Abstract
Background
Adrenal tumours are associated with familial adenomatous polyposis (FAP). In the literature, most studies use the clinical definition of FAP (more than 100 adenomatous polyps found in endoscopic studies). However, not all patients that meet clinical criteria for FAP carry pathogenic mutations in the adenomatous polyposis coli (APC) gene, as there is genetic heterogeneity responsible for FAP with the polyposis sometimes explained by genetic and environmental factors other than pathogenic APC mutations. Reciprocally, not all the patients with pathogenic APC variants will fulfil the classic criteria of FAP.
Objective
This study aims to investigate the characteristics of adrenal tumours in patients with pathogenic or likely pathogenic APC variants and explore the hormonal function of these patients.
Method
This is a retrospective cohort study. Patients with pathogenic or likely pathogenic APC variants were recruited and their radiological assessments were reviewed. Patient demographic data, APC variants, adrenal mass characteristics and hormonal testing results were collected.
Result
The prevalence of adrenal mass was 26.7% (24/90) among patients with pathogenic or likely pathogenic APC variants. Using the classic definition, the prevalence was 32.4% (22/68). Four patients had adrenal hormone testing, two of which had Conn’s syndrome and two had nonspecific subclinical results.
Conclusion
In our cohort, the prevalence of adrenal tumours among patients with pathogenic and likely pathogenic APC mutations is at least twice to three times higher than the general population prevalence reported from international population-based studies. The hormonal functions of patients with pathogenic APC variants and adrenal tumours can be investigated with routine testing in further research.
Keywords: Genetics, Colorectal cancer, APC, Familial adenomatous polyposis, Adrenal tumour
Background
Familial adenomatous polyposis (FAP) is an autosomal dominant genetic disorder that is caused by a germline mutation in the adenomatous polyposis coli (APC) gene [1, 2]. The classic clinical sign is the development of more than 100 colorectal adenomas throughout the colon with or without extraintestinal manifestations [2]. The incidence of FAP at birth is 1 in 8,300, based on a genetic study in the United Kingdom [3].
One of the recognised extraintestinal manifestations of FAP is adrenal tumours. Here we use the term “tumour” as a mass lesion identified on imaging or through surgical pathology. Most are considered to be adenomas [4]. The prevalence of adrenal tumours among FAP patients has been estimated to be 16% from a Canadian study [4], in comparison to a 7% prevalence among the public in Canada [5]. This result is consistent with small case series in the US, UK and Netherlands [6–8].
There are two main clinical concerns regarding adrenal tumours: the malignancy potential, and hormonal function abnormalities. From the four largest retrospective studies so far, the malignancy rate of adrenal tumours found in FAP patients seems to be consistent with those in the general population [4, 6–8]. Therefore, the authors from the four registries suggest that no special considerations are required for the surveillance and management of adrenal tumours for FAP patients. Regarding the functional status of these lesions, hyperfunction appears to be uncommon, based on the small group of patients who have undergone adrenal hormone testing [4, 8].
In the literature, most of the studies use the clinical definition of FAP, where more than 100 colonic polyps were found in endoscopic studies. However, not all patients meeting clinical criteria for FAP carry pathogenic APC mutations, as there is genetic heterogeneity responsible for FAP with the polyposis explained by genetic and environmental factors other than pathogenic APC mutations. Reciprocally, not all the patients with pathogenic APC variants will fulfil the classic criteria of FAP. FAP is increasingly now a genotypically defined disorder, as variability of the phenotype even in those with pathogenic APC mutations is common.
This study aims to investigate the characteristics of adrenal tumours in patients with pathogenic or likely pathogenic APC variants and the degree of adrenal hormonal testing among these patients.
Method
Records of patients aged 18 to 90, who attended the Parkville Familial Cancer Clinic and who carried a constitutional pathogenic or likely pathogenic APC variant were reviewed.
The variant of APC was detected using the Colorectal and Polyps assay by the Peter MacCallum Cancer Institute DNA diagnostic laboratory that targeted familial cancer-related genes including APC. Targeted gene sequencing of coding regions and splice sites was performed on DNA extracted from blood. Libraries were prepared and enriched using SureSelect XT target enrichment. Indexed libraries were pooled and sequenced to a targeted coverage of 700 reads/base (Illumina Next Seq500, 2*75 bp). Sqlinerv0.5 was used to generate aligned reads and call variants against the hg19 human reference genome. The sequence variants were classified according to ClinVar or InSiGHT LOVD database.
Patients with benign variants, likely benign variants, variants of unknown significance or where no APC variants were identified were excluded. Patients without available radiological reports in our database were also excluded. Data were collected regarding patients’ demographics, age of diagnosis, APC genotypes, presence and characteristics of adrenal tumours, CT or MRI findings and findings from adrenal hormone testing. Patients with adrenal tumours were defined as those who had radiological evidence of an adrenal lesion equal to or larger than 1 cm in maximal diameters. This is in concordance with previous studies [5–9].
The endocrinological investigations of interest included but were not limited to morning serum cortisol level, 24-hour urine cortisol level, dexamethasone suppression test, serum/urine metanephrine level, aldosterone to renin ratio and gonadocorticoids level.
Descriptive statistics were computed for all variables using medians and percentiles for continuous factors and frequencies and percentages for categorical variables. A Chi-sqaure test was performed to investigate whether there was an association between genotype and presence of adrenal tumours. The analyses were performed by using IBM SPSS software, version 25.
Result
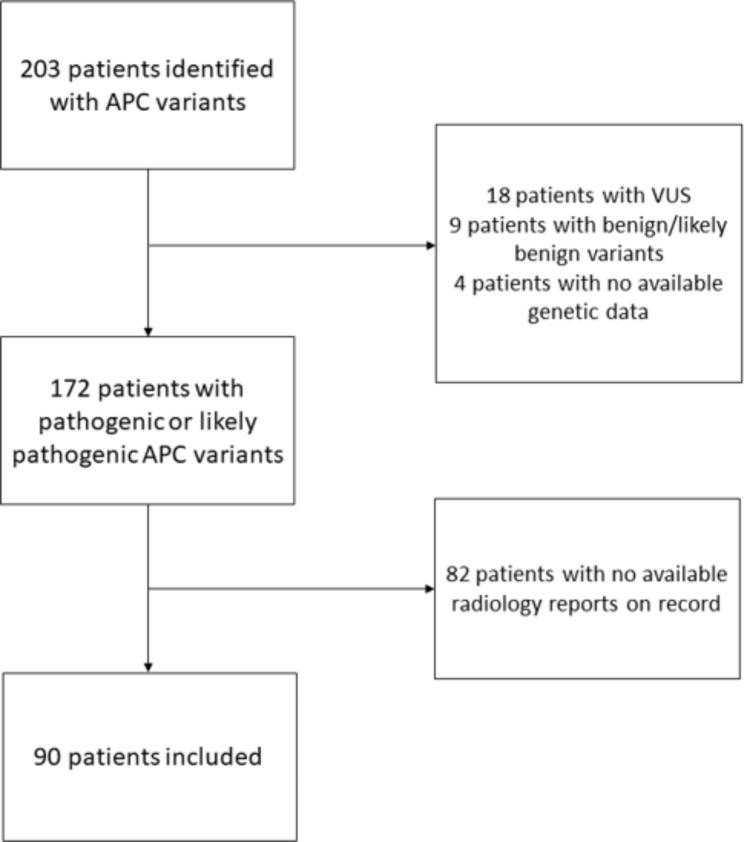
172 patients had pathogenic or likely pathogenic APC variants. 82 of the 172 patients were externally managed and thus no radiology records are available in our database. In total, 90 patients met the inclusion criteria with pathogenic or likely pathogenic variants and available CT/MRI scans covering the adrenal glands’ regions (see Fig. 1). The CT and MRI scans were conducted from January 2009 to January 2023. The majority of CT or MRI scans were conducted for colorectal cancer staging, desmoids tumour screening or planning for colorectal surgery.
Fig. 1.
Flow chart of the patient selection process
66 out of 90 (73.3%) had normal adrenal glands and 24 (26.7%) had adrenal tumours. The demographic information of these patients can be seen in Table 1. In total, 34 adrenal tumours were found in the 24 patients. The characteristics of these tumours are summarised in Table 2. No malignant features of adrenal glands were detected among the cohort based on CT or MRI scans. No significant correlation is found between genotype and the presence of adrenal tumours (p = 0.552).
Table 1.
Characteristics of adrenal tumours among patients with pathogenic or likely pathogenic mutations
| PARAMETERS | PATIENTS, n = 90 | |
|---|---|---|
| No adrenal tumours, n = 66 | Adrenal tumours, n = 24 | |
| Sex | ||
| Male | 36 (54.5%) | 11 (45.8%) |
| Female | 30 (45.5%) | 13 (54.2%) |
| The median age of FAP diagnosis, years (IQR) | 22 (18–31) | 29 (20–43) |
| The median age when the radiological assessment was conducted, years (IQR) | 39 (29–50) | 49 (35–61.75) |
| Modality | ||
| CT | 49 (74.2%) | 17 (70.8%) |
| MRI | 17 (25.8%) | 7 (29.2%) |
| Laterality | ||
| Left only | 11 (45.8%) | |
| Right only | 3 (12.5%) | |
| Bilateral | 9 (37.5%) | |
| Unknown | 1 (4.2%) | |
| Median maximal diameter, mm (IQR) | 17 (12.5–23) | |
| Type of adrenal tumours | 34 tumours in total | |
| Adenoma | 25 (73.5%) | |
| Myelolipoma | 3 (8.8%) | |
| Indeterminate | 6 (17.6%) | |
Table 2.
Clinical details of the cases
| Sex | Age | Variant | Variant type | Pathogenicity | Adrenal mass type | Maximal diameter (mm) |
|---|---|---|---|---|---|---|
| Male | 47 | NM_000038.5:c.1958 + 1_1958 + 2dupGT | Splice donor variant | Likely Pathogenic | Adrenal adenoma | 20 |
| Male | 34 | NM_000038.5:c.531 + 1G > T | Splice donor variant | Pathogenic | Bilateral adrenal adenomas | L) 17, R) 12 |
| Male | 52 | NM_000038.5:c.3924dup | Frameshift | Pathogenic | Bilateral adrenal adenomas | L) 26, R) 33 |
| Male | 29 | NM_000038.5:c.5826_5829del | Frameshift | Pathogenic | Bilateral adrenal adenomas | R) 22, L) unknown |
| Female | 36 | NM_000038.5:c.3444_3447del | Frameshift | Pathogenic | Bilateral adrenal adenomas | L) 16, R) 32 |
| Female | 62 | NM_000038.5:c.1885_1886insA | Frameshift | Pathogenic | Bilateral adrenal adenomas (3 in total) | L) inferior 31, L) superior 12, R) 6 |
| Male | 24 | NM_000038.5:c.2805 C > A | Nonsense | Pathogenic | Left adrenal adenoma | 20 |
| Female | 30 | NM_000038.5:c.5952_5955del | Frameshift | Pathogenic | Left adrenal adenoma | 12 |
| Female | 14 | NM_000038.5:c.487 C > T | Nonsense | Pathogenic | Bilateral indeterminate adrenal masses | L) 15, R) 10 |
| Female | 29 | NM_000038.5:c.1259_1269del | Frameshift | Pathogenic | Left adrenal adenoma | 18 |
| Male | 20 | NM_000038.5:c.904 C > T | Nonsense | Pathogenic | Bilateral adrenal adenomas | L) 12, R) 12 |
| Female | 63 | NM_000038.5:c.7016_7064del | Frameshift | Pathogenic | Left adrenal adenoma | 15 |
| Female | 19 | NM_000038.5:c.2805 C > A | Nonsense | Pathogenic | Left adrenal myelolipoma | 17 |
| Female | 43 | NM_000038.5:c.3183_3187del | Frameshift | Pathogenic | Left adrenal myelolipoma and right adrenal adenoma | L) unknown, R) 15 |
| Female | 40 | ** | Pathogenic | Left adrenal nodule | ||
| Female | 26 | NM_000038.5:c.423-1G > A | Splice donor variant | Pathogenic | Left adrenal myelolipoma | 41 |
| Female | 46 | NM_000038.5:c.3183_3187del | Frameshift | Pathogenic | Left adrenal nodule | 13 |
| Male | 20 | NM_000038.5:c.487 C > T | Nonsense | Pathogenic | Left adrenal nodule | 35 |
| Female | N/A | NM_000038.5:c.487 C > T | Nonsense | Pathogenic | Right adrenal adenoma | 21 |
| Female | 17 | NM_000038.5:c.348_352del | Frameshift | Pathogenic | Right adrenal adenoma | 11 |
| Male | 43 | NM_000038.5:c.348_352del | Frameshift | Pathogenic | Right adrenal nodule | 15 |
| Male | 18 | Truncated protein from exon 15* | Pathogenic | Bilateral adrenal adenomas | L) unknown, R) 18 | |
| Male | 23 | NM_000038.5:c.2805 C > A | Nonsense | Pathogenic | Left adrenal adenoma | 12 |
| Male | 18 | NM_000038.5:c.2805 C > A | Nonsense | Pathogenic | Left adrenal adenoma | 29 |
*Patient had protein testing only when genetic sequencing was not widely available at the time in clinical practice
**Patients had the genetic testing externally. The exact variant was not available but was labelled as pathogenic on the record
The classic definition of FAP was the development of more than 100 colorectal adenomas throughout the colon detected by endoscopic studies [2]. Notably, 4 patients in our cohort that fulfil the clinical diagnosis of classic FAP had non-pathogenic APC variants (one with likely benign variants and three with VUS), and they were excluded as per our criteria. None of them have radiological evidence of adrenal masses on scans. In contrast, 24 patients with pathogenic or likely pathogenic APC variants did not strictly fulfil the classic definition of FAP but were considered in our study. 13 of them had available radiological records and 2 out of 13 (15.4%) had evidence of adrenal tumours. When the classic definition of FAP is used 22 out of 68 (32.4%) FAP patients had adrenal tumours and 46 (67.6%) had normal adrenal glands.
Routine hormonal screening was not conducted for adrenal incidentaloma in our practice. There were only four patients who had adrenal hormone testing for various reasons (Table 3). Two patients were tested as part of a secondary hypertension screen and both had Conn’s Syndrome. The other two were tested because they presented with systemic symptoms of weight loss and fatigue in the context of their adrenal tumours. They both had mild abnormalities in hormone testing, not considered responsible for their clinical presentation.
Table 3.
Adrenal hormone testing results
| Age | Type of adrenal mass | Early morning cortisol level, nmol/L (Ref: 100–540 nmol/L) |
Urine metanephrine/creatinine ratio (Ref: <0.10 mmol/mol) | Plasma metanephreines, pmol/L (Ref: < 500pmol/L) | Plasma normetanephrine, pmol/L (Ref: <900 pmol/L) | Aldosterone/renin ratio (Ref: <70) | 17-hydroxyprogesterone, nmol/L (Ref: <70) | Adrenocorticotropic hormone, ng/L (Ref: 7.2–63.3 ng/L) | Estradiol, pmol/L | Follicle-stimulating hormone, IU/L | Luteinising hormone, IU/L | Indication for testing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 75 | Bilateral adrenal adenomas | 67 | - | 362 | 1148 | 42 | 0.4 | 22.1 | < 18 | 2.4 | 0.5 | Fatigue in the context of adrenal tumour |
| 46* | Bilateral adrenal adenomas | 153 | 0.14 | 148 | 674 | 96 | - | - | - | - | - | Secondary hypertension screen |
| 36 | Left adrenal adenomas | 78 | - | - | - | 45 | - | - | 142 | 13 | 4 | Fatigue in the context of adrenal tumour |
| 44* | Bilateral adrenal adenomas | - | - | - | - | 98 | - | - | - | - | - | Secondary hypertension screen |
*: both patients were diagnosed with Conn’s syndrome
Discussion
The inclusion criteria for patients in this study are based on the pathogenicity of the APC variants they carry, in contrast to other international studies where a clinical definition of FAP was used (> 100 adenomatous polyps in endoscopic studies). In our cohort, some patients presented with polyposis on colonoscopy but had VUS or benign APC variants. These patients were excluded from the study to limit the heterogeneity of the studied population. Patients with pathogenic APC variants who did not strictly fulfil the classic clinical criteria of FAP in endoscopic studies were included as per our criteria. Rather than regarding adrenal tumours as an extraintestinal manifestation of FAP, we focus on whether the pathogenicity of APC variants would affect the formation of adrenal tumours.
There is no Australian data on the prevalence of adrenal tumours in FAP. From recent international studies, the prevalence of adrenal tumours is 3–10% in the general population [10–14] and 7.4–26% among FAP patients defined phenotypically [4, 6–8]. The prevalence of adrenal tumours among patients with pathogenic or likely pathogenic APC mutations in this study (26.7%) is so far the highest.
There was no significant correlation between genotype and the presence of adrenal tumours among those with pathogenic variants. This result is consistent with the Canadian study [4]. Most of the variants in our patients with adrenal tumours involve frameshift or premature stop codons, typical of pathogenic variants described in APC in the literature and on the LOVD and ClinVar databases.
No malignant features of adrenal glands were detected among the cohort based on CT or MRI scans. This is consistent with the four previous retrospective studies where only two cases of malignancy were found among them, and it was hypothesised that the prevalence of malignant adrenal tumours among FAP is similar to that in the general population [4, 6–8].
There are some limitations identified in this study. This study is retrospective in nature and a control group was not included. Also, the prevalence of adrenal tumours in the general Australian population is unavailable. Therefore, we were unable to directly compare the prevalence of adrenal tumours in our cohort against the Australian population.
The CT or MRI scans were conducted at no specific patient age. Rather, they were done for various reasons, including screening for desmoid tumours, staging for malignancy, and investigating abdominal pain. The percentage of the patients who received the scans due to presentation with gastrointestinal symptoms was similar between those with and without adrenal tumours (41.7% vs. 43.9%) supporting the likelihood that the findings from this study were representative of all patients with FAP. No initial CT or MRI scans of the patients in our cohort were conducted related to clinical suspicion of adrenal tumours (like symptoms of hormonal disturbance). All adrenal lesions we found were considered to be adrenal incidentalomas.
No routine adrenal hormonal testing was conducted for adrenal incidentalomas. Only four patients in this study had adrenal hormonal testing due to secondary hypertension screening and constitutional symptoms of weight loss and fatigue in the context of the adrenal tumours. Thus, it is difficult to assess the pattern of hormonal function for patients with pathogenic APC variants and adrenal tumours. In the general population, isolated or combined subclinical hormonal abnormalities can occur in 37.4% of patients with adrenal tumours [15]. However, no studies in the literature systematically investigate hormonal abnormalities among FAP patients with adrenal tumours. Given the increased prevalence of adrenal tumours among those with pathogenic APC variants, future research can investigate the functional consequences of these adrenal tumours. This will provide meaningful information on whether routine hormonal testing on this cohort will be beneficial.
Conclusion
The prevalence of adrenal tumours among patients with pathogenic and likely pathogenic APC mutations in our cohort is likely to be twice to three times higher than the general population prevalence reported from international population-based studies. More data on adrenal tumours in classic FAP patients with non-pathogenic APC variants is needed, like in those with MUTYH and HTHL1 variants. The hormonal functions of patients with pathogenic APC variants and adrenal tumours should be investigated in further research to better understand whether there are any clinical endocrine implications of the increased prevalence of adrenal tumours in these patients.
Author contributions
LL - Acquisition of data; drafting of the manuscript and critical revision of the manuscript for important intellectual content. VB - Critical revision of the manuscript for important intellectual content. FM - Manuscript concept and critical revision of the manuscript for important intellectual content.
Funding
No specific funding has been received for this work.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study has been approved by the Royal Melbourne Hospital Human Research Ethics Committee (NHMRC accredited). Reference: QA2022140.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dinarvand P, Davaro EP, Doan JV, Ising ME, Evans NR, Phillips NJ, Lai J, Guzman MA. Familial adenomatous polyposis syndrome: an update and review of Extraintestinal manifestations. Arch Pathol Lab Med. 2019;143(11):1382–98. 10.5858/arpa.2018-0570-RA [DOI] [PubMed] [Google Scholar]
- 2.Aihara H, Kumar N, Thompson CC. Diagnosis, surveillance, and treatment strategies for familial adenomatous polyposis: rationale and update. Eur J Gastroenterol Hepatol. 2014;26(3):255–62. 10.1097/MEG.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed TE, Neel JV. A genetic study of multiple polyposis of the colon with an appendix deriving a method of estimating relative fitness. Am J Hum Genet. 1955;7(3):236–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Shiroky JS, Lerner-Ellis JP, Govindarajan A, Urbach DR, Devon KM. Characteristics of adrenal masses in familial adenomatous polyposis. Dis Colon Rectum. 2018;61(6):679–85. 10.1097/DCR.0000000000001008 [DOI] [PubMed] [Google Scholar]
- 5.Granger P, Genest J. Autopsy study of adrenals in unselected normotensive and hypertensive patients. Can Med Assoc J. 1970;103(1):34–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Marchesa P, Fazio VW, Church JM, McGannon E. Adrenal masses in patients with familial adenomatous polyposis. Dis Colon Rectum. 1997;40(9):1023–8. 10.1007/BF02050923 [DOI] [PubMed] [Google Scholar]
- 7.Smith TG, Clark SK, Katz DE, Reznek RH, Phillips RK. Adrenal masses are associated with familial adenomatous polyposis. Dis Colon Rectum. 2000;43(12):1739–42. 10.1007/BF02236860 [DOI] [PubMed] [Google Scholar]
- 8.Kallenberg FGJ, Bastiaansen BAJ, Nio CY, Soeters MR, Boermeester MA, Aalfs CM, Bossuyt PMM, Dekker E. Adrenal lesions in patients with (attenuated) familial adenomatous polyposis and MUTYH-Associated polyposis. Dis Colon Rectum. 2017;60(10):1057–64. 10.1097/DCR.0000000000000809 [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekar T, Goldberg H, Klaassen Z, Wallis CJD, Woon DTS, Herrera-Caceres JO, Kulkarni GS, Fleshner NE. The who, when, and why of primary adrenal malignancies: insights into the epidemiology of a rare clinical entity. Cancer. 2019;125(7):1050–9. 10.1002/cncr.31916 [DOI] [PubMed] [Google Scholar]
- 10.Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–10. 10.1056/NEJMcp065470 [DOI] [PubMed] [Google Scholar]
- 11.Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, Hartman RP, Dean DS, Thomas MA, Shah MZ, Herndon J, McKenzie TJ, Arlt W, Young WF Jr, Bancos I. Clinical, biochemical, and radiological characteristics of a single-Center Retrospective Cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2(1):30–9. 10.1016/j.mayocpiqo.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao JJ, Dages KN, Suresh M, Bancos I. Presentation, disease progression and outcomes of adrenal gland metastases. Clin Endocrinol (Oxf). 2020;93(5):546–54. 10.1111/cen.14268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tallis PH, Rushworth RL, Torpy DJ, Falhammar H. Adrenal insufficiency due to bilateral adrenal metastases - a systematic review and meta-analysis. Heliyon. 2019;5(5):e01783. 10.1016/j.heliyon.2019.e01783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubomski A, Falhammar H, Torpy DJ, Rushworth RL. The epidemiology of primary and secondary adrenal malignancies and associated adrenal insufficiency in hospitalised patients: an analysis of hospital admission data, NSW, Australia. BMC Endocr Disord. 2021;21(1):141. 10.1186/s12902-021-00787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernini GP, Moretti A, Oriandini C, Bardini M, Taurino C, Salvetti A. Long-term morphological and hormonal follow-up in a single unit on 115 patients with adrenal incidentalomas. Br J Cancer. 2005;92(6):1104–9. 10.1038/sj.bjc.6602459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.