Abstract
Objective.
To determine population-average risk profiles for severe and non-severe periodontitis in US adults (30 years and older) using optimal surveillance measures and standard case definitions.
Methods.
We analyzed data from the 2009–2012 National Health and Nutrition Examination Survey (NHANES), which for the first time used the “gold standard” full-mouth periodontitis surveillance protocol to classify severity of periodontitis following the suggested CDC/AAP case definitions. The probabilities of periodontitis by socio-demographics, behavioral factors, and co-morbid conditions, were assessed using prevalence ratios (PR) estimated by the predicted marginal probability from multivariable generalized logistic regression models. The analyses were further stratified by gender and severity of periodontitis.
Results.
For adults 65 years and older, the likelihood of periodontitis was greater in females (aPR=3.15; 95% CI 2.63 – 3.77) than males (aPR=2.07; 1.76 – 2.43). The likelihood of severe periodontitis was relatively level across all age groups, while non-severe periodontitis increased with age. Compared to non-Hispanic whites, periodontitis was more likely among Hispanics (aPR=1.38; 1.26–1.52) and non-Hispanic blacks (aPR=1.35; 1.22–1.50), and severe periodontitis was most likely among non-Hispanic blacks (aPR=1.82; 1.44–2.31). There was a 50% greater likelihood of periodontitis among current smokers compared to non-smokers. For females, the likelihood of periodontitis was not different between former smokers and non-smokers. Periodontitis was not more likely in persons with pre-diabetes or diabetes, but was more likely only among persons with un-controlled diabetes compared to persons with no diabetes and this association was restricted to only males. Periodontitis was not associated with obesity status.
Conclusions.
An assessment of risk profiles for periodontitis in US adults based on gold standard periodontal measures show important differences by severity of disease and gender. Cigarette smoking, specifically among current smokers remains an important modifiable risk for all levels of periodontitis severity. The higher burden of periodontitis in older women and in males with uncontrolled diabetes is noteworthy. These findings could improve the identification of target populations for effective public health interventions and improve periodontal health of US adults.
Keywords: Periodontitis, dental research, epidemiology, oral health surveys, dental health surveys, nutrition surveys, oral health, periodontal attachment loss, population surveillance, probability, risk, tooth loss, periodontal pocket, surveillance
Introduction
Periodontitis is a chronic disease of the hard and soft tissues supporting the teeth (Pihlstrom et al., 2005). Monitoring and reducing periodontitis in the adult US population through national disease surveillance and public health interventions is a significant concern highlighted in the Surgeon General’s Report on Oral Health (USDHHS 2000b), and is an objective highlighted in Healthy People 2020 (USDHHS 2010) (Eke et al 2012c). To address this concern, it is crucial to better understand the burden and population characteristics, including modifiable and non-modifiable risk factors, associated with periodontitis in US adults.
Recent advances in surveillance of periodontitis in the US, including recent changes in the clinical examination protocols used and the development and use of standard case definitions, have improved our understanding of the burden of periodontitis in the adult population (Page and Eke, 2007; Eke et al., 2012a). Historically, representative population estimates of periodontitis for US adults were based on data from the National Health and Nutrition Examination Survey (NHANES). Prior to 2009, surveillance of periodontitis in NHANES was based on data collected by various Partial Mouth Periodontal Examination (PMPE) protocols, (Dye et al., 2007; Dye and Thornton-Evans, 2007). Identification of periodontitis cases based on PMPE measurements can result in false negatives, impacting the accuracy of prevalence estimates and risk assessments due to misclassification bias (Eke et al., 2010). Using the Full Mouth Periodontal Examination (FMPE) protocol optimizes measurements for the most accurate classification of periodontitis in surveillance. Since 2009, the NHANES has used the FMPE protocol. Similarly, standardized case definitions of periodontitis are critical to the proper characterization of true disease burden in populations, and for comparison between studies. In 2007, an expert workgroup developed and suggested standard case definitions for surveillance of periodontitis (Page and Eke, 2007; Eke et al., 2012a). Using data from the 2009–2012 NHANES and applying these case definitions, we have revised the prevalence of periodontitis in US adults, reporting for the first time direct evidence that almost 50% of the US adults aged 30 years and older have periodontitis (Eke et al., 2012b).
The availability of periodontal measures from a FMPE protocol and simultaneous collection of information on putative risk factors such as socio-demographic, behavioral, and comorbid conditions in the 2009–2012 NHANES for the first time affords the opportunity to revisit and determine a more valid population-average risk profiles for periodontitis in US adults. Several studies have previously reported putative population risk factors for periodontitis, notably socio-demographic characteristics such as age, gender, race/ethnicity, and income; risk behavior such as smoking; and co-morbid conditions such as diabetes and obesity (Albandar, 2005; Genco and Borgnakke, 2013). However, many of these risk indicator studies are subject to limitations such as using periodontitis cases determined from partial mouth periodontal measures and study samples that are not generalizable to the adult US population. (Albandar 2005; Beck and Offenbacher 2005; Genco and Borgnakke 2013). Also, these studies have often used different and inconsistent case definitions that did not capture severity of periodontitis (Albandar 2005; Beck and Offenbacher 2005; Genco and Borgnakke 2013). (Saremi 2005; Taylor 1999).
Identifying population risk factors is critical to developing effective preventative interventions of disease in populations. Thus, we analyzed data from the 2009–2012 NHANES, based on the FMPE and classification of severity of periodontitis based on the suggested Centers for Disease Control/American Academy of Periodontology (CDC/AAP) case definitions for surveillance of periodontitis, to determine population-average risk profiles for periodontitis in US adults at least 30 years of age.
Methods
This study used the publically released NHANES 2009–2010 and 2011–2012 data. NHANES is a stratified, multistage probability sample of the civilian non-institutionalized population in the 50 states of the US and the District of Columbia. The technical details of the survey, including sampling design, periodontal data collection protocols, and data availability can be accessed at www.cdc.gov/nchs/nhanes.htm. Additional information on the oral health data collection and quality of the data during the 2009–2010 cycle are described (Dye et al., 2014). Oral health data collection protocols for the NHANES 2009–2012 were approved by the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) Research Ethics Review Board (equivalent to Institutional Review Boards), and all survey participants provided written informed consent.
Among participants 30 years and older, periodontal examinations were conducted in a mobile examination center (MEC). Gingival recession (distance between the free gingival margin (FGM) and the cemento-enamel junction [CEJ]), followed by periodontal probing depth (PPD) (= distance from FGM to the bottom of the sulcus or periodontal pocket) were measured at six sites around each tooth (mesio-, mid-, and disto-buccal; mesio-, mid-, and disto-lingual) for all teeth, excluding third molars. For measurements at each site, a periodontal probe (Hu-Friedy PCP 2™) with 2–4-6-8-10-12mm graduations was positioned parallel to the long axis of the tooth at each site. Each measurement was rounded to the lower whole millimeter. Data were recorded directly into a NHANES oral health data management program that instantly calculated clinical attachment loss (CAL) as PPD minus gingival recession. Adults aged 30 years or older, who had one or more natural teeth and did not have a health condition that required antibiotic prophylaxis before periodontal probing, were eligible for the periodontal examination during NHANES 2009–2012.
Periodontitis cases were defined following the suggested CDC/AAP case definitions for surveillance of periodontitis (Page and Eke 2007, Eke et al 2012a). Severe periodontitis was defined as having two or more interproximal sites with ≥ 6 mm CAL (not on the same tooth) AND one or more interproximal site(s) with ≥ 5mm PPD. Second, non-severe periodontitis combined two levels of disease: moderate periodontitis, defined as two or more interproximal sites with ≥ 4 mm clinical CAL (not on the same tooth) OR two or more interproximal sites with PPD ≥ 5 mm, but not on the same tooth; and mild periodontitis, defined as ≥ 2 interproximal sites with ≥ 3mm CAL and ≥ 2 interproximal sites with ≥ 4mm PPD (not on the same tooth) or 1 site with ≥ 5mm. Both categories are not truly ordinal as the label suggests because many of the “moderate” cases had insufficient pocket depth to qualify as “mild” and therefore we combined them under the label “non-severe” periodontitis (Eke et al, 2015). Finally, Total periodontitis (reported as periodontitis) was defined as the presence of severe or non-severe periodontitis.
Consistent with previous studies (Borrell and Crawford, 2011, 2012; Dye et al., 2007; Genco and Borgnakke, 2013), we selected for our analyses previously reported potential socio-demographic, behavior and co-morbid risk indicators for periodontitis. Age (categorized as 30 – 44, 45 – 54, 55 – 65, and > 65) and gender (male/female) were included in the analyses as collected by NHANES. Race/ethnicity was analyzed in four groups: non-Hispanic Whites, non-Hispanic Blacks, Hispanics (i.e., anyone who self-identifies as “Hispanic” which is a combination of Mexican-Americans and other Hispanics, of which the majority self-identify as Mexican-Americans), and other race/ethnicity, including multi-racial. Education was classified as less than high school, high school graduate or General Education Development (GED) high school equivalency test, and greater than high school. Poverty status categories or percentage of poverty relative to the federal poverty levels were derived from family income, family size, and the number of children in the family for families with two or fewer adults, and on the ages of the adults in the household. The poverty level was based on definitions originally developed by the Social Security Administration (Fisher 1992). Families or individuals with income below their appropriate thresholds are classified as below the Federal Poverty Level (FPL). These thresholds are updated annually by the U.S. Census Bureau (.http://aspe.hhs.gov/poverty/11poverty.shtml). Marital status was self-reported married, divorced or separated, widowed, living with a partner, and single/never married.
Smoking status was constructed from responses to two questions: (1) Have you smoked at least 100 cigarettes in your life? and (2) Do you now smoke cigarettes? Respondents who reported smoking every day or some days and had smoked at least 100 cigarettes were categorized as current smokers; respondents who reported currently not smoking but having smoked more than 100 cigarettes in the past were categorized as former smokers; and respondents who reported not having smoked at least 100 cigarettes were categorized as non-smokers.
Diabetes status was defined by self-report, levels of fasting plasma glucose (FPG) or blood levels of glycosylated hemoglobin A1c (HbA1c or A1c). Participants who responded “Yes” to the question “have you ever been told by your doctor or other care provider that you had diabetes?” were considered to have diagnosed diabetes, and those who answered “No” were classified as not diagnosed. Un-controlled diabetes was defined as diagnosed diabetes and FPG ≥ 126 mg/dL or HbA1c ≥ 7.0; controlled diabetes was defined as diagnosed diabetes and FPG <126 mg/dL or HbA1c < 7.0; pre-diabetes was defined as no diagnosed diabetes and 100 ≤ FPG ≤ 126 mg/dL or 5.7 ≤ HbA1c ≤ 6.5; and non-diabetes was defined as self-reported no diagnosed diabetes and FPG < 100 mg/dL or HbA1c <5.7. Un-diagnosed diabetes was defined as no diagnosed diabetes and FPG ≥ 125 or HbA1c ≥ 7.0. Duration of diabetes was categorized as 0 – 2, 3 – 6, 7 – 10, 11 – 17 and > 18 years, since told by a doctor you had diabetes. Body mass index (BMI) was used to determine levels of obesity status. An individual with BMI ≥ 30 was considered obese, between ≥25 - < 30 overweight, between 18.5 - < 25 normal weight, and <18.5 as underweight (Keys 1972, CDC 2011).
In NHANES 2009–2012, adults aged 30 years or older with at least one natural tooth and not suffering from any health condition requiring antibiotic prophylaxis prior to periodontal probing were eligible for the periodontal examination yielding a combined total of 9402 adults aged 30 years. Out of these, 1,631 were excluded from the oral health assessment in the MEC due to medical exclusions or did not complete their examination, and 705 were identified as edentulous (edentate, having no teeth) and were excluded. Our analyses were based on the remaining 7,066 participants representing a weighted population of approximately 141.0 million civilian non-institutionalized American adults 30 years of age and older, and further restricted the analysis to respondents with non-missing values for all covariates and the dependent variable (see footnote for each table for sample size).
Descriptive statistics were calculated by severity of periodontitis (i.e., severe and non-severe periodontitis, and total periodontitis) and by gender. Our preliminary analyses suggested that gender modified the effect of several putative risk factors on periodontitis hence we further stratified our analyses by gender. Accounting for the high prevalence of periodontitis (> 10%) in the US adult population, we used Prevalence Ratios (PR) to avoid overestimation of the association between each exposure and periodontitis. All analyses were conducted using SAS-callable SUDAAN and NHANES MEC examination weights to account for complex survey sampling design. The adjusted weighted predicted prevalence ratios (PR) and 95% confidence interval (CI) for the likelihood of having periodontitis (Total, severe or non-severe) by selected co-variates was calculated using (Proc RLOGIST) log-linear regression models.
Results
Overall, a total of 46% US adults aged 30 and older had periodontitis, distributed as 8.9% with severe periodontitis and 37.1% with non-severe periodontitis. More males than females had severe periodontitis (13.3% vs. 4.7%) and non-severe periodontitis (41.6% vs. 32.7%). The study population was about evenly distributed by gender (51% females), but more males than females were current and former smokers, had diabetes and were overweight (Table 1).
Table 1.
Population Characteristics and Unadjusted Prevalence Ratios for Periodontitis among US adults 30 years and older: NHANES 2009–2012
| Groups | N | Weighted N (in millions) | Percent | SE | Male | Female | Total Periodontitis Crude PR (95% CI) |
Severe Periodontitis Crude PR (95% CI) |
Non-Severe Periodontitis Crude PR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| CDC Severe | 879 | 12.5 | 8.9 | 0.59 | 13.3 | 4.7 | |||
| CDC Non-Severe | 3005 | 52.2 | 37.1 | 1.51 | 41.6 | 32.7 | |||
| CDC Non Cases | 3182 | 76.2 | 54.0 | 1.60 | 45.1 | 62.6 | |||
| Total | 7066 | 140.9 | 100.0 | 100.0 | 100.0 | ||||
| Age Groups | |||||||||
| Age 65+ | 1511 | 23.8 | 16.9 | 0.81 | 16.1 | 17.6 | 2.21(1.98–2.47) | 2.26(1.75–2.93) | 2.35(2.08–2.66) |
| Age 55–64 | 1397 | 28.1 | 19.9 | 0.77 | 19.5 | 20.3 | 1.79(1.63–1.96) | 2.58(2.03–3.30) | 1.78(1.60–1.98) |
| Age 45–54 | 1619 | 36.0 | 25.6 | 0.74 | 25.6 | 25.6 | 1.52(1.34–1.73) | 2.19(1.77–2.70) | 1.49(1.27–1.74) |
| Age 30–44 (Ref) | 2539 | 53.0 | 37.6 | 1.31 | 38.8 | 36.5 | |||
| Gender | |||||||||
| Male | 3515 | 69.0 | 49.0 | 0.64 | NA | NA | 1.47(1.37–1.58) | 2.84(2.29–3.53) | 1.40(1.29–1.51) |
| Female (Ref) | 3551 | 71.9 | 51.0 | 0.64 | NA | NA | |||
| Race/Ethnicity | |||||||||
| Hispanics | 1774 | 18.8 | 13.4 | 1.81 | 13.8 | 12.9 | 1.45(1.30–1.62) | 1.89(1.47–2.43) | 1.45(1.29–1.63) |
| NH Black | 1512 | 15.1 | 10.7 | 1.16 | 9.8 | 11.7 | 1.45(1.29–1.64) | 2.31(1.72–3.09) | 1.41(1.24–1.62) |
| Others | 762 | 10.0 | 7.1 | 0.74 | 6.7 | 7.6 | 1.26(1.09–1.47) | 1.73(1.17–2.57) | 1.24(1.04–1.47) |
| NH White (Ref) | 3018 | 97.0 | 68.8 | 2.41 | 69.7 | 67.8 | |||
| Education | |||||||||
| < High School | 1784 | 23.1 | 16.4 | 1.04 | 17.0 | 15.8 | 1.80(1.64–1.98) | 2.98(2.46–3.61) | 1.81(1.61–2.02) |
| High School | 1514 | 29.5 | 20.9 | 0.99 | 21.9 | 20.0 | 1.50(1.39–1.62) | 2.08(1.63–2.64) | 1.49(1.36–163) |
| >High School (Ref) | 3757 | 88.3 | 62.7 | 1.52 | 61.1 | 64.2 | |||
| Poverty Level | |||||||||
| Missing Value on FPL | 618 | 9.6 | 6.8 | 0.55 | 6.5 | 7.1 | 1.65(1.42–1.91) | 2.41(1.46–3.97) | 1.63(1.39–1.92) |
| Less than 100% FPL | 1257 | 15.5 | 11.0 | 0.72 | 10.2 | 11.7 | 1.89(1.67–2.13) | 3.00(2.11–4.28) | 1.89(1.64–2.17) |
| 100–199% FPL | 1683 | 24.9 | 17.7 | 0.89 | 17.1 | 18.2 | 1.73(1.58–1.88) | 2.76(1.97–3.87) | 1.70(1.54–1.88) |
| 200–399% FPL | 1660 | 37.6 | 26.6 | 1.45 | 26.5 | 26.8 | 1.46(1.27–1.68) | 1.63(1.14–2.33) | 1.48(1.28–1.71) |
| ≥ 400% FPL (Ref) | 1848 | 53.4 | 37.9 | 1.54 | 39.6 | 36.2 | |||
| Marital Status | |||||||||
| Married | 4049 | 88.4 | 62.7 | 1.04 | 65.3 | 60.2 | 0.93(0.82–1.06) | 0.95(0.67–1.35) | 0.93(0.81–1.06) |
| Divorced or Separated | 1170 | 20.9 | 14.9 | 0.59 | 12.6 | 17.0 | 1.23(1.08–1.39) | 1.71(1.20–2.44) | 1.20(1.03–1.39) |
| Widowed | 525 | 7.3 | 5.2 | 0.26 | 2.4 | 7.8 | 1.37(1.15–1.63) | 1.42(0.94–2.15) | 1.41(1.17–1.70) |
| Living with Partner | 495 | 9.5 | 6.7 | 0.50 | 7.9 | 5.7 | 1.21(1.01–1.43) | 1.62(1.15–2.29) | 1.18(0.97–1.44) |
| Never Married (Ref) | 821 | 14.8 | 10.5 | 0.69 | 11.9 | 9.2 | |||
| Smoking | |||||||||
| Current Smoker | 1338 | 24.6 | 17.4 | 0.62 | 20.5 | 14.5 | 1.74(1.63–1.85) | 3.45(2.78–4.29) | 1.69(1.58–1.82) |
| Former Smoker | 1769 | 37.0 | 26.3 | 1.12 | 29.5 | 23.2 | 1.27(1.18–1.37) | 1.74(1.28–2.35) | 1.25(1.15–1.35) |
| Non-Smoker (Ref) | 3956 | 79.3 | 56.3 | 1.01 | 50.0 | 62.3 | |||
| Diabetes | |||||||||
| Diabetes Not Controlled | 481 | 7.1 | 5.0 | 0.35 | 5.7 | 4.4 | 1.72(1.55–1.92) | 2.13(1.64–3.27) | 1.77(1.55–2.02) |
| Diabetes Controlled | 733 | 10.7 | 7.6 | 0.39 | 8.3 | 6.9 | 1.67(1.52–1.83) | 2.12(1.48–3.04) | 1.71(1.53–1.92) |
| Pre-Diabetes | 1904 | 33.9 | 24.0 | 0.62 | 23.2 | 24.8 | 1.43(1.31–.155) | 1.66(1.34–2.06) | 1.45(1.32–1.59) |
| Non-Diabetes (Ref) | 3948 | 89.3 | 63.4 | 0.79 | 62.8 | 63.9 | |||
| BMI Groups | |||||||||
| Obese | 2690 | 51.4 | 37.1 | 1.02 | 35.9 | 38.2 | 1.11(1.02–1.21) | 0.98(0.77–1.25) | 1.14(1.03–1.28) |
| Overweight | 2464 | 50.5 | 36.4 | 0.87 | 41.4 | 31.6 | 1.09(1.00–1.19) | 1.06(0.81–1.39) | 1.11(0.99–1.23) |
| Normal Weight (Ref) | 1790 | 36.8 | 26.5 | 0.85 | 22.8 | 30.2 | |||
For Non-Severe Periodontitis, severe cases were excluded from the denominator. NA=not applicable.
Using multi-variable analyses (Table 2), the likelihood of having periodontitis or non-severe periodontitis increased steadily with increasing age, while the likelihood of having severe periodontitis was relatively level across age groups. By gender, the likelihood of severe periodontitis was two times more likely among males compared to females (aPR=2.68, 2.22 – 3.23). By race/ethnicity, having periodontitis was most likely among Hispanics (aPR=1.38; 1.26–1.52) and non-Hispanic blacks (aPR=1.35; 1.22–1.50) compared to non-Hispanic whites, and severe periodontitis was most likely among non-Hispanic blacks (aPR=1.82; 1.44–2.31). Periodontitis did not vary significantly by education. Overall, there was a steady increase in the likelihood of periodontitis with increasing poverty (lower FPL). Periodontitis was significantly more likely among current and formers smokers compared to non-smokers. The risk for periodontitis was highest among current smokers (aPR 1.54, 1.45 – 1.65), and smoking was more strongly associated with severe forms of periodontitis (aPR = 2.46, 1.87 – 3.24). By diabetes status, periodontitis was more likely in persons with un-controlled diabetes, specifically among those with severe periodontitis (aPR = 1.42, 1.02 – 1.98) and this association was restricted to only males. Finally, the likelihood of periodontitis did not significantly vary by obesity status (i.e., normal weight, overweight and obese).
Table 2.
Prevalence Ratios of Periodontitis by Socio-demographic factors, Smoking, and co-morbid conditions: NHANES 2009–2012
| Effect | Total Periodontitis (N=6924) | Severe Periodontitis (N=6924) | Non-Severe Periodontitis (N=6061) | |||
|---|---|---|---|---|---|---|
| PR (95% CI) | P-Value | PR (95% CI) | P-Value | PR (95% CI) | P-Value | |
| Age 65+ vs. Age 30–44 | 2.46(2.14–2.82) | <0.0001 | 2.61(1.88–3.62) | <0.0001 | 2.73(2.34–3.19) | <0.0001 |
| Age 50–64 vs. Age 30–44 | 2.01(1.80–2.24) | 2.80(2.07–3.79) | 2.13(1.90–2.40) | |||
| Age 45–54 vs. Age 30–44 | 1.62(1.41–1.87) | 2.22(1.78–2.77) | 1.66(1.41–1.96) | |||
| Male vs. Female | 1.47(1.38–1.58) | <0.0001 | 2.68(2.22–3.23) | <0.0001 | 1.47(1.36–1.58) | <0.0001 |
| Hispanics vs. NH Whites | 1.38(1.26–1.52) | <0.0001 | 1.56(1.23–2.00) | <0.0001 | 1.44(1.29–1.60) | <0.0001 |
| NH Blacks vs. NH Whites | 1.35(1.22–1.50) | 1.82(1.44–2.31) | 1.36(1.19–1.54) | |||
| Others vs. NH Whites | 1.34(1.19–1.51) | 1.69(1.16–2.45) | 1.37(1.19–1.58) | |||
| <High School vs. > High School | 1.29(1.17–1.42) | <0.0001 | 1.63(1.26–2.12) | 0.0015 | 1.29(1.15–1.45) | <0.0001 |
| High School vs.>High School | 1.20(1.12–1.30) | 1.50(1.14–1.98) | 1.21(1.10–1.32) | |||
| Missing FPL vs. >=400% FPL | 1.27(1.10–1.48) | <0.0001 | 1.69(1.03–2.77) | 0.0347 | 1.26(1.07–1.49) | <0.0001 |
| Less than 100% FPL vs. >=400% FPL | 1.41(1.25–1.59) | 1.71(1.14–2.57) | 1.44(1.26–1.65) | |||
| 100–199% FPL vs. >=400% FPL | 1.35(1.23–1.48) | 1.82(1.22–2.71) | 1.36(1.22–1.52) | |||
| 200–399% FPL vs. >=400% FPL | 1.25(1.12–1.39) | 1.31(0.91–1.90) | 1.27(1.13–1.44) | |||
| Married vs. Never Married | 0.96(0.87–1.06) | 0.0301 | 1.09(0.78–1.52) | 0.0866 | 0.93(0.83–1.04) | 0.1063 |
| Divorced vs. Never Married | 1.10(0.99–1.23) | 1.44(1.02–2.04) | 1.05(0.92–1.20) | |||
| Widowed vs. Never Married | 0.92(0.77–1.11) | 1.15(0.76–1.75) | 0.88(0.72–1.09) | |||
| Living with Partner vs. Never Married | 1.06(0.91–1.23) | 1.21(0.86–1.69) | 1.04(0.87–1.25) | |||
| Current Smoker vs. Non Smoker | 1.54(1.45–1.65) | <0.0001 | 2.46(1.87–3.24) | <0.0001 | 1.56(1.44–1.68) | <0.0001 |
| Former Smoker vs. Non Smoker | 1.09(1.02–1.17) | 1.39(1.03–1.87) | 1.07(0.99–1.16) | |||
| Diabetes Not Controlled vs. Non Diabetes | 1.16(1.04–1.29) | 0.0366 | 1.42(1.02–1.98) | 0.1922 | 1.14(1.00–1.29) | 0.1693 |
| Diabetes Controlled vs. Non Diabetes | 1.14(1.03–1.27) | 1.27(0.83–1.94 | 1.13(1.00–1.27) | |||
| Pre-Diabetes vs. Non Diabetes | 1.07(0.98–1.16) | 1.17(0.93–1.45) | 1.06(0.97–1.16) | |||
| Obese vs. Normal Weight | 1.02(0.94–1.10) | 0.8907 | 0.93(0.74−−1.16) | 0.7083 | 1.03(0.94–1.14) | 0.7690 |
| Overweight vs. Normal Weight | 1.02(0.94–1.11) | 1.02(0.81–1.27) | 1.03(0.93–1.15) | |||
6924 respondents were included in the model for total and severe periodontitis; 6061 respondents were included in the model for non-severe periodontitis. For non-severe periodontitis, severe periodontitis cases were excluded from the denominator.
When stratified by gender (Table 3, figures 1 a–c), periodontitis and non-severe periodontitis were more likely among females 65 years and older. Periodontitis was equally likely in female former smokers and non-smokers. In females, periodontitis and non-severe periodontitis were not significantly associated with diabetes status. In males, all levels of severity of periodontitis were more likely among un-controlled diabetes when compared with persons without diabetes.
Table 3.
Gender Stratified Prevalence Ratios for Periodontitis by Socio-demographic, Smoking and Co-morbid Conditions: NHANES 2009–2012
| Total periodontitis (N=6924) | Severe Periodontitis (N=6924) | Non-Severe Periodontitis (N=6061) | ||||
|---|---|---|---|---|---|---|
| Effect | Male (N=3458) | Female(N=3466) | Male(N=3458) | Female(N=3466) | Male(N=2836) | Female(N=3225) |
| PR (CI) | PR (CI) | PR (CI) | PR (CI) | PR (CI) | PR (CI) | |
| Age 65+ vs. Age 30–44 | 2.07(1.76–2.43) | 3.15(2.63–3.77) | 2.62(1.95–3.53) | 2.71(1.23–5.94) | 2.26(1.85–2.75) | 3.44(2.83–4.17) |
| Age 55–64 vs. Age 30–44 | 1.82(1.64–2.02) | 2.34(1.97–2.79) | 2.95(2.18–3.99) | 2.65(1.28–5.48) | 1.91(1.71–2.13) | 2.47(2.06–2.95) |
| Age 45–54 vs. Age 30–44 | 1.54(1.29–1.85) | 1.77(1.47–2.14) | 2.28(1.68–3.10) | 2.11(1.24–3.57) | 1.57(1.25–1.97) | 1.82(1.50–2.20) |
| NH Hispanics vs. NH Whites | 1.33(1.22–1.45) | 1.45(1.25–1.68) | 1.51(1.14–2.00) | 1.74(1.25–2.42) | 1.40(1.26–1.56) | 1.47(1.25–1.72) |
| NH Blacks vs. NH Whites | 1.30(1.19–1.42) | 1.39(1.17–1.66) | 1.72(1.32–2.26) | 2.15(1.36–3.42) | 1.32(1.18–1.49) | 1.37(1.14–1.65) |
| Others vs. NH Whites | 1.19(1.04–1.36) | 1.55(1.28–1.87) | 1.89(1.27–2.83) | 1.21(0.64–2.30) | 1.17(0.99–1.38) | 1.61(1.31–1.97) |
| <High School vs. > High School | 1.29(1.17–1.43) | 1.29(1.09–1.52) | 1.65(1.19–2.29) | 1.60(1.18–2.16) | 1.30(1.14–1.48) | 1.28(1.07–1.53) |
| High School vs.>High School | 1.22(1.11–1.34) | 1.18(1.03–1.35) | 1.64(1.19–2.26) | 1.18(0.75–1.87) | 1.21(1.07–1.38) | 1.19(1.02–1.39) |
| Missing FPL vs. ≥400% FPL | 1.18(0.99–1.42) | 1.43(1.17–1.75) | 1.75(1.01–3.05) | 1.69(0.83–3.46) | 1.14(0.92–1.41) | 1.45(1.16–1.81) |
| Less than 100% FPL vs. ≥400% FPL | 1.32(1.15–1.51) | 1.58(1.29–1.92) | 1.70(1.05–2.74) | 1.93(0.90–4.14) | 1.34(1.15–1.56) | 1.60(1.29–1.97) |
| 100–199% FPL vs. ≥400% FPL | 1.24(1.12–1.36) | 1.54(1.27–1.88) | 1.77(1.08–2.91) | 2.11(1.06–4.20) | 1.23(1.12–1.36) | 1.55(1.26–1.91) |
| 200–399% FPL vs. ≥400% FPL | 1.14(1.02–1.28) | 1.42(1.17–1.72) | 1.30(0.87–1.92) | 1.47(0.67–3.24) | 1.14(1.00–1.31) | 1.45(1.18–1.79) |
| Married vs. Never Married | 1.06(0.93–1.20) | 0.84(0.72–0.97) | 1.06(0.72–1.55) | 1.23(0.78–1.93) | 1.05(0.90–1.24) | 0.80(0.68–0.95) |
| Divorced vs. Never Married | 1.20(1.04–1.37) | 0.97(0.81–1.18) | 1.45(0.90–2.33) | 1.48(0.83–2.61) | 1.15(0.96–1.38) | 0.92(0.76–1.13) |
| Widowed vs. Never Married | 0.92(0.72–1.17) | 0.80(0.62–1.04) | 1.08(0.57–2.05) | 1.29(0.70–2.35) | 0.86(0.66–1.12) | 0.76(0.57–1.01) |
| Living with Partner vs. Never Married | 1.21(1.00–1.45) | 0.84(0.68–1.04) | 1.16(0.78–1.73) | 1.43(0.77–2.66) | 1.24(0.99–1.55) | 0.80(0.62–1.03) |
| Current Smoker vs. Non Smoker | 1.50(1.37–1.63) | 1.61(1.45–1.80) | 2.33(1.73–3.14) | 2.97(1.87–4.70) | 1.54(1.39–1.71) | 1.59(1.41–1.80) |
| Former Smoker vs. Non Smoker | 1.11(1.02–1.21) | 1.07(0.93–1.23) | 1.45(1.02–2.06) | 1.19(0.77–1.84) | 1.09(0.98–1.21) | 1.05(0.91–1.22) |
| Diabetes Not Controlled vs. Non Diabetes | 1.23(1.09–1.40) | 1.04(0.82–1.33) | 1.41(1.01–1.96) | 1.61(0.90–2.88) | 1.25(1.07–1.46) | 1.00(0.75–1.32) |
| Diabetes Controlled vs. Non Diabetes | 1.12(1.00–1.26) | 1.17(0.96–1.43) | 1.17(0.70–1.95) | 1.58(0.83–2.99) | 1.12(0.96–1.31) | 1.13(0.911.41) |
| Pre-Diabetes vs. Non Diabetes | 1.06(0.96–1.16) | 1.07(0.93–1.21) | 1.16(0.92–1.46) | 1.19(0.74–1.92) | 1.05(0.94–1.17) | 1.06(0.92–1.22) |
| Obese vs. Normal Weight | 0.99(0.89–1.09) | 1.06(0.92–1.22) | 1.08(0.78–1.50) | 0.65(0.46–0.93) | 0.98(0.85–1.12) | 1.10(0.94–1.29) |
| Overweight vs. Normal Weight | 1.02(0.91–1.14) | 1.02(0.87–1.19) | 1.20(0.87–1.64) | 0.67(0.44–1.01) | 1.01(0.87–1.16) | 1.04(0.88–1.24) |
6924 respondents were included in the model for total and severe periodontitis; 6061 respondents were included in the model for non-severe periodontitis. For non-severe periodontitis, severe periodontitis cases were excluded from the denominator.
Figure 1.
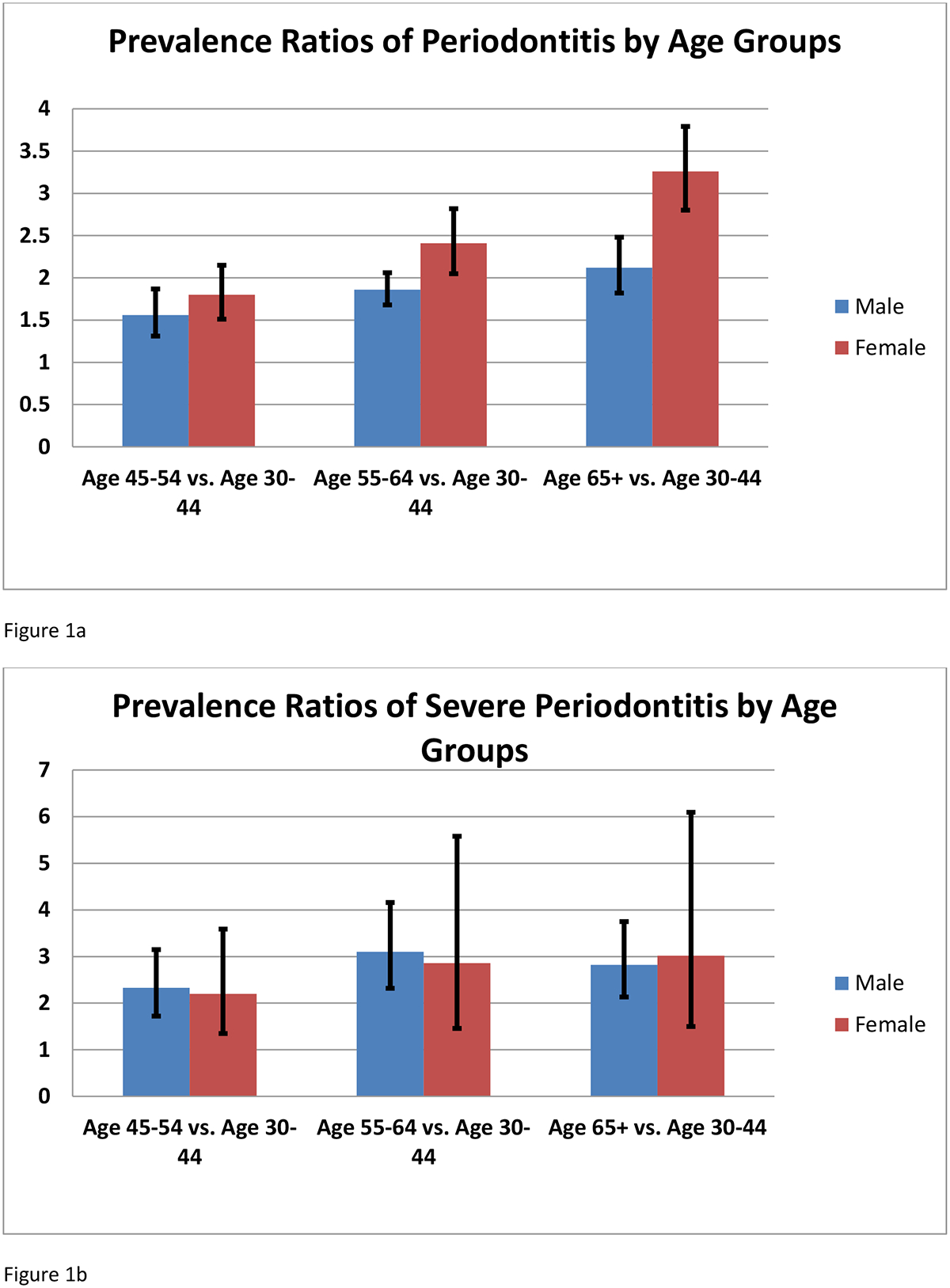
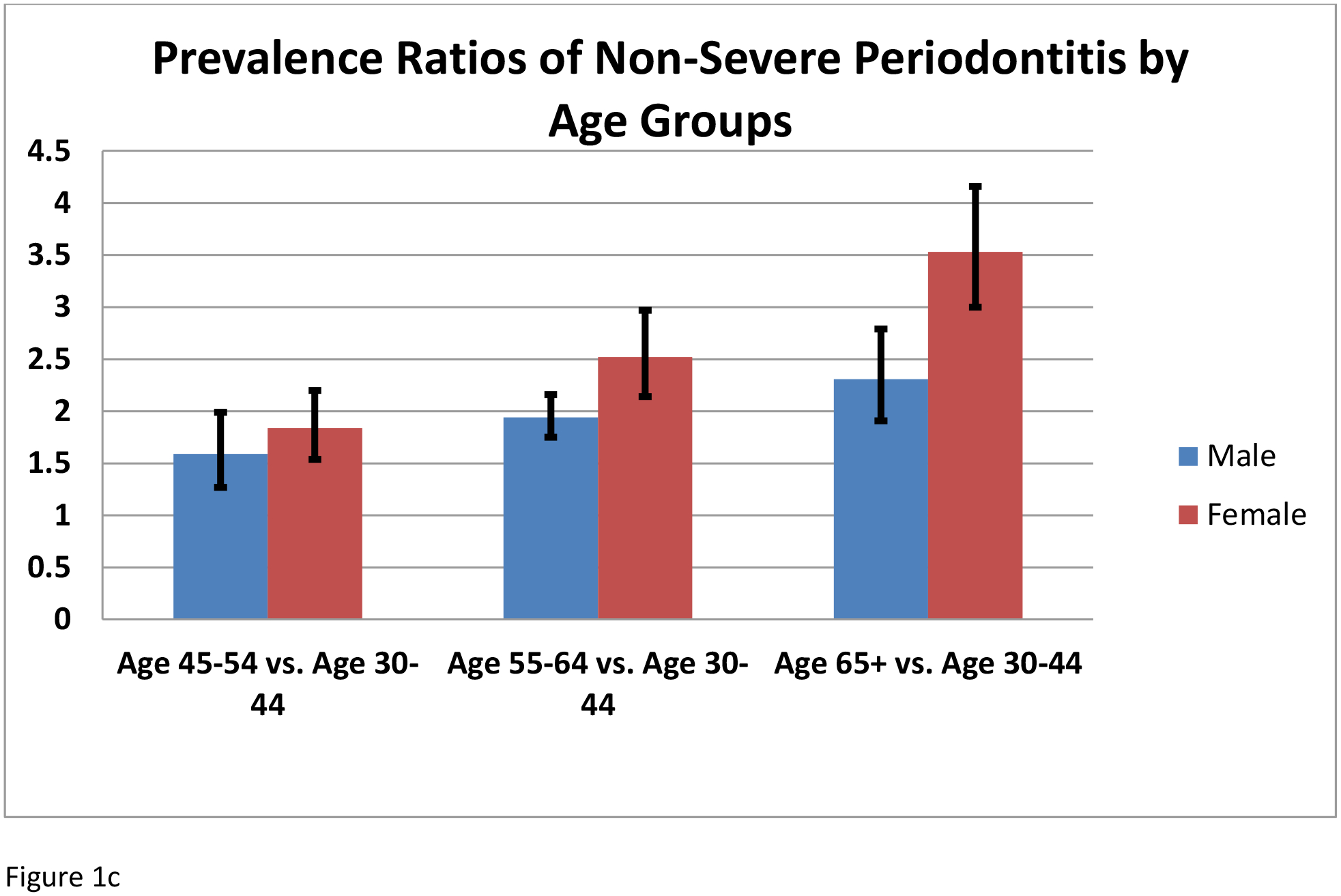
Prevalence ratios by age groups and sex: A) TP; B) SP; and C) NSP (mild or moderate).
Further analyses of periodontitis and diabetes also suggested that the duration of diabetes did not significantly correlate with the likelihood of periodontitis after adjusting for all covariates. Similarly, no trend was observed between periodontitis and fasting glucose amongst persons with diabetes. In females without diabetes, total periodontitis and non-severe periodontitis (but not severe periodontitis) significantly increased with increasing levels of fasting glucose levels. In males without diabetes, no trend was observed for any level of severity of periodontitis and increasing fasting glucose levels. Periodontitis were significantly associated with increasing levels of HbA1c in both male and females without diabetes. Among persons with diabetes, only severe periodontitis was significantly correlated with increasing levels of HbA1c in males (results not included).
Discussion
This study finds new and important risk profiles for periodontitis by severity of periodontitis and gender. Notably, we report differences in the likelihood of non-severe periodontitis and severe periodontitis by increasing age, and a greater likelihood of periodontitis in older females than males (≥ 65 years old). While the probability of periodontitis was consistently associated with current smoking, we find that periodontitis was only significant in male former smokers and not in females. Periodontitis was not more likely in persons with controlled diabetes, only more likely among males with uncontrolled diabetes. Among females, this relationship was observed only between severe periodontitis and uncontrolled diabetes. Finally, this study did not find that periodontitis was more likely associated with obesity status regardless of gender.
In this study, we report a higher likelihood of periodontitis in older women compared to males at ≥65years old. This finding is not widely recognized in periodontal epidemiology even though a similar US national study had also reported that older men had better periodontal status then females (Albandar, 2002). The higher likelihood of periodontitis in older women can be attributed to women keeping more of their teeth and a possible higher prevalence of tooth loss in males, and also that older women at this age are likely to be post-menopausal when production of estrogen has decreased. Lower estrogen production has been associated with increased risk for periodontitis (Friedlander, 2002). In 2000, approximately 45.6 million women in the United States were in the postmenopausal phase of life, and more women are living beyond 65 (USDHHS 2000a; US census 2002). Gender dimorphism is known for several chronic diseases and a recent study on the classification of periodontitis based on gene expression has demonstrated that severity and extent of periodontitis are most strongly linked with gender compared to other putative risk factors for periodontitis (Kebschull et al., 2014, Alam, Misha and Chandrasekaran, 2012).
Consistent with previous studies, we find that the likelihood of periodontitis is highest among current smokers (Albandar 2002, Tomar and Asma 2000). The likelihood of periodontitis dropped significantly among former smokers, and was not significantly different from non-smokers in females. This finding supports the potential benefits of smoking cessation in preventing and controlling periodontitis. In 2013, an estimated 1 in 5 (17.8%) of US adults were current smokers (CDC, 2014, Agaku et al., 2014). Also, it is worth noting that we reported a higher but non-significant likelihood for periodontitis among female current smokers compared to men. Postmenopausal female smokers have been reported to experience more severe alveolar bone loss than non-smokers, as smoking and osteoporosis/osteopenia combine to exacerbate bone loss (Payne JB et al., 2000).
Our determination of diabetes status was based on results from actual laboratory tests of fasting plasma glucose and HbA1C, an objective measure of diabetes. Whereas relationships between periodontitis and diabetes have been widely reported in clinical studies conducted among persons with diabetes, evidence for this association by severity of disease and by gender at the population level is scarce. Our findings are consistent with reports that severe periodontitis is more prevalent in people with diabetes. Specifically, about one-third of people with diabetes have severe forms of periodontal disease and adults aged 45 years or older with poorly controlled diabetes are three times more likely to have severe periodontitis than those without diabetes (Saremi et al, 2005., Taylor, Loesche and Terpenning 2000). Also, a recent prospective population study similarly report significant relationships between periodontitis and diabetes only in persons with uncontrolled diabetes (Demmer et al., 2012).
In the US population, 9.3% of adults have diagnosed (21.0 million) and undiagnosed (8.1 million) diabetes, with 12.3% of adults aged 20 and over having diabetes (CDC, 2014). Among the latter with diagnosed diabetes, about 86% use insulin (14%), anti-diabetes oral medication (57%) or both (15%), so only about 14% do not take medications to control their diabetes (CDC 2014). This distribution may help explain our findings of no significant risk for periodontitis associated with controlled diabetes, and may indicate that the risk is mediated by the level of successful diabetes management that decreases the proportion of uncontrolled diabetes cases in the US population.
We report no increased likelihood of periodontitis associated with obesity status regardless of gender or severity of periodontitis. This finding is consistent with the finding for another recent population-based study (de Castilos et al., 2012,). However, a recent systemic review of five studies reported an association between weight gain and obesity and higher risk for new incident cases of periodontitis. Notably, the US population studies included in this review used self-report periodontitis and a single measure of probing depth as the outcome. The importance of obesity to and its influence on periodontitis is attributed to BMI being a main risk factor for pre-diabetes (Arora et al., 2014). In this study, we did not find an increase likelihood of periodontitis among pre-diabetes. While some studies have implicated obesity as a putative risk factor for periodontitis, we find that most of these studies were conducted in hospital patients or insmall samples (Moura-Grec et al 2014, Palle 2013).
We confirm that several socioeconomic indicators, such as race/ethnicity, poverty level, and education, are associated with increased risk for periodontitis and therefore may account for disparities in periodontitis across US adult communities. For these indicators however, we did not find that the effects were modified by gender. Borrell and Crawford have discussed how these socioeconomic variables interact to influence racial/ethnic disparities in populations (Borrell and Crawford 2012). Particularly, they conclude that periodontal disease is inversely related to education and income after controlling for age and gender, and that the differences in education and income explain most, if not all, of the observed disparities in periodontitis by race and ethnicity.
This study has some notable limitations. The study is cross-sectional and therefore does not allow any deduction of causality. The included population was limited to adults 30 years and older and did not include institutionalized persons, such as older adults in nursing home settings and adults in prisons, which may introduce some selection bias. There are possibilities of misclassified periodontitis cases because third molars were excluded, furcation involvement status and bleeding upon probing were not assessed (a parameter that could indicate active inflammation). Finally, a small number of non-severe cases may be misclassified as non-cases based on the classification criteria used.
In conclusion, this study using optimal surveillance measures and standardized case definitions, provides new information on potential risk indicators for public health action to prevent periodontitis in the contemporary US adult population 30 years and over. Cigarette smoking, specifically current smoking remains an important modifiable risk for all levels of periodontitis severity. The higher burden of periodontitis in older women and the differences in potential risk profiles for severe and non-severe periodontitis by gender and co-morbid conditions (i.e. uncontrolled diabetes) is noteworthy, especially in light of the higher burden of multiple chronic conditions occurring in women compared to men (Lochner and Cox, 2013). Our findings provides potential areas of focus to prevent and control periodontitis to improve the oral health, general health and wellbeing of our adults US population.
Acknowledgments
The authors acknowledge the contributions from the CDC Periodontal Disease Surveillance Workgroup (Eke and Genco, 2007). They have no conflict of interest with this article.
Conflict of interest and source of funding:
The authors declared no conflict of interest and received no external funding for this study.
Footnotes
Disclaimer:
The findings and conclusion in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
References
- ADA (American Diabetes Association) (2014). Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- Agaku IT, King BA, Dube SR (2014). Current cigarette smoking among adults — United States, 2005–2012. Morb Mortal Wkly Rep 63:29–34. [PMC free article] [PubMed] [Google Scholar]
- Albandar JM (2002). Global Risk Factors and Risk Indicators for Periodontal Disease. Perio 2000 29:177–206. [DOI] [PubMed] [Google Scholar]
- Albandar JM (2005). Epidemiology and risk factors of periodontal diseases. Dent Clin North Am 49:517–532. [DOI] [PubMed] [Google Scholar]
- Arora N, Papapanou PN, Rosenbaum M, Jacobs DR Jr., Desvarieux M, Demmer RT (2014). Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. J Clin Periodontol 41:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S (2005). Systemic effects of periodontitis epidemiology of periodontal disease and cardiovascular diseases. J Perio 76:2089–2100 [DOI] [PubMed] [Google Scholar]
- Borrell LN, Crawford ND (2011). Social disparities in periodontitis among US adults: the effect of allostatic load. J Epidemiol Community Health 65:144–149. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Crawford ND (2012). Socioeconomic position indicators and periodontitis: examining the evidence. Periodontol 2000 58:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Current Cigarette Smoking Among Adults—United States, 2005–2013. Morbidity and Mortality Weekly Report 2014; 63 (47):1108–12 [accessed 2015 Aug 19]. [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) (2011). Healthy weight - it’s not a diet, it’s a lifestyle! About BMI for adults; interpretation of BMI for adults. Available at: http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. Accessed July 2, 2014.
- CDC (Centers for Disease Control and Prevention) (2014). National diabetes statistics report: estimates of diabetes and its burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed July 2, 2014. [Google Scholar]
- De castilhos ED, Horta BL, Gigante DP, Demarco FF, Peres KG and Peres MA. Assocaition between obesity and periodntal disease in young adults: a popualtion based birth cohort. J Clin Periodontol 2012. 39(8):717–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Holtfreter B, Desvarieux M, Jacobs DR Jr., Kerner W, Nauck M, et al. (2012). The influence of type 1 and type 2 diabetes on periodontal disease progression: prospective results from the Study of Health in Pomerania (SHIP). Diabetes Care 35:2036–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BA, Li X, Lewis BG, Iafolla T, Beltran-Aguilar ED, Eke PI (2014). Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2009–2010. J Public Health Dent [E-pub ahead of print May 22, 2014] In press. [DOI] [PubMed] [Google Scholar]
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al. (2007). Trends in oral health status: United states, 1988–1994 and 1999–2004. National Center for Health Statistics. Vital Health Stat 11 (248). [PubMed] [Google Scholar]
- Dye BA, Thornton-Evans GO (2007). A brief history of national surveillance efforts for periodontal disease in the United States. J Periodontol 78(7 Suppl):S1373–S1379. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, on behalf of the participating members of the CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washington, Seattle, USA), Gary Slade (University of North Carolina, Chapel Hill, USA), George W. Taylor (University of Michigan, Ann Arbor, USA), Wenche Borgnakke (University of Michigan, Ann Arbor, USA), and representatives of the American Academy of Periodontology (2012b). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914–920. [DOI] [PubMed] [Google Scholar]
- Eke PI, Genco RJ (2007). CDC periodontal disease surveillance project: background, objectives, and progress report. J Periodontol 78(7 Suppl 1):S1366–S1371. [DOI] [PubMed] [Google Scholar]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ (2012a). Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83:1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans G, Dye B, Genco R (2012c). Advances in surveillance of periodontitis: the Centers for Disease Control and Prevention (or CDC) periodontal disease surveillance project. J Periodontol 83:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA (2010). Accuracy of NHANES periodontal examination protocols. J Dent Res 89:1208–1213. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ (2015). Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol 86:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GM (1992). Poverty Guidelines for 1992. Social Security Bullentin. 55:43–46. [PubMed] [Google Scholar]
- Friedlander AH. The physiology, medical amangement and oral implications of menopause. J Am Dent Assoc. 2002;133(1);73–81 [DOI] [PubMed] [Google Scholar]
- Genco RJ, Borgnakke WS (2013). Risk factors for periodontal disease. Periodontol 2000 62:59–94. [DOI] [PubMed] [Google Scholar]
- Kebschull M, Demmer RT, Grun B, Guarnieri P, Pavlidis P, Papapanou PN (2014). Gingival tissue transcriptomes identify distinct periodontitis phenotypes. J Dent Res 93:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A, Idanza F, Marttij T, Karvonen MJ, Imura O, Taylor HL (1972). Indices of relative weight and obesity. J Chron Dis 25: 329–343. [DOI] [PubMed] [Google Scholar]
- Lochner KA and SC Cox. Prevalence of multiple chronic conditions among medicare beneficiaries, United States, 2010. Prev Chronic Dis 2013; 10:120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura-Grec PG, Marsicano JA, Carvalho CA, Sales-Peres SH. Obsesity and periodontitis: systemic revierw and meta-analysis. Cien Saude Colet 2014. 19(6): 1763–72 [DOI] [PubMed] [Google Scholar]
- Page RC, Eke PI (2007). Case definitions for use in population-based surveillance of periodontitis. J Periodontol 78(7 Suppl):S1387–S1399. [DOI] [PubMed] [Google Scholar]
- Palle AR, Reddy CM, Shankar BS, Gelli V, Sudhakar J and Reddy KK. Assocaiations between obesity and chronic periodontitis: a cross-sectional study. J Contemp Dent Pract 2013. 14(2):168–73 [DOI] [PubMed] [Google Scholar]
- Payne JB, Reinhart RA, Nummikoski PV, Dunning DG, and Patil KD. The assocaition of cigarette smoking with alveolar bone loss in postmenopausal females. J Clin Periodontol. 2000; 27(9):658–64 [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW (2005). Periodontal diseases. Lancet 366:1809–1820. [DOI] [PubMed] [Google Scholar]
- Saremi A, Nelson RF, Tulloch-Reid M, Hanson RL, Sievers M, Taylor GW, Schlossman M, Bennett PH, Genco R, Knowler WC (2005). Periodontal Disease and Mortality in Type 2 Diabetes. Diabetes Care. 28:27–32. [DOI] [PubMed] [Google Scholar]
- Taylor GW (1999). Periodontal Treatment and its effects on glycemi control-A review of the Literature. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 87:311–316. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Loesche WJ, Terpenning WS (2000). Impact of oral diseases on systemic health in the elderly: diabetes and aspiration pneumonia. J Public Health Dent. 60-313-320. [DOI] [PubMed] [Google Scholar]
- Tomar AL, Asma S (2000). Smoking Attributable periodontitis in the United States: Findings from NHANES III. National Health and Nutrition and Examination Survey. J Periodontol 71:743–51. [DOI] [PubMed] [Google Scholar]
- USDHHS Administration on Aging. A Profile of Older Americans: 2000. Washington, DC: US Department of Health and Human Services, Administration on Aging, 2000a. [Google Scholar]
- USDHHS (U. S. Department of Health and Human Services) (2010). Healthy People 2020; objectives: oral health. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=32. Accessed July 2, 2014.
- USDHHS (U. S. Department of Health and Human Services), Public Health Service, Office of the Surgeon General (2000b). Oral health in America: a report of the Surgeon General. Rockville, MD: National Institutes of Health, National Institute of Dental and Craniofacial Research; p. 33–59. Available at: http://silk.nih.gov/public/hck1ocv.@www.surgeon.fullrpt.pdf. Accessed on July 2, 2014. [Google Scholar]
- US Census Bureau. Population survey: female population by age, sex, and race and Hispanic origin: March 2002. Available at: http://www.census.gov/population/socdemo/race/api/ppl-163/tab01.pdf.
- WHO (World Health Organization) (2011). Use of glycated hemoglobin (HbA1c) in the diagnosis of diabetes mellitus; abbreviated report of a WHO consultation. WHO. Available at: http://www.who.int/diabetes/publications/report-hba1c_2011.pdf. Accessed July 2, 2014. [PubMed] [Google Scholar]


