Abstract
Background
The coronavirus 2019 (COVID-19) pandemic impacted cancer health care in several countries, with delays in the detection and treatment of breast and cervical cancer. The objective of this study is to analyze and compare the screening, diagnosis and treatment of breast and cervical cancer in the pre-COVID period and during the COVID-19 period.
Methods
Cross-sectional study with secondary data collected from the Mortality Information System (SIM), Hospital Information System (SIH), Ambulatory Information System (SIA) and the Oncology Panel (PO) of breast cancer notifications with ICD C50.0 to C50.9 and cervix ICD C53.0 to C53.9, The analyzed period before the pandemic was from March 1 to October 1, 2019, and during the pandemic from March 1 to October 1, 2020. The period from 2013 to 2022 was also analyzed with the same information, including the number of diagnoses, treatments, and deaths from breast cancer and cervical cancer. The study population consisted of Brazilian women aged 25 to 70 years. In order to compare categorical variables between periods, the Chi-Square or Fisher’s Exact tests, and Mann-Whitney U tests were applied, and the Poisson Regression model was applied to model the number of reported cases of COVID-19 and the amount of procedures.
Results
There was a decrease in the number of mammograms and cytopathological exams during COVID-19, as well as a decrease in cases of breast and cervical cancer. The Poisson regression showed that the increase in the number of COVID-19 cases caused a decrease in the number of breast cytopathological examinations, cervical-vaginal cytopathological examinations/microflora and screening, diagnosis, initiation of treatment for breast cancer and deaths from this disease. Meanwhile, in some regions of Brazil, as the number of Covid-19 increased, there was a significantly increase in the number of mammograms performed and cervical cancer diagnoses.
Conclusions
The COVID-19 period in 2020 significantly impacted screening, diagnosis, treatment for breast and cervical cancer.
Keywords: Cervical cancer, Breast cancer, COVID-19
Introduction
Breast cancer is the most common cancer in women worldwide. It is the leading cause of death in women in the Americas. In 2020, there were 68 thousand deaths and 210 thousand new cases of breast cancer diagnosed in this region. The estimated incidence for the Americas in 2040 is 684,174 new cases and 162,044 deaths [1]. Another important type of cancer that affects women is cervical cancer, also considered one of the main causes of death in Latin America and the Caribbean. Every year around 35,700 women die worldwide. An incidence of 51,500 new cases of this type of cancer is predicted for 2030 [2].
In Brazil, the most common cancer in women is breast cancer, in first place, with a greater number of cases, and in third place, cervical cancer. For each year of the 2023–2025 period, there are an estimated 73,610 cases of breast cancer, corresponding to an estimated risk of 66.54 new cases for every 100,000 women. For the same time period, cervical cancer has an estimated 17,010 cases, with an estimated risk of 15.38 cases per 100,000 women [3]. The decrease in these data is associated with early diagnosis and effective screening.
The effective screening strategies available to the population are mammography for breast cancer screening and oncotic colpocytology (Pap smear) for cervical cancer screening. Biennial mammography is recommended for women aged 50 to 69 years and oncotic cytology for women between 25 and 64 years of age who have already had sexual activity, at an annual frequency or every three years after two consecutive normal exams [3].
After the emergence of the COVID-19 pandemic in 2020, health services adopted measures to prevent the transmission and contamination of this disease, including the interruption of care and primary and secondary prevention procedures, such as the reduction of elective care, and the postponement of exams, cancer screening and elective surgeries [4–6].Cancer patients were the most affected by these measures, as the delay between diagnosis and the start of treatment may have negatively affected the prognosis of the disease [7]. National cancer screening programs were temporarily suspended as an alternative to reduce demand on health services [8, 9] This suspension resulted in a drastic decrease in the number of cancer screening exams and, consequently, in the diagnosis of the disease during this period [5, 6, 10].
According to the Brazilian Society of Clinical Oncology (SBOC), a rate of treatment abandonment and avoidance of cancer diagnoses of around 75% was identified in the population during the pandemic in 2020 [8]. Studies show that during the pandemic, in 2020, there was a 44.60% reduction in cervical cancer screening (cytopathological), 42.6% in mammograms, 35.3% in biopsies, 15.7% oncological surgeries and 0.7% in radiotherapy procedures, when compared to 2019]. This reduction may have had a negative impact on the diagnosis of both breast and cervical cancer.
After the start of the pandemic (2020), there was a reduction in the number of breast and cervical cancer diagnoses [13, 14]. Given the above, it is possible that the COVID-19 pandemic has had consequences for women’s health, thus being considered a public health problem. Therefore, the objective of this study was to analyze and compare the screening, diagnosis and treatment of breast and cervical cancer in the pre-COVID period and during the COVID-19 pandemic.
Methods
Study design and population
Cross-sectional study, using secondary data to determine the magnitude of the effects of the COVID-19 pandemic on diagnosis, treatment and death from breast and cervical cancer comparing the pre-pandemic period in 2019 and during the pandemic in 2020. Furthermore, we included information on diagnoses, treatments and deaths from 2013 to 2022 to identify possible trends and variations over time, aiming to avoid conclusions based on random fluctuations in records. The study size was determined based on the total number of observations recorded in the notification systems used for this research. This approach ensured that all available data were utilized, providing a comprehensive analysis of the entire population under study. According to the Brazilian Institute of Geography and Statistics (IBGE), in 2022 Brazil had an estimated population of 203,062,512 inhabitants, of which approximately 103,561,881, that is, 51.1% (the majority of the Brazilian population) is represented by females [15]. In relation to the female age group, in 2021 it was estimated that the largest stratum was represented by the population between 35 and 39 years old, followed by 30 to 34 and later 25 to 29 years old [16]. The population over 50 and under 70 years old represents 2 to 2.5% of the total number of women.
Data source
Data were collected from 2013 to 2022 from the Mortality Information System (SIM), Hospital Information System (SIH), Ambulatory Information System (SIA) and the Oncology Panel (PO). In SIM, death certificates (DO) were selected as the underlying cause according to the International Classification of Diseases 10 (ICD 10) C50.0 to C50.9 which refers to malignant neoplasms of the breast, being C50.0: malignant neoplasm of the nipple and areola, C50.1: malignant neoplasm of the central portion of the breast, C50.2: malignant neoplasm of the upper-inner quadrant of breast, C50.3: malignant neoplasm of the lower-inner quadrant of breast, C50.4: malignant neoplasm of the upper-outer quadrant of breast, C50.5: malignant neoplasm of the lower-outer quadrant of breast, C50.6: Malignant neoplasm of axillary tail of breast, C50.8: Malignant neoplasm of overlapping sites of breast, and C50.9: Malignant neoplasm of breast of unspecified site. In addition, the ICDs from C53.0 to C53.9 were also selected, which refer to malignant neoplasm of the cervix, being C53.0: malignant neoplasm of the endocervix, C53.1: malignant neoplasm of the exocervix, C53.8: Malignant neoplasm of overlapping sites of cervix uteri, and C53.9: Malignant neoplasm of cervix uteri, unspecified.
The SIH was used to collect information from hospitalized patients according to the ICDs mentioned above. Regarding SIA, information was collected about the procedures performed such as mammography (0204030030), breast cytopathology (0203010043) and cervical-vaginal cytopathology/microflora (0203010019) and cervical-vaginal cytopathology/microflora-tracking (0203010086). The procedure codes come from the Procedure Table Management System (SIGTAP). The information in the PO refers to patients who started treatments. Data regarding COVID-19 cases were collected from Brasil.io, in a public data repository made available in an accessible format [17]. Finally, information was collected from population estimates for 2013 and 2022 from DATASUS [18].
All variables used in the study, including their categorization and structure, are detailed in the variable dictionary available in the online repository (10.6084/m9.figshare.c.7392682.v1).
Data analysis
To assess how the COVID-19 pandemic affected the detection, treatment and deaths related to breast and cervical cancer, the study was divided into two periods. The first, called “pre COVID-19”, covered the period from March 1, 2019 to October 1, 2019. The second, called “during COVID-19”, covered the period from March 1, 2020 to October 1, 2020. The date choices were based on research that also investigated the impacts of the pandemic on women’s health in similar periods [5, 19, 20].
Next, the distribution of new cases, treatment and deaths was verified among the five regions of Brazil (North, Northeast, Midwest, Southeast and South) and by race (white, black, brown, yellow and indigenous), using absolute frequencies arising from notifications. To compare categorical variables between periods, Chi-Square or Fisher’s Exact tests were employed. For comparing age medians between groups, the Mann-Whitney U test was used. Statistical significance was considered for p-values < 0.05.
A Poisson Regression model was created using the number of reported cases of COVID-19 and the number of procedures as independent variables, while the number of new cases of breast or cervical cancer, as well as the number of deaths, served as dependent variables. To ensure the robustness of the analysis and minimize bias in variable selection, we conducted a Variance Inflation Factor (VIF) analysis to detect multicollinearity between variables, with a cutoff point set at 5. A statistically significant p value was considered when < 0.05.
All analyzes were conducted using the R software (version 4.12) together with the read.dbc, lubridade, dplyr, gtsummary and blorr packages.
Data availability
The databases used in this study can be accessed from an online repository: 10.6084/m9.figshare.c.7392682.v1.
Ethics
The study was approved by the Permanent Ethics Committee for Research with Human Beings – COPEP, of the State University of Maringá, under protocol: 123.456/2021.
Results
Between the months of March and October, from 2019 to 2020, there were a total of 39,731 cases of type C50 breast cancer, and 21,782 type C53 cervical cancer. Specifically, regarding breast cancer, when analyzing the periods before and during the COVID-19 pandemic comparatively, it was found that there was a decrease of approximately 13% in the number of diagnoses, which represented significant differences across races and regions. Overall, the white race is the most common, followed by brown and black. However, the p-value of < 0.001 indicates that there is a statistically significant association between race and breast cancer, suggesting that breast cancer rates may vary among different racial groups. In this sense, it was found that the yellow race showed the biggest decrease, with around 18%, while black and indigenous people had an increase in the number of diagnoses. Regarding the regions, it was found that there were significant differences between the distribution of cases, in which the Southeast and Northeast have the highest number of cases, followed by the South. Furthermore, although all regions showed a decrease in cases, the Southeast was the one with the greatest reduction, around 16% (Table 1). In relation to cervical cancer, there was also a difference in proportions between races, with white being the most prevalent. But when evaluated by period, there was a decrease of approximately 9% during COVID-19, while the incidence of the disease in the black race increased by approximately 21%.
Table 1.
Description of breast and cervical cancer diagnoses during the study period
| Variables | Breast cancer | Cervical cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Total N = 39.731 |
Pre N = 21.202 |
During N = 18.529 |
p-value | Total N = 21.782 |
Pre N = 11.458 |
During N = 10.324 |
p-value | |
| Age |
58 (54–63) |
59 (54–63) |
58 (54–63) |
0.048 |
44 (37–53) |
44 (37–53) |
44 (37–53) |
0.7 |
| Race | < 0.001 | < 0.001 | ||||||
| White |
18,028 (45.38%) |
9,688 (45.69%) |
8,340 (45.01%) |
7,869 (36.13%) |
4,125 (36.00%) |
3,744 (36.27%) |
||
| Black |
2,633 (6.63%) |
1,267 (5.98%) |
1,366 (7.37%) |
1,155 (5.30%) |
522 (4.56%) |
633 (6.13%) |
||
| Brown |
14,733 (37.08%) |
7,957 (37.53%) |
6,776 (36.57%) |
9,428 (43.28%) |
5,093 (44.45%) |
4,335 (41.919%) |
||
| Yellow |
512 (1.29%) |
281 (1,33%) |
231 (1,25%) |
351 (1.61%) |
167 (1.46%) |
184 (1.78%) |
||
| Indigenous |
3 (0.01%) |
1 (0.00%) |
2 (0.01%) |
31 (0.14%) |
15 (0.13%) |
16 (0.15%) |
||
| Ignores |
3,822 (9.62%) |
2,008 (9.47%) |
1,814 (9.79%) |
2,948 (13.53%) |
1,536 (13.41%) |
1,412 (13.68%) |
||
| Region | < 0.001 | 0.13 | ||||||
| Midwest |
2,311 (5.82%) |
1,239 (5.84%) |
1,072 (5.79%) |
1,800 (8.26%) |
978 (8.54%) |
822 (7.96%) |
||
| Northeast |
8,326 (20.96%) |
4,293 (20.25%) |
4,033 (21.77%) |
5,694 (26.14%) |
3,021 (26.37%) |
2,673 (25.89%) |
||
| North |
1,183 (2.98%) |
619 (2.92%) |
564 (3.04%) |
1,746 (8.02%) |
876 (7.65%) |
870 (8.43%) |
||
| Southest |
20,466 (51.51%) |
11,139 (52.54%) |
9,327 (50.34%) |
8,404 (38.58%) |
4,423 (38.60%) |
3,981 (38.56%) |
||
| South |
7,445 (18.74%) |
3,912 (18.45%) |
3,533 (19.07%) |
4,138 (19.00%) |
2,160 (18.85%) |
1,978 (19.16%) |
||
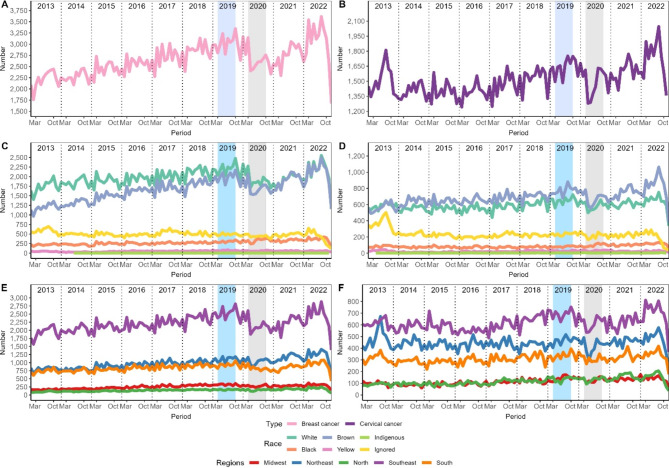
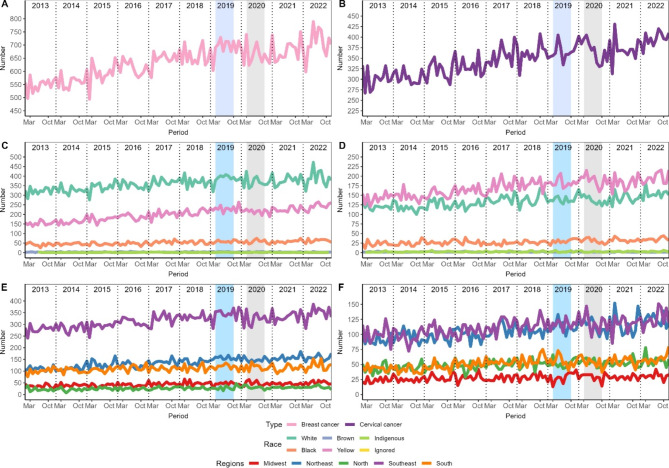
Figure 1 shows the temporal evolution of diagnosed cases. All panels showed the same pattern of increase until the pre-COVID period, followed by a sharp decline during COVID-19. However, in subsequent years there was a rapid increase again reaching levels above the pre-COVID period. Furthermore, both diseases had the most diagnosed cases in the Southeast region, followed by the Northeast, in the last 10 years. In relation to races, breast cancer was more prevalent in white people followed by mixed race people, but this difference decreased during COVID-19 and has remained so. In cervical cancer, something similar was observed during the COVID-19 period, but after that the difference increased.
Fig. 1.
Temporal evolution of diagnosed cases of breast and cervical cancer. The left column refers to breast cancer and cervical cancer is on the right. Panels A and B show the number of diagnosed cases, C and D the races, E and F the regions
Regarding the number of procedures for detecting breast cancer (Table 2), it was found that there was a decrease of 26% in mammograms and 44% in cytopathology. In both periods, race and region were the significant variables that showed differences in frequencies. The distribution of races varied in the mammography groups before and during the pandemic, in which the majority of patients were white (41.73% in the total sample), followed by mixed race (18.86%) and yellow (7.04%). However, the biggest reduction was in the yellow race, with approximately 31%. In relation to regions, there was a significant association with the performance of mammography, in which the majority of participants in the pre-pandemic period were from the Southeast region (62.33%), followed by the South region (17.39%) and Northeast region (14.11%), and this pattern continued during the pandemic period again with the Southeast region (62.34%) being the most frequent, followed by the South region (18.61%) and Northeast (12.85%). However, it was in the Midwest region where the greatest reduction was observed (37.73%).
Table 2.
Description of the number of procedures performed during the study period to detect breast cancer
| Variables | Mammograms | Cytopathological | ||||||
|---|---|---|---|---|---|---|---|---|
| Total N = 139,507 |
Pre N = 80,221 |
During N = 59,286 |
p-value | Total N = 6,835 |
Pre N = 4,398 |
During N = 2,437 |
p-value | |
| Age |
59.0 (54–64) |
59.0 (54–64) |
59.0 (54–64) |
0.2 |
56.0 (52–61) |
55.0 (52–61) |
56.0 (52–61) |
0.036 |
| Race | < 0.001 | < 0.001 | ||||||
| White |
58,220 (41.73%) |
32,730 (40.80%) |
25,490 (42.99%) |
1,983 (29.01%) |
1,237 (28.13%) |
746 (30.61%) |
||
| Black |
5,945 (4.26%) |
3,350 (4.18%) |
2,595 (4.38%) |
425 (6.22%) |
253 (5,75%) |
172 (7.06%) |
||
| Brown |
26,312 (18.86%) |
15,153 (18.89%) |
11,159 (18.82%) |
2,008 (29.38%) |
1,300 (29.56%) |
708 (29.05%) |
||
| Yellow |
9,821 (7.04%) |
5,821 (7.26%) |
4,000 (6.75%) |
1,011 (14.79%) |
696 (15.83%) |
315 (12.93%) |
||
| Indigenous |
25 (0.02%) |
13 (0.02%) |
12 (0.02%) |
2 (0.03%) |
1 (0.02%) |
1 (0.04%) |
||
| Ignored |
39,184 (28.09%) |
23,154 (28.86%) |
16,030 (27.04%) |
1,406 (20.57%) |
911 (20.71%) |
495 (20.31%) |
||
| Region | < 0.001 | < 0.001 | ||||||
| Midwest |
5,225 (3.75%) |
3,220 (4.01%) |
2,005 (3.38%) |
723 (10.58%) |
426 (9.69%) |
297 (12.19%) |
||
| Northeast |
18,940 (13.58%) |
11,322 (14.11%) |
7,618 (12.85%) |
2,872 (42.02%) |
2,016 (45.84%) |
856 (35.13%) |
||
| North |
3,396 (2.43%) |
1,728 (2.15%) |
1,668 (2.81%) |
197 (2.88%) |
107 (2.43%) |
90 (3.69%) |
||
| Southeast |
86,964 (62.34%) |
50,004 (62.33%) |
36,960 (62.34%) |
2,387 (34.92%) |
1,412 (32.11%) |
975 (40.01%) |
||
| South |
24,982 (17.91%) |
13,947 (17.39%) |
11,035 (18.61%) |
656 (9.60%) |
437 (9.94%) |
219 (8.99%) |
||
The cytopathological breast exam had far fewer procedures performed compared to mammograms and the age difference was statistically significant between the two periods (p = 0.036). Among the races, browns and whites were most represented in the breast cytopathology groups before and during the pandemic. However, the most noticeable difference was in relation to the yellow race, with approximately 54%. Regarding the regions, the Northeast and Southeast had the highest number of procedures performed overall, although, the Northeast showed the greatest reduction during the pandemic period with more than 57%.
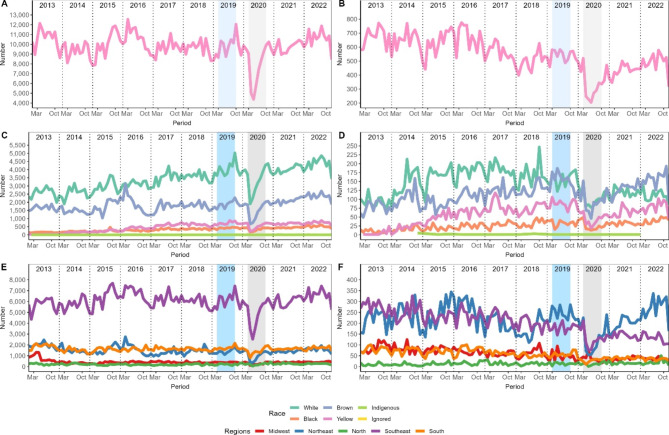
In Fig. 2 it is possible to observe the trends in procedures over the last 10 years. Although mammograms had a negative impact during the pandemic, it apparently returned to pre-pandemic levels. The same could not be observed in the cytology, which showed difficulties in reaching pre-pandemic levels, and a downward trend. Furthermore, there was a shift in races that underwent the most procedures, with the white race being the most prevalent until the period before and during the pandemic, but after that, the brown race surpassed it and the difference increased in the coming years.
Fig. 2.
Temporal evolution of procedures for detecting breast cancer. The left column refers to mammograms, and cytopathology is on the right. Panels A and B show the number of procedures, C and D the races, E and F the regions
Table 3 provides detailed information on the number of procedures performed during the study period to detect cervical cancer, considering variables such as age, race and region. No significant differences were found in age between the cervical-vaginal cytopathology/microflora screening groups before and during the pandemic (p = 0.2), however, a significant difference was observed between the cervical-vaginal cytopathology/microflora-screening groups (p < 0.001), with the screening group presenting the most frequent median age of 43 years. The white and brown races were the ones that underwent the most procedures, but the yellow and black races had the greatest reduction during the pandemic (both 56%) in the first exam, and 61% and 57%, respectively, in the second exam. When analyzing the regional distribution, it was observed that the Southeast represented the region with the highest number of procedures, with a significantly high proportion of 75.13%. This indicates a substantial concentration of procedures in this region. The South region also had a considerable share, with 5.23% of procedures, while the Northeast, North and Midwest regions had lower proportions of procedures, ranging from 3.71 to 8.25%. When looking at the regional distribution of the second procedure, the Southeast once again stood out, representing a significant proportion of 32.97% of screening procedures. This indicates a considerable concentration of tracking efforts in this region. The biggest reduction between the 2 periods is in the Midwest region, which varied between 64% and 66%.
Table 3.
Description of the number of procedures performed during the study period to detect cervical cancer
| Variables | Cervical-Vaginal Cytopathology/Microflora | Cervical Vaginal Cytopathology/Microflora-Tracking | ||||||
|---|---|---|---|---|---|---|---|---|
| Total N = 1,789,5261 |
Pre N = 1,230,423 |
During N = 559,103 |
p-value | Total N = 4,873,496 |
Pre N = 3,422,169 |
During N = 1,451,327 |
p-value | |
| Age |
42 (33–52) |
42 (33–52) |
42 (33–52) |
0.2 |
43 (35–52) |
43 (35–52) |
43 (35–52) |
< 0.001 |
| Race | 0.051 | 0.051 | ||||||
| Whte |
426,225 (23.82%) |
298,892 (24.29%) |
127,333 (22.77%) |
2,063,132 (42.33%) |
1,434,759 (41.93%) |
628,373 (43.30%) |
||
| Black |
33,092 (1.85%) |
22,991 (1.87%) |
10,101 (1.81%) |
290,929 (5.97%) |
205,205 (6.00%) |
85,724 (5.91%) |
||
| Brown |
149,822 (8.37%) |
103,855 (8.44%) |
45,967 (8.22%) |
1,282,980 (26.33%) |
887,508 (25.93%) |
395,472 (27.25%) |
||
| Yellow |
46,093 (2.58%) |
32,229 (2.62%) |
13,864 (2.48%) |
874,323 (17.94%) |
631,465 (18.45%) |
242,858 (16.73%) |
||
| Indigenous |
1,083 (0.06%) |
739 (0.06%) |
344 (0.06%) |
23,244 (0.48%) |
16,383 (0.48%) |
6,861 (0.47%) |
||
| Ignored |
1,133,211 (63.32%) |
771,717 (62.72%) |
361,494 (64.66%) |
338,888 (6.95%) |
246,849 (7.21%) |
92,039 (6.34%) |
||
| Region | < 0.001 | < 0.001 | ||||||
| Midwest |
66,408 (3.71%) |
49,689 (4.04%) |
16,719 (2.99%) |
368,065 (7.55%) |
271,112 (7.92%) |
96,953 (6.68%) |
||
| Northeast |
147,702 (8.25%) |
106,214 (8.63%) |
41,488 (7.42%) |
1,479,526 (30.36%) |
1,057,261 (30.89%) |
422,265 (29.10%) |
||
| North |
137,271 (7.67%) |
92,928 (7.55%) |
44,343 (7.93%) |
298,650 (6.13%) |
210,217 (6.14%) |
88,433 (6.09%) |
||
| Southeast |
1,344,514 (75.13%) |
915,403 (74.40%) |
429,111 (76.75%) |
1,606,613 (32.97%) |
1,099,955 (32.14%) |
506,658 (34.91%) |
||
| South |
93,631 (5.23%) |
66,189 (5.38%) |
27,442 (4.91%) |
1,120,642 (22.99%) |
783,624 (22.90%) |
337,018 (23.22%) |
||
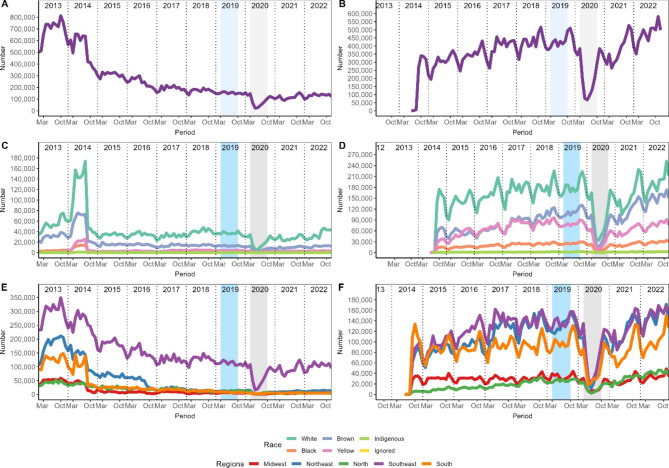
Figure 3 shows an inversion in the number of procedures performed for the first and second exam, with a reduction in the first exam while the second exam increases over time. However, a greater decline was observed during the pandemic in relation to the second procedure. Regarding races, it was necessary to remove ignored data, that is, information in which patients did not declare their race or which was not recorded by the notifier to adapt the scale of the graph. The pattern was maintained in both procedures. In relation to the regions, the Southeast remains with high numbers of the first procedure and is on a similar level with the Northeast and South in relation to the second procedure.
Fig. 3.
Temporal evolution of procedures for detecting cervical cancer. The left column refers to cervical-vaginal cytopathology/microflora, and cervical-vaginal cytopathology/microflora-tracking is on the right. Panels A and B show the number of procedures, C and D the races, E and F the regions
Regarding the start of treatment, stage III had the highest frequencies for both breast and cervical cancer. And when analyzing the regions, only for the breast cancer group there were significant differences, with the most prevalent region being the Southeast. Nonetheless, the Midwest showed the greatest reduction during the pandemic (11%), while the North and South showed an increase of 0.01 and 0.03% respectively.
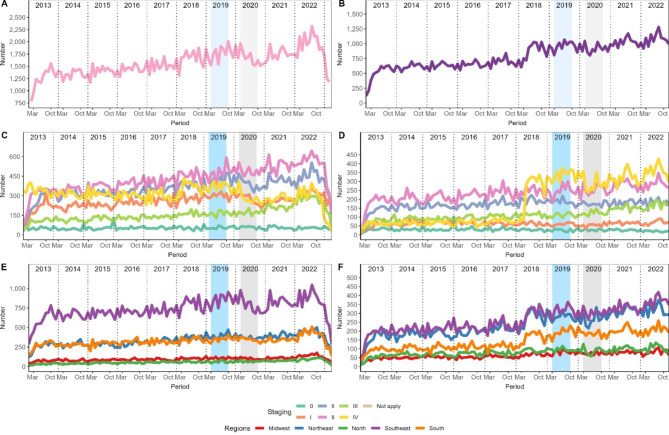
From 2013 to 2022, there was an increase in the number of treatments initiated for breast cancer (Fig. 4). However, in 2020, during the pandemic, there was a temporary drop in these numbers, and it took a while to recover the pre-pandemic levels. In 2022, there was a resume in growth. Stage II stood out with consistent behavior over the years and remained stable in periods before and during the pandemic. On the other hand, the other stages showed a decrease. For cervical cancer, we observed a similar pattern to that of breast cancer. Starting in 2018, there was a rapid increase in the number of treatments for cervical cancer, and this trend remained strong, even during the pandemic. In regional terms, we observed that the Southeast registered the highest number of treatment initiations for breast cancer. However, when it comes to cervical cancer, both the Southeast and Northeast led in terms of starting treatment. These regional differences may reflect variations in the incidence of these types of cancer as well as access to health services.
Fig. 4.
Temporal evolution of treatments initiated over the years for breast and cervical cancer. The left column refers to breast cancer, and cervical cancer is on the right. Panels A and B show the number of treatments initiated, C and D the staging, E and F the regions
Table 4 analyzed the number of deaths related to breast and cervical cancer. We observed a greater number of deaths resulting from breast cancer, mainly among individuals of white ethnicity and residents of the Southeast region. Although there was an increase in the number of deaths among people of black and indigenous ethnicity, as well as in the Midwest and North regions, this difference did not reach statistical significance. When considering deaths associated with cervical cancer, a higher occurrence was also observed among individuals of white ethnicity and residents of the Southeast region. Furthermore, there was an increase in the number of deaths among people of black and yellow ethnicity, residing in the Midwest, Northeast and North regions. However, it is important to highlight that this difference was also not significant. Table 4 also displays the results of the analysis of 10 dependent variables in relation to the number of COVID-19 cases, considered as an independent variable. Notably, some variables showed negative associations with the number of COVID-19 cases. Breast Cytopathology, Cervical-Vaginal Cytopathology/Microflora and Cervical Vaginal Cytopathology/Microflora-Screening, as well as Breast Cancer and Breast Cancer Treatment Initiation, have all shown reductions as the incidence of COVID-19 increased. Additionally, Cervical Cancer Deaths also had a negative association, suggesting a decrease in death rates as COVID-19 cases increased. Constrastingly, two variables showed positive associations with the number of COVID-19 cases. Mammography and Cervical Cancer Diagnosis have seen an increase as COVID-19 cases have increased. This indicates that, during the period analyzed, there was an increase in the number of mammograms performed and diagnosis of cervical cancer in regions with a higher incidence of COVID-19.
Table 4.
Description of treatments initiated and number of deaths during the analyzed period of the study
| Varibles | Breast | Cervical | ||||||
|---|---|---|---|---|---|---|---|---|
| Total N = 50,439 |
Pre N = 25,666 |
During N = 24,7731 |
p-value | Total N = 19,539 |
Pre N = 9,907 |
DUring N = 9,632 |
p-value | |
| Treatments | ||||||||
| Age |
53 (45–60) |
53 (45–60) |
53 (45–60) |
0.5 |
47 (38–56) |
46 (38–56) |
47 (38–56) |
0.7 |
| Staging | < 0.001 | < 0.001 | ||||||
| 0 |
1,219 (2.99%) |
574 (2.77%) |
645 (3.22%) |
423 (2.94%) |
199 (2.71%) |
224 (3.19%) |
||
| I |
6,167 (15.12%) |
3,180 (15.33%) |
2,987 (14.89%) |
884 (6.15%) |
466 (6.34%) |
418 (5.96%) |
||
| II |
9,441 (23.14%) |
4,731 (22.81%) |
4,710 (23.48%) |
2,801 (19.49%) |
1,450 (19.73%) |
1,351 (19.25%) |
||
| III |
12,622 (30.94%) |
6,140 (29.61%) |
6,482 (32.31%) |
3,871 (26.94%) |
1,909 (25.97%) |
1,962 (27.95%) |
||
| IV |
3,914 (9.59%) |
1,927 (9.29%) |
1,987 (9.91%) |
1,849 (12.87%) |
885 (12.04%) |
964 (13.73%) |
||
| Not apply |
7,436 (18.23%) |
4,187 (20.19%) |
3,249 (16.20%) |
4,541 (31.60%) |
2,441 (33.21%) |
2,100 (29.92%) |
||
| Region | 0,004 | 0,2 | ||||||
| Midwest |
2,742 (6.72%) |
1,451 (7.00%) |
1,291 (6.44%) |
1,169 (8.14%) |
621 (8.45%) |
548 (7.81%) |
||
| Northeast |
9,446 (23.15%) |
4,851 (23.39%) |
4,595 (22.91%) |
4,153 (28.90%) |
2,145 (29.18%) |
2,008 (28.61%) |
||
| North |
1,665 (4.08%) |
826 (3.98%) |
839 (4.18%) |
1,409 (9.81%) |
734 (9.99%) |
675 (9.62%) |
||
| Southeast |
18,681 (45.79%) |
9,540 (46.00%) |
9,141 (45.57%) |
4,796 (33.38%) |
2,443 (33.24%) |
2,353 (33.52%) |
||
| South |
8,265 (20.26%) |
4,071 (19.63%) |
4,194 (20.91%) |
2,842 (19.78%) |
1,407 (19.14%) |
1,435 (20.44%) |
||
| Number of deaths | ||||||||
| Age |
59.0 (55–64) |
59.0 (55–64.0) |
59.0 (55–64) |
0.8 |
48 (40–56) |
48 (40–56) |
48 (40–56) |
0.5 |
| Race | 0.8 | 0.9 | ||||||
| White |
5,408 (57.48%) |
2,778 (57.69%) |
2,630 (57.25%) |
1,946 (38.57%) |
968 (39.02%) |
978 (38.14%) |
||
| Black |
826 (8.78%) |
405 (8.41%) |
421 (9.16%) |
439 (8.70%) |
207 (8.34%) |
232 (9.05%) |
||
| Brown |
56 (0.60%) |
28 (0.58%) |
28 (0.61%) |
17 (0.34%) |
9 (0.36%) |
8 (0.31%) |
||
| Yellow |
3,108 (33.03%) |
1,599 (33.21%) |
1,509 (32.85%) |
2,615 (51.83%) |
1,283 (51.71%) |
1,332 (51.95%) |
||
| Indigenous |
11 (0.12%) |
5 (0.10%) |
6 (0.13%) |
28 (0.56%) |
14 (0.56%) |
14 (0.55%) |
||
| Region | 0.11 | 0.5 | ||||||
| Miwest |
691 (7.17%) |
338 (6.86%) |
353 (7.49%) |
395 (7.64%) |
181 (7.08%) |
214 (8.19%) |
||
| Northeast |
2,128 (22.08%) |
1,112 (22.57%) |
1,016 (21.56%) |
1,616 (31.26%) |
801 (31.33%) |
815 (31.20%) |
||
| North |
408 (4.23%) |
188 (3.82%) |
220 (4.67%) |
735 (14.22%) |
356 (13.92%) |
379 (14.51%) |
||
| Southeast |
4,727 (49.05%) |
2,441 (49.55%) |
2,286 (48.51%) |
1,651 (31.94%) |
829 (32.42%) |
822 (31.47%) |
||
| South |
1,684 (17.47%) |
847 (17.19%) |
837 (17.76%) |
772 (14.94%) |
390 (15.25%) |
382 (14.62%) |
||
Figure 5 shows an increasing trend in the number of deaths related to breast and cervical cancer over the years, although there were some annual variations. Both types of cancer experienced a sudden increase followed by a decrease in 2021, after which they resumed their upward trend. Interestingly, it was observed that more white people die from breast cancer, while for cervical cancer, yellow people register higher rates. In relation to regions, it was also highlighted that the Southeast region had a higher number of deaths related to breast cancer. On the other hand, for cervical cancer, a high number of deaths was observed in the Southeast and Northeast regions.
Fig. 5.
Temporal evolution of deaths from breast and cervical cancer. The left column refers to breast cancer, and cervical cancer is on the right. Panels A and B show the number of deaths, C and D by race, E and F the regions
Discussion
The COVID-19 pandemic period had a negative impact on the screening, diagnosis and treatment of breast and cervical cancer around the world, which could result in an increase in preventable deaths from these types of cancer in the coming years. Statistical modeling studies predict increased tumor staging rates [21]. The results of this study show a decrease in diagnosis and treatment of breast and cervical cancer during the pandemic period, which may be associated with a decrease in the number of screening tests for these diseases, as well as a decrease in supply of health services. This reduction in screening for these diseases was evidenced in other studies, where the average overall decrease in breast cancer screening reached − 44.9% and − 51.8% for cervical cancer [22–26].
In 2020, during the COVID-19 pandemic, a reduction was observed in the number of screening tests carried out to detect breast and cervical cancer, as well as in the number of diagnoses. After the pandemic period, the number of tests for cervical cancer screening did not reach the levels seen in the years prior to the COVID-19 period. The yellow race showed a greater reduction in the number of diagnoses and in the performance of mammography and cytopathological exams. The Northeast region showed a greater reduction in the number of cytopathological tests performed and, the Midwest region, in the number of mammograms. The impact of the COVID-19 pandemic has been observed in other studies [11, 12]. For the same period, the National Cancer Institute (INCA) announced a 41% reduction in the number of mammograms, however in 2021 this number increased again, returning to the same levels as in the years before COVID-19 in the South and Southeast regions and higher numbers in the North, Northeast and Midwest regions [27].
Interruptions in elective health care occurred in most countries in order to avoid the risk of spreading the disease caused by the new coronavirus (SARS-CoV-2), ease the workload for healthcare professionals who were overloaded with caring for COVID-19 patients, with crowded health institutions and with lack of materials and medicines, thus, prioritizing only urgent and emergency care. The recommendations at the beginning of the pandemic were for early detection actions for cancer to be postponed. As COVID began to be controlled, the guidelines instructed health services to resume elective care under conditions favorable in order not to spread the disease [21–28].
During the period analyzed, breast cancer was more prevalent in the Southeast and Northeast regions, and cervical cancer in the Southeast region. The Southeast region also stood out in terms of the highest number of mammograms and tests to detect cervical cancer. According to the National Cancer Institute (INCA) released in 2023, breast cancer is the most common in all regions, with the Southeast region having the highest incidence rate (52.83/100,000 women) [29]. In 2022, the Southeast and Northeast regions were predicted to have the highest number of cervical cancer detection tests, however the North region was predicted to have the highest mortality and incidence rates (26.24/100,000 thousand) for this type of cancer in the country, with a temporal trend of growth in recent years [30].
The residential area of a population can influence the reduction in demand and supply of health services related to the prevention of breast cancer and cervical cancer, with populations of lower socioeconomic status being the most affected, as they have a greater tendency to live in more distant areas. This means that the coverage of these health services does not reach the goals established by the Ministry of Health (MS) and consequently there is insufficient care to assist the women [31].
An increasing trend in the number of deaths related to breast and cervical cancer was also found in this study. A similar result was identified in Korea, where mortality from breast cancer has tended to increase in recent years, while mortality from cervical cancer has decreased [32]. In Peru, a trend study showed a decrease in the number of deaths from both types of cancer [33]. These data reflect the social and health disparities found in different countries, where socioeconomic, racial and ethnic differences stand out. This defines the process of illness and death from such diseases, which are unfair and avoidable differences in the health conditions observed in populations between different countries or even within the same country [34].
According to the results of this study, the start of treatment for breast and cervical cancer was more frequent for stage III, that is, an already advanced stage of the disease. This late diagnosis is the main factor that directly reflects the high mortality rate from these diseases [35]. A study carried out with data from hospital records between 2000 and 2012 showed a trend towards an increase in diagnosis of stage III and IV breast cancer [36]. Research shows that the factors that lead to late diagnosis are associated with low income, race/skin color, age, low education, sociocultural barriers, difficulties in accessing health services and a long timespan between suspected tumor and biopsy [35, 37, 38].
This study has limitations that deserve to be highlighted. Firstly, given that this is research based on secondary data, the presence of underreporting is inherent, resulting in an underestimation of the true number of cases. Furthermore, it is common to find gaps in the data in this type of study, and the absence of this information plays a notable role in the exploratory analysis of the relationships between variables. Secondly, the model developed to assess the impacts of COVID-19 was based on general information from Brazil, without considering regional variations. Therefore, although the study captured the overall impact of COVID-19 in the country, it is important to mention that certain regions may have been more affected, less affected, or not significantly affected. It is also worth considering that there are strengths to this study, such as the use of data from the government, representing the best source of information currently available. Incidentally, the work provides detailed information on the impacts suffered in diagnosis, treatment and deaths during the most critical period of the COVID-19 pandemic.
Conclusions
This study showed that the COVID-19 pandemic period had a negative impact on the screening, diagnosis and treatment of breast cancer and cervical cancer, drastically reducing the number of procedures performed in all Brazilian states. Hence, by presenting a general overview, with relevant information regarding the screening, diagnosis and treatment of breast and cervical cancer in the pre-COVID period and during the COVID-19 pandemic, we hope this in turn may provide the groundwork needed for planning new healthcare policies by the government.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author contributions
Conceptualization: Stevenato KP, Pelloso SM. Formal analysis: dos Santos L. Funding acquisition: None. Methodology: dos Santos L, Stevanato KP, Silva LL. Project administration: Pelloso SM. Visualization: Pelloso FC. Writing – original draft: Stevenato KP. Writing – review & editing: Borghesan DHP, Consolaro EL, de Almeida R, Huber IMT, Egger P, Alarcão ACJ, de Souza RR, Ribeiro HF, Silva LL, Camparoto CW, Bitencourt MR, Borba PB, Carvalho MDB. All authors reviewed the manuscript.
Funding
None.
Data availability
All data is available to the public at the link: https://doi.org/10.6084/m9.figshare.
Declarations
Ethics approval and consent to participate
The study was approved by the Permanent Ethics Committee for Research with Human Beings – COPEP, of the State University of Maringá, under protocol: 123.456/2021.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) a. Cervical Cancer. https://www.paho.org/en/topics/cervical-cancer. Acess. 09 november 2023.
- 2.World Health Organization (WHO) b. Breast Cancer. https://www.paho.org/en/topics/breast-cancer. Acess. 09 november 2023.
- 3.Instituto Nacional do Câncer (INCA). Estimativa 2023 – Incidência de Câncer no Brasil. Rio de Janeiro: INCA. 2023. https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2023.pdf. Acess. 09 november 2023.
- 4.Aquino EML, Silveira IH, Pescarini JM, Aquino R, de Souza-Filho JA, Rocha A, dos Ciência S et al. & Saúde Coletiva [Internet]. 25(1):2423–46. https://www.scielosp.org/article/csc/2020.v25suppl1/2423-2446/pt/.
- 5.Bonadio RC, Messias AP, Moreira OA, Vecchi Leis L, Zanin Orsi B, Testa L et al. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil. Ecancermedicalscience. 2021;15. [DOI] [PMC free article] [PubMed]
- 6.Tachibana BMT, Ribeiro RL, de Federicci M, Feres ÉEF, Lupinacci R, Yonekura FAS. I, The delay of breast cancer diagnosis during the COVID-19 pandemic in São Paulo, Brazil. Einstein (São Paulo). 2021;19. [DOI] [PMC free article] [PubMed]
- 7.del Pilar Estevez-Diz M, Colombo Bonadio R, Costa Miranda V, Paula Carvalho J. Management of cervical cancer patients during the COVID-19 pandemic: a challenge for developing countries. Ecancermedicalscience. 2020;14. [DOI] [PMC free article] [PubMed]
- 8.SBOC, Pesquisa SBOC. november : 74% dos oncologistas observaram interrupção do tratamento durante a pandemia [Internet]. sboc.org.br. https://sboc.org.br/noticias/item/2099-pesquisa-sboc-74-dos-oncologistas-observaram-interrupcao-do-tratamento-durante-a-pandemia. Acess. 09 2023.
- 9.Vista do Recomendações para detecção precoce de câncer durante a pandemia de covid-19 em 2021 [Internet]. Ufjf.br. 2021. https://periodicos.ufjf.br/index.php/aps/article/view/33510/22826. Acess. 09 november 2023.
- 10.Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer Screening tests and Cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021. [DOI] [PMC free article] [PubMed]
- 11.Ribeiro CM, Correa FM, Migowski A. Efeitos de curto prazo da pandemia de COVID-19 na realização de procedimentos de rastreamento, investigação diagnóstica e tratamento do câncer no Brasil: estudo descritivo, 2019–2020. Epidemiologia e Serviços de Saúde, 2022, 31. [DOI] [PMC free article] [PubMed]
- 12.Rigon FP, Plewka J, Turkiewicz M, Santos MA. Dos. Dados do programa do Câncer do colo do útero na pandemiaCOVID-19. Arquivos De Ciências Da Saúde Da UNIPAR. Umuarama. 2022;26(3):794–808. [Google Scholar]
- 13.Figueiredo BQ, Souza ACB, Machado BG, et al. Queda no número de diagnósticos de cânceres durante pandemia de Covid-19: estadiamento e prognóstico prejudicados. Res Soc Dev. 2021;10(11):273101119762–e273101119762. 10.33448/rsd-v10i11.19762 [DOI] [Google Scholar]
- 14.Neves LR, Eustáquio VM, Araújo RL. A influência da covid 19 no diagnóstico de neoplasias de colo uterino e de mama no Brasil. JNT- Facit Bus Technol J. 2022;1(34):297–311. [Google Scholar]
- 15.IBGE. Instituto Brasileiro de Geografia e Estatística. Séries estatísticas & séries históricas. Rio de Janeiro. 2018. Avaibe from: https://www.ibge.gov.br/estatisticas/sociais/habitacao/25089-censo-1991-6.html?edicao=25091. Acess. 09 november 2023.
- 16.Instituto Brasileiro de Geografia e E. Pesquisa nacional por amostra de domicílios contínua. Estatísticas sociais. 2019. Avaible from: https://www.ibge.gov.br/estatisticas/sociais/trabalho/17270-pnad-continua.html. Acess. 22 november 2023.
- 17.Brasil. Departamento de Informática do Sistema Único de Saúde (DATASUS). Avaible from: https://datasus.saude.gov.br/. Acess. 10 september 2023.
- 18.Solla Negrao EM, Cabello C, Conz L, Mauad EC, Zeferino LC, Vale DB. The impact of the COVID-19 pandemic on breast cancer screening and diagnosis in a Brazilian metropolitan area. J Med Screen. 2023;30(1):42–6. 10.1177/09691413221122055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negrao EMS, Cabello C, Conz L, Mauad EC, Zeferino LC, Vale DB. The COVID-19 pandemic impact on breast Cancer diagnosis: a retrospective study. Rev Bras Ginecol Obstet. 2022;44(9):871–7. 10.1055/s-0042-1749207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinmohamed AG, Cellamare M, Visser O, de Munck L, Elferink MAG, Westenend PJ, Wesseling J, Broeders MJM, Kuipers EJ, Merkx MAW, Nagtegaal ID, Siesling S. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J Hematol Oncol. 2020;13(1):147. 10.1186/s13045-020-00984-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantellini P, Battisti F, Armaroli P, Giubilato P, Ventura L, Zorzi M, et al. [Oncological organized screening programmes in the COVID-19 era: an Italian survey on accrued delays, reboot velocity, and diagnostic delay estimates]. Epidemiol Prev. 2020;44(5):344–52. [DOI] [PubMed] [Google Scholar]
- 22.Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of Cancer Screening Deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878–84. 10.1001/jamaoncol.2021.0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedewa SA, Cotter MM, Wehling KA, Wysocki K, Killewald R, Makaroff L. Changes in breast cancer screening rates among 32 community health centers during the COVID-19 pandemic. Cancer. 2021;127(23):4512–5. 10.1002/cncr.33859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MJ, Xu L, Qin J, et al. Impact of COVID-19 on Cervical Cancer Screening Rates among women aged 21–65 years in a Large Integrated Health Care System - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(4):109–13. 10.15585/mmwr.mm7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu P, Lucas E, Zhang L, Muwonge R, Murillo R, Nessa A. Leveraging vertical COVID-19 investments to improve monitoring of cancer screening programme - a case study from Bangladesh. Prev Med. 2021;151:106624. 10.1016/j.ypmed.2021.106624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luiz OC, Nisida V, Silva Filho. et at,. Iniquidade racial na mortalidade por câncer de colo de útero no Brasil: estudo de séries temporais de 2002 a 2021. [Internet]. https://cienciaesaudecoletiva.com.br/artigos/iniquidade-racial-na-mortalidade-por-cancer-de-colo-de-utero-no-brasil-estudo-de-series-temporais-de-2002-a-2021/18828?id=18828&id=18828. Acess. 10 september 2023. [DOI] [PubMed]
- 27.Mitchell EP. Declines in Cancer Screening during COVID-19 pandemic. J Natl Med Assoc. 2020;112(6):563–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tait SD, Ren Y, Horton CC, Oshima SM, et al. ,.Characterizing participants in the North Carolina Breast and cervical Cancer Control Program: a retrospective review of 90,000 women. Cancer. 2021;127(14):2515–24. 10.1002/cncr.33473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Instituto Nacional do Câncer - INCA. Dados e números sobre o câncer de colo de útero – relatório anual 2022. Rio de janeiro. 2022. Avaible from: https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/dados_e_numeros_colo_22setembro2022.pdf. Acess. 10 september 2023.
- 30.Duarte DAP, Bustamante-Teixeira MT. Social Iniquity and mortality related to breast and cervical cancers: an integrative review. Rev Fund Care Online. 2018;10(3):877–88. [Google Scholar]
- 31.Kweon SS. Updates on Cancer Epidemiology in Korea, 2018. Chonnam Med J. 2018;54(2):90–100. 10.4068/cmj.2018.54.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luna-Abanto J, Ruiz LG, Laura-Martinez J, Tairo-Cerron T. Cancer incidence and mortality trends in young adults in Metropolitan Lima young adults, 1990–2012. Ecancermedicalscience. 2020;14:1025. 10.3332/ecancer.2020.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira NPD, Santos Siqueira CAD, Lima KYN, de Camargo Cancela M, Souza DLB. Association of cervical and breast cancer mortality with socioeconomic indicators and availability of health services. Cancer Epidemiol. 2020;64:101660. 10.1016/j.canep.2019.101660 [DOI] [PubMed] [Google Scholar]
- 34.Santos TB, Borges AKM, Ferreira JD et al. Prevalência E fatores associados ao diagnóstico de câncer de mama em estágio avançado. Ciênc saúde Coletiva, 2022; 27(2). [DOI] [PubMed]
- 35.Renna NLJ, Silva GA. Late-stage diagnosis of breast cancer in Brazil: analysis of data from hospital-based cancer registries (2000–2012). Rev Bras Ginecol Obstet. 2018;40(3):127–36. 10.1055/s-0038-1624580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smail HM, Mokhtar S, El-Mansy H. Factors associated with late-stage diagnosis of breast Cancer among Egyptian women. J Public Health Res. 2022;10(1):20212874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebremariam A, Dereje N, Addissie A, et al. Factors associated with late-stage diagnosis of breast cancer among women in Addis Ababa, Ethiopia. Breast Cancer Res Treat. 2021;185(1):117–24. 10.1007/s10549-020-05919-5 [DOI] [PubMed] [Google Scholar]
- 38.Darré T, Tchandikou L, Simgban P, et al. Factors associated with late diagnosis of breast cancer in women in Togo, Sub-saharan Africa. BMC Womens Health. 2023;23(1):106. 10.1186/s12905-023-02257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The databases used in this study can be accessed from an online repository: 10.6084/m9.figshare.c.7392682.v1.
All data is available to the public at the link: https://doi.org/10.6084/m9.figshare.