 3.72 | < 0.001 | 0.541* | 0.218* | < 0.001* |
| Family history of gout (%) | 247 (39.5) | 27 (39.1) | 175 (41.1) | 45 (34.9) | 0.450 | |||
| Lifestyles | ||||||||
| Smoking history (%) | 260 (41.5) | 29 (42.0) | 167 (39.0) | 64 (49.6) | 0.101 | |||
| Drinking history (%) | 412 (65.8) | 43 (62.3) | 286 (66.8) | 83 (64.3) | 0.707 | |||
| Coexisting conditions | ||||||||
| Hypertension (%) | 120 (19.2) | 25 (36.2) | 71 (16.6) | 24 (18.6) | 0.001 | < 0.001# | 0.006# | 0.340# |
| Diabetes (%) | 13 (2.1) | 2 (2.9) | 9 (2.1) | 2 (1.6) | 0.816 | |||
| Cardiovascular disease (%) | 14 (2.2) | 3 (4.3) | 9 (2.1) | 2 (1.6) | 0.423 | |||
| Hyperlipidemia (%) | 125 (20.0) | 17 (24.6) | 81 (18.9) | 27 (20.9) | 0.520 | |||
| Chronic kidney disease (%) | 2 (0.3) | 0 (0.0) | 1 (0.2) | 1 (0.8) | 0.560 | |||
| Urinary calculi (%) | 43 (6.9) | 8 (11.6) | 24 (5.6) | 11 (8.5) | 0.138 | |||
| Tophus (%) | 110 (17.6) | 21 (30.4) | 67 (15.7) | 22 (17.1) | 0.011 | 0.004# | 0.024# | 0.398# |
| Obesity/overweight (%) | 486 (77.6) | 54 (78.3) | 318 (74.3) | 114 (88.4) | 0.003 | 0.294# | 0.048# | < 0.001# |
| Blood pressure | ||||||||
| SBP (mmHg) | 137.04 ± 17.74 | 142.83 ± 19.06 | 135.64 ± 16.97 | 138.60 ± 18.85 | 0.004 | 0.005* | 0.326* | 0.284* |
| DBP (mmHg) | 86.68 ± 12.21 | 90.54 ± 12.44 | 86.01 ± 11.67 | 86.85 ± 13.45 | 0.016 | 0.013* | 0.127* | 1.000* |
| Blood chemistry parameters | ||||||||
| ALT (U/L) | 28.00 (19.00, 43.00) | 27.50 (18.25, 36.00) | 27.00 (19.00, 39.00) | 33.00 (23.00, 48.00) | 0.295 | |||
| AST (U/L) | 21.00 (18.00, 27.00) | 20.50 (18.00, 26.75) | 21.00 (18.00, 26.00) | 23.00 (18.00, 29.00) | 0.276 | |||
| FPG (mmol/L) | 5.67 ± 0.87 | 5.85 ± 1.19 | 5.62 ± 0.79 | 5.72 ± 0.92 | 0.115 | |||
| TG (mmol/L) | 1.89 (1.31, 2.71) | 1.96 (1.28, 2.67) | 1.77 (1.29, 2.58) | 2.17 (1.47, 3.03) | 0.070 | |||
| TC (mmol/L) | 4.95 ± 0.96 | 5.23 ± 1.07 | 4.84 ± 0.93 | 5.08 ± 0.97 | 0.004 | 0.011* | 0.851* | 0.094* |
| BUN (mmol/L) | 4.94 ± 1.40 | 5.25 ± 1.52 | 4.86 ± 1.43 | 5.04 ± 1.21 | 0.068 | |||
| Scr (μmol/L) | 84.66 ± 13.82 | 87.62 ± 17.24 | 84.77 ± 13.36 | 82.71 ± 13.86 | 0.056 | |||
| eGFR [mL min−1 (1.73 m2)−1] | 95.91 ± 19.96 | 89.46 ± 17.11 | 95.46 ± 17.97 | 100.188 ± 25.78 | < 0.001 | 0.058* | < 0.001* | 0.019 |
| sUA (μmol/L) | 576.43 ± 91.29 | 562.59 ± 99.37 | 569.16 ± 85.39 | 607.92 ± 97.58 | < 0.001 | 1.000* | 0.002* | < 0.001* |
Blood pressure normal refers to systolic blood pressure 90–139 mmHg and diastolic pressure 60–89 mmHg, elevated refers to systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. Fasting glucose normal refers to 3.9–6.1 mmol/L, elevated refers to > 6.1 mmol/L. Hepatic function normal refers to alanine aminotransferase (ALT) and aspartate aminotransferase (AST) 10-40U/L, abnormal refers to ALT or AST > 40U/L. p-values were calculated by one-way ANOVA or Kruskal–Wallis ANOVA for continues variables and Chi-square (Bonferroni adj. p‐value) for categorical variables. *p < 0.05 was considered statistically significant. #p < 0.017 was considered statistically significant
Comparison of outcome measures and clinical characteristics in total population
Overall, SBP, DBP, BMI, ALT, AST, TG, TC, Scr, BUN and sUA of 626 gout patients were decreased significantly after 2-week low-purine diet. Serum urate dropped from 576.43 ± 91.29 μmol/L to 514.01 ± 88.17 μmol/L (Table 2).
Table 2.
Comparison of parameters before and after 2-week LPD in total patients
| PRE | Week 2 | t/Z | p-value | |
|---|---|---|---|---|
| SBP (mmHg) | 137.04 ± 17.74 | 133.28 ± 16.66 | 6.973 | < 0.001 |
| DBP (mmHg) | 86.68 ± 12.21 | 84.42 ± 11.82 | 5.985 | < 0.001 |
| BMI (kg/m2) | 27.53 ± 3.63 | 27.35 ± 3.51 | 4.625 | < 0.001 |
| ALT (U/L) | 28.00 (19.00, 43.00) | 26.00 (19.00, 41.00) | − 4.793 | < 0.001 |
| AST (U/L) | 21.00 (18.00, 27.00) | 20.00 (17.00, 26.00) | − 5.239 | < 0.001 |
| FPG (mmol/L) | 5.67 ± 0.87 | 5.63 ± 0.83 | 1.483 | 0.139 |
| TG (mmol/L) | 1.89 (1.31,2.71) | 1.64 (1.19, 2.45) | − 5.111 | < 0.001 |
| TC (mmol/L) | 4.95 ± 0.96 | 4.89 ± 0.96 | 2.886 | 0.004 |
| BUN (mmol/L) | 4.94 ± 1.40 | 4.37 ± 1.27 | 12.286 | < 0.001 |
| Scr (μmol/L) | 84.66 ± 13.82 | 83.63 ± 13.59 | 2.552 | 0.011 |
| sUA (μmol/L) | 576.43 ± 91.29 | 514.01 ± 88.17 | 26.453 | < 0.001 |
| eGFR [mL min−1 (1.73 m2)−1] | 95.91 ± 19.96 | 96.67 ± 20.28 | − 1.753 | 0.080 |
Data are expressed as the mean ± SD, median (quartile)
sUA serum uric acid, SBP systolic blood pressure, (1 mmHg = 0.133 kPa), SDP diastolic blood pressure, (1 mmHg = 0.133 kPa), BMI body mass index, ALT serum alanine aminotransferase, AST serum aspartate aminotransferase, FPG fasting plasma glucose, TG serum triglyceride, TC serum total cholesterol, BUN blood urea nitrogen, Cr serum creatinine, eGFR = glomerular filtration rate
Comparison of primary outcomes in subtypes
After two weeks of low-purine diet, we observed that sUA were significantly decreased in overproduction type, underexcretion type, and combined type with mean change value of 88.81 ± 63.01, 57.32 ± 61.19, 65.22 ± 44.13 μmol/L, respectively (Table 3, Fig. 2). The reduction of sUA in overproduction type was significantly greater than that in underexcretion type and the combined type (p < 0.001; p < 0.05), but there was no significant difference between underexcretion type and the combined type (p > 0.05). Age, BMI and baseline sUA and eGFR have a great influence on level changes, but after adjusting for age, BMI, sUA and eGFR, we still found statistically significant differences in sUA decline among different types.
Table 3.
Comparison of parameters during the test in 3 types
| Overproduction type 1 n = 69 |
Underexcretion type 2 n = 428 |
Combined type 3 n = 129 |
p-value† | |||
|---|---|---|---|---|---|---|
| 1 vs 2 | 1 vs 3 | 2 vs 3 | ||||
| ΔsUA (μmol/L) | ||||||
| Unadjusted | − 88.81 ± 63.01 | − 57.32 ± 61.19 | − 65.22 ± 44.13 | < 0.001 | 0.021 | NS |
| Adjusted for age | − 89.94 ± 7.06 | − 57.29 ± 2.82 | − 64.71 ± 5.14 | < 0.001 | 0.012 | NS |
| Adjusted for BMI | − 88.59 ± 7.01 | − 57.69 ± 2.83 | − 64.07 ± 5.19 | < 0.001 | 0.015 | NS |
| Adjusted for sUA | − 92.27 ± 6.49 | − 59.13 ± 2.61 | − 57.34 ± 4.80 | < 0.001 | < 0.001 | NS |
| Adjusted for eGFR | − 87.73 ± 7.06 | − 57.24 ± 2.82 | − 66.05 ± 5.16 | < 0.001 | 0.042 | NS |
| ΔSBP (mmHg) | − 4.58 ± 14.38 | − 4.38 ± 13.14 | − 1.26 ± 14.02 | NS | ||
| ΔDBP (mmHg) | − 2.48 ± 10.58 | − 2.61 ± 8.83 | − 0.98 ± 10.60 | NS | ||
| ΔBMI (kg/m2) | 0.00 (− 0.34, 0.00) | 0.00 (− 0.34, 0.00) | 0.00 (− 0.34, 0.00) | NS | ||
| ΔALT (U/L) | 0.00 (− 6.00, 9.00) | − 1.00 (− 8.00, 3.00) | − 4.00 (− 11.00, 3.00) | NS | ||
| ΔAST (U/L) | 0.00 (− 4.00, 3.00) | − 1.00 (− 4.00, 2.00) | − 1.00 (− 4.50, 1.00) | NS | ||
| ΔFPG (mmol/L) | 0.35 ± 1.38 | − 0.10 ± 0.72 | − 0.02 ± 0.68 | < 0.001 | 0.007 | NS |
| ΔTG (mmol/L) | − 0.31 (− 0.81, 0.13) | − 0.07 (− 0.53, 0.34) | − 0.25 (− 0.83, 0.12) | 0.008 | NS | 0.004 |
| ΔTC (mmol/L) | − 0.17 ± 0.72 | − 0.05 ± 0.58 | − 0.09 ± 0.57 | NS | ||
| ΔBUN (mmol/L) | − 0.32 ± 1.13 | − 0.63 ± 1.17 | − 0.51 ± 1.11 | NS | ||
| ΔScr (μmol/L) | 0.11 ± 15.82 | − 0.70 ± 12.58 | 1.25 ± 13.07 | NS | ||
| ΔeGFR [mL min−1 (1.73 m2)−1] | 0.72 ± 14.44 | 0.43 ± 10.33 | 1.85 ± 9.72 | NS | ||
Data are expressed as the mean ± SD, median (quartile)
NS no significance, p > 0.05
†p-values are from one-way ANOVA and Kruskal–Wallis ANOVA, accompanying correspond to post hoc multiple comparison test (Bonferroni adj.) performed if significant ANOVA p-values
Fig. 2.
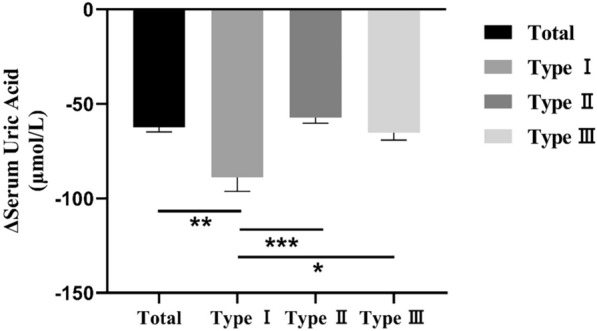
Changes of serum uric acid of total and different types. Type I: overproduction type, Type II: underexcretion type, Type III: combined type. Data are expressed as mean and standard error of mean (SEM). *p < 0.05, **p < 0.01 and ***p < 0.001
Comparison of secondary outcomes in subtypes
FPG slightly increased by 0.35 ± 1.38 mmol/L in overproduction type and decreased by 0.10 ± 0.72 mmol/L in underexcretion type and 0.02 ± 0.68 mmol/L in combined type. However, there was no statistically significant difference between underexcretion type and the combined type. The serum TG concentration decline in overproduction type and combined type was greater than that in underexcretion type (0.31 vs. 0.07 mmol/L; 0.25 vs. 0.07 mmol/L, p < 0.01 for both), but the difference between overproduction type and combined type was not statistically significant (p > 0.05). In terms of renal function, there was no significant difference in BUN, Scr and eGFR before and after 2-week low-purine diet in patients with above types (p > 0.05).
Analysis of influence factors of change of uric acid levels
For the whole population and overproduction type, linear stepwise regression analysis showed that the factors associated with decrease in sUA levels after 2-week LPD were baseline sUA (standardized β − 0.368, p < 0.001 and standardized β − 0.604, p < 0.001, respectively) and BUN levels (standardized β − 0.087, p = 0.019 and standardized β − 0.230, p = 0.022, respectively). For underexcretion type, baseline sUA (standardized β − 0.309, p < 0.001), BUN (standardized β − 0.138, p = 0.002) and ALT (standardized β − 0.116, p = 0.013) levels were independent predictors of ΔsUA while the only independent predictor in combined type was baseline sUA (standardized β − 0.400, p < 0.001). The whole patients and all the four types have common influencing factor—baseline sUA, although they have respective unique factors (Table 4).
Table 4.
Multiple stepwise linear regression analysis
| Dependent variable | Independent variable (before LPD) | β | t | p | 95%CI | Model | |
|---|---|---|---|---|---|---|---|
| Total | ΔsUA | sUA | − 0.368 | − 9.908 | < 0.001 | − 0.285, − 0.191 |
After adjustment R2 = 0.148; F = 5.513 p < 0.001 |
| BUN | − 0.087 | − 2.348 | 0.019 | − 6.751, − 0.602 | |||
| Overproduction type | sUA | − 0.604 | − 6.170 | < 0.001 | − 0.487, − 0.249 |
After adjustment R2 = 0.366; F = 5.496 p < 0.001 |
|
| BUN | − 0.230 | − 2.344 | 0.022 | − 1.417, − 17.710 | |||
| Underexcretion type | sUA | − 0.309 | − 6.615 | < 0.001 | − 0.288, − 0.156 |
After adjustment R2 = 0.149; F = 6.199 p < 0.001 |
|
| BUN | − 0.138 | − 3.053 | 0.002 | − 9.713, − 2.105 | |||
| ALT | − 0.116 | − 2.490 | 0.013 | − 0.565, − 0.066 | |||
| Combined type | sUA | − 0.400 | − 0.419 | < 0.001 | − 0.245, − 0.108 |
After adjustment R2 = 0.153; F = 9.183 P < 0.001 |
ΔsUA sUAweek 2 − sUApre, LPD low-purine diet
Discussion
Lifestyle interventions are commonly used in the management of HUA and gout, although the evidence and extent of their benefits has not been proved emphatically [23, 24]. Our study found that 2-week low-purine diet decreased serum uric acid levels, and the changes were dependent on clinical subtypes, which might provide some evidence for dietary modification in patients with gout.
The suspicion that there is a link between purine-rich diets and gout has been based on metabolic experiments in animals and humans that examined the effect of the artificial short-term loading of purified purine on the sUA level. A random, stratified cluster sampling survey in the Shandong coastal cities of eastern China found urban residents had much higher prevalence of hyperuricemia than rural residents due to their much more daily consumption of meat and seafood [25].
Previous studies have demonstrated a low-purine diet will reduce serum uric acid, but whether a low-purine diet is beneficial to a specific classification was rarely reported. The main purpose of this study was to evaluate a 2-week purine-restricted diet test on uric acid level of gout patients in different classifications in a real-world setting, taking into account possible influencing factors (i.e., age, BMI, original uric acid level, etc.). In this study, diet led to decreased levels of sUA which dropped from 579.11 ± 90.73 μmol/L to 514.53 ± 87.61 μmol/L, reducing sUA by approximately 64.58 μmol/L (11.2%). This result is basically consistent with that in previous researches, of which rich-purine diet produced a rise of serum uric acid by about 60–120 µmol/L and an isocaloric purine-free diet lower serum uric acid by about 60–120 µmol/L for 7–10 days [14, 26]. As expected, gout patients of overproduction type decreased mostly in this study (− 93.61 ± 61.12 μmol/L), followed by patients with combined type (− 69.08 ± 62.16 μmol/L), and patients with underexcretion type benefit the least (− 59.19 ± 41.06 μmol/L). Thus, we suggest that the overproduction type patients can benefit the most from dietary treatment. Excessive endogenous production and exogenous intake of purines are the main causes of elevated uric acid in patients with overproduction type. Inchida and Matsuo found that the decrease in urate exporter ABCG2 function in hyperuricemic patients is associated with the increase of UUE and frequency of overproduction hyperuricemia [21]. The administration of an oral purine load to humans can increase the sUA level by 1.0–2.0 mg/dL (59–118 µmol/L) within 24 h [27, 28]. The Dutch Nutritional Surveillance Study suggested that, in women, higher consumption of meat and fish was associated with increased sUA level [29]. The results of the Third National Health and Nutrition Examination Survey showed that the higher the intake of high-purine foods such as total meat, pork and beef, processed meat, fish and seafood, the higher the blood uric acid level [30]. It can be seen that implementing low-purine dietary intervention and limiting exogenous purine intake has the greatest benefit in reducing uric acid in gout patients with overproduction type.
Meanwhile, the 2-week low-purine diet resulted in reduction of plasma levels of TG, TC, BUN and Scr, consistent with past research of Cardona Fernando et al. [31], which were influenced by both diet and the allele X2 of XmnI polymorphism of apolipoprotein AI gene. A cross-sectional data from a large-scale cohort study in China has revealed that the Dietary Approaches to Stop Hypertension (DASH) diet was associated with a low likelihood of having hyperuricemia in Chinese adults [32]. The DASH diet encompasses many of the content in accordance with low-purine diet, implying the similar dietary pattern for reducing blood pressure. There is a strong association between hyperuricemia/gout and overeating, obesity and insulin resistance syndrome (IRS) [15, 33, 34]. Actually, a previous study reported that in 5 coastal cities of Shandong province in Eastern China, 58.6% of the hyperuricemic subjects were overweight or obese, while only 18.2% of the normal subjects showed overweight or obesity (OR = 2.53, 95%CI 2.14–2.98, p < 0.001) [25]. In this study, we found that 86.3% of the gout subjects were overweight or obese. Obesity is associated with both increased production and decreased renal excretion of urate [14]. Obese patients often have insulin resistance. If there is a significant impairment of glucose tolerance, improving insulin resistance through low-energy diet or insulin sensitizer can reduce the sUA level of overweight patients [35]. In this study, the 2-week low-purine diet slightly decreased BMI (27.48 ± 3.50 to 27.29 ± 3.40, p < 0.001) and blood pressure (SBP: 136.33 ± 17.28 to 132.90 ± 16.45; DBP: 86.27 ± 12.07 to 83.83 ± 11.63, p for all < 0.001), indicating the potential benefit of low-purine diet in improving BMI and blood pressure. Subgroup analysis showed the different effects of LPD on glucolipid metabolism in patients with different clinical types. However, probably due to the short observation time and insufficient sample size in this study, the changes in the above indicators were small, and subsequent expansion of the sample size and extended follow-up time is required for in-depth research.
Our study found that baseline sUA level was an independent predictor in all types which is strongly consistent with the finding of Juraschek et al. reporting that baseline sUA concentrations were positively correlated (B > 0) with the urate-lowing effect of low-purine diet [36]. Notably, ALT is a common factor for total and underexcretion type patients, suggesting liver function may affect the reduction of sUA after low-purine diet. Previous research data show that ALT can reflect the severity of fat deposition in the liver [37], and is one of the markers of nonalcoholic fatty liver disease (NAFLD). A recent case–control study found that the sUA level in the NAFLD group was higher than that of the control group, and the NAFLD stage was positively correlated with the sUA level [38]. Therefore, we speculated that patients with poorer basal liver function and NAFLD had higher levels of sUA and more pronounced improvement in uric acid after a low-purine diet. In addition, for total, overproduction type, and underexcretion type patients, the higher the baseline BUN level, the more obvious the decrease in uric acid after a low-purine diet. It is known that renal function is an important factor affecting the change of uric acid [39]. However, we did not find that Scr and eGFR are independent predictors that affect the changes of uric acid in patients after low-purine diet, and these need to be further studied.
An observational study found that a strict purine-free diet will reduce the sUA by 15–20% [40]. However, it is difficult to stick to a strict purine-free diet for a long time. Moderate intake of purine rather than a strict purine-free diet may improve patient compliance and help to reduce uric acid [15, 33, 40]. Unfortunately, there are few studies on low-purine foods at present, including the mechanism and function of processing technology, the interaction between nutritional components, and the removal method of food purine, etc. Actively carrying out research in this area will improve the quality of life of patients with hyperuricemia and gout, improve patient compliance and the effect of dietary intervention, which has important medical significance.
There are several limitations of our study. The study is a single center, single race that should be confirmed in multiple centers. And the study lacks a separate control group, with a relatively small sample size of the overproduction type, and a relatively short duration of the trial (2 weeks). They will be taken into consideration in future research.
Conclusions
We have demonstrated that the improvement of serum uric acid level with gout patients has been recorded after a 2-week low-purine diet, especially overproduction type. We speculate that there is potential influence of clinical classification on the response to LPD in patients with primary gout, but the mechanisms deserve further investigation.
Acknowledgements
Not applicable.
Abbreviations
- LPD
Low-purine diet
- sUA
Serum uric acid
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- BMI
Body mass index
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- TG
Triglycerides
- TC
Total cholesterol
- BUN
Blood urea nitrogen
- Scr
Serum creatinine
- HUA
Hyperuricemia
- UUE
Urinary urate excretion
- FEUA
Fractional excretion of urate clearance
- ULT
Urate-lowering therapy
- Ccr
Creatinine clearance rate
- Cr-U
Urine creation
- UA-U
Urine uric acid
- DASH
Dietary Approaches to Stop Hypertension
- IRS
Insulin resistance syndrome
- NAFLD
Nonalcoholic fatty liver disease
Author contributions
ZC, XX, CL and YC were major contributors in writing the manuscript; ZC, XX, LM, SZ, KL, CW and WS worked on the sample processing; XX and ZC contributed in the interpretation of data; YC and CL were the designers of the study. YC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
This work was supported by research project grants from National Natural Science Foundation of China (81600601) and Natural Science Foundation of Shandong Province (ZR2021MH363).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was in accordance with Declaration of Helsinki. The study was approved by the ethics committee of the Affiliated Hospital of Qingdao University. Informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhaoying Chen and Xiaomei Xue contributed equally to this article.
References
- 1.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388(10055):2039–52. 10.1016/S0140-6736(16)00346-9 [DOI] [PubMed] [Google Scholar]
- 2.Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123. 10.1186/s12916-017-0890-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas-Santos AB, Taylor WJ, Neogi T. Gout classification criteria: update and implications. Curr Rheumatol Rep. 2016;18(7):46. 10.1007/s11926-016-0594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed F, Tscharke B, O’Brien JW, et al. Wastewater-based prevalence trends of gout in an Australian community over a period of 8 years. Sci Total Environ. 2021;759: 143460. 10.1016/j.scitotenv.2020.143460 [DOI] [PubMed] [Google Scholar]
- 5.Roddy E, Choi HK. Epidemiology of gout. Rheum Dis Clin North Am. 2014;40(2):155–75. 10.1016/j.rdc.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in Mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int. 2015;2015: 762820. 10.1155/2015/762820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen LB. Role of the intestinal tract in the elimination of uric acid. Arthritis Rheum. 1965;8(5):694–706. 10.1002/art.1780080429 [DOI] [PubMed] [Google Scholar]
- 8.Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, et al. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. 1998;57(9):545–9. 10.1136/ard.57.9.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300–600 mg/day versus benzbromarone 100–200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892–7. 10.1136/ard.2008.091462 [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb DS, MacDonald PA, Hunt B, et al. Febuxostat in gout: serum urate response in uric acid overproducers and underexcretors. J Rheumatol. 2011;38(7):1385–9. 10.3899/jrheum.101156 [DOI] [PubMed] [Google Scholar]
- 11.Yan F, Xue X, Lu J, et al. Superiority of low-dose benzbromarone to low-dose febuxostat in a prospective, randomized comparative effectiveness trial in gout patients with renal uric acid underexcretion. Arthritis Rheumatol. 2022;74(12):2015–23. 10.1002/art.42266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol. 2007;3(8):443–9. 10.1038/ncprheum0556 [DOI] [PubMed] [Google Scholar]
- 13.Schlesinger N. Dietary factors and hyperuricaemia. Curr Pharm Des. 2005;11(32):4133–8. 10.2174/138161205774913273 [DOI] [PubMed] [Google Scholar]
- 14.Emmerson BT. The management of gout. N Engl J Med. 1996;334(7):445–51. 10.1056/NEJM199602153340707 [DOI] [PubMed] [Google Scholar]
- 15.Fam AG. Gout, diet, and the insulin resistance syndrome. J Rheumatol. 2002;29(7):1350–5. [PubMed] [Google Scholar]
- 16.Georgel PT, Georgel P. Where epigenetics meets food intake: their interaction in the development/severity of gout and therapeutic perspectives. Front Immunol. 2021;12: 752359. 10.3389/fimmu.2021.752359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu TH, Huang HY, Chiu YF, et al. Taiwanese vegetarians and omnivores: dietary composition, prevalence of diabetes and IFG. PLoS ONE. 2014;9(2): e88547. 10.1371/journal.pone.0088547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlich MJ, Jaceldo-Siegl K, Sabate J, et al. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr. 2014;112(10):1644–53. 10.1017/S000711451400261X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu THT, Liu CH, Chang CC, et al. Vegetarian diet and risk of gout in two separate prospective cohort studies. Clin Nutr. 2020;39(3):837–44. 10.1016/j.clnu.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 20.Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol. 2005;32(5):773–7. [PubMed] [Google Scholar]
- 21.Ichida K, Matsuo H, Takada T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. 10.1038/ncomms1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peixoto MR, Monego ET, Jardim PC, et al. Diet and medication in the treatment of hyperuricemia in hypertensive patients. Arq Bras Cardiol. 2001;76(6):463–72. 10.1590/S0066-782X2001000600004 [DOI] [PubMed] [Google Scholar]
- 23.Moi JH, Sriranganathan MK, Falzon L, et al. Lifestyle interventions for the treatment of gout: a summary of 2 Cochrane systematic reviews. J Rheumatol Suppl. 2014;92:26–32. 10.3899/jrheum.140459 [DOI] [PubMed] [Google Scholar]
- 24.Andres M, Sivera F, Buchbinder R, et al. Dietary supplements for chronic gout. Cochrane Database Syst Rev. 2021;11(11): CD010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao Z, Li C, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol. 2008;35(9):1859–64. [PubMed] [Google Scholar]
- 26.Yu T, Yu TF. Milestones in the treatment of gout. Am J Med. 1974;56(5):676–85. 10.1016/0002-9343(74)90634-2 [DOI] [PubMed] [Google Scholar]
- 27.Zollner N. Influence of various purines on uric acid metabolism. Bibl Nutr Dieta. 1973;19:34–43. [PubMed] [Google Scholar]
- 28.Zollner N, Griebsch A. Diet and gout. Adv Exp Med Biol. 1974;41:435–42. 10.1007/978-1-4757-1433-3_8 [DOI] [PubMed] [Google Scholar]
- 29.Loenen HM, Eshuis H, Lowik MR, et al. Serum uric acid correlates in elderly men and women with special reference to body composition and dietary intake (Dutch Nutrition Surveillance System). J Clin Epidemiol. 1990;43(12):1297–303. 10.1016/0895-4356(90)90095-7 [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. 10.1002/art.30520 [DOI] [PubMed] [Google Scholar]
- 31.Cardona F, Tinahones FJ, Collantes E, et al. Response to a urate-lowering diet according to polymorphisms in the apolipoprotein AI-CIII-AIV cluster. J Rheumatol. 2005;32(5):903–5. [PubMed] [Google Scholar]
- 32.Gao Y, Cui LF, Sun YY, et al. Adherence to the dietary approaches to stop hypertension diet and hyperuricemia: a cross-sectional study. Arthritis Care Res (Hoboken). 2021;73(4):603–11. 10.1002/acr.24150 [DOI] [PubMed] [Google Scholar]
- 33.Myers AR, Epstein FH, Dodge HJ, et al. The relationship of serum uric acid to risk factors in coronary heart disease. Am J Med. 1968;45(4):520–8. 10.1016/0002-9343(68)90168-X [DOI] [PubMed] [Google Scholar]
- 34.Roubenoff R, Klag MJ, Mead LA, et al. Incidence and risk factors for gout in white men. JAMA. 1991;266(21):3004–7. 10.1001/jama.1991.03470210072035 [DOI] [PubMed] [Google Scholar]
- 35.Tsunoda S, Kamide K, Minami J, et al. Decreases in serum uric acid by amelioration of insulin resistance in overweight hypertensive patients: effect of a low-energy diet and an insulin-sensitizing agent. Am J Hypertens. 2002;15(8):697–701. 10.1016/S0895-7061(02)02953-9 [DOI] [PubMed] [Google Scholar]
- 36.Juraschek SP, White K, Tang O, et al. Effects of a dietary approach to stop hypertension (dash) diet intervention on serum uric acid in African Americans with hypertension. Arthritis Care Res (Hoboken). 2018;70(10):1509–16. 10.1002/acr.23515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotronen A, Juurinen L, Hakkarainen A, et al. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care. 2008;31(1):165–9. 10.2337/dc07-1463 [DOI] [PubMed] [Google Scholar]
- 38.Oral A, Sahin T, Turker F, et al. Relationship between serum uric acid levels and nonalcoholic fatty liver disease in non-obese patients. Medicina (Kaunas). 2019;55(9):600. 10.3390/medicina55090600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava A, Kaze AD, McMullan CJ, et al. Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis. 2018;71(3):362–70. 10.1053/j.ajkd.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholls A, Scott JT. Effect of weight-loss on plasma and urinary levels of uric acid. Lancet. 1972;2(7789):1223–4. 10.1016/S0140-6736(72)92271-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.