Abstract
Brain computer interfaces (BCIs) have the potential to restore communication to people who have lost the ability to speak due to neurological disease or injury. BCIs have been used to translate the neural correlates of attempted speech into text1–3. However, text communication fails to capture the nuances of human speech such as prosody, intonation and immediately hearing one’s own voice. Here, we demonstrate a “brain-to-voice” neuroprosthesis that instantaneously synthesizes voice with closed-loop audio feedback by decoding neural activity from 256 microelectrodes implanted into the ventral precentral gyrus of a man with amyotrophic lateral sclerosis and severe dysarthria. We overcame the challenge of lacking ground-truth speech for training the neural decoder and were able to accurately synthesize his voice. Along with phonemic content, we were also able to decode paralinguistic features from intracortical activity, enabling the participant to modulate his BCI-synthesized voice in real-time to change intonation, emphasize words, and sing short melodies. These results demonstrate the feasibility of enabling people with paralysis to speak intelligibly and expressively through a BCI.
Introduction:
Speaking is an essential human ability, and losing the ability to speak is devastating for people living with neurological disease and injury. Brain computer interfaces (BCIs) are a promising therapy to restore speech by bypassing the damaged parts of the nervous system through decoding neural activity4. Recent demonstrations of BCIs have focused on decoding neural activity into text on a screen2,3 with high accuracy1. While these approaches offer an intermediate solution to restore communication, communication with text alone falls short of providing a digital surrogate vocal apparatus with closed-loop audio feedback and fails to restore critical nuances of human speech, including prosody, intonation, and tone.
These additional capabilities can be restored with a “brain-to-voice” BCI that decodes neural activity into sounds in real-time that the user can hear as they attempt to speak, rather than words or word-components like phonemes. Developing such a speech synthesis BCI poses several unsolved challenges: the lack of ground truth training data, i.e., not knowing how and when a person with speech impairment is trying to speak; causal low-latency decoding for instantaneous voice synthesis that provides continuous closed-loop audio feedback; and a flexible decoder framework for producing unrestricted vocalizations and modulating paralinguistic features in synthesized voice.
A growing literature of studies have reconstructed voice offline from able speakers using previously recorded neural signals measured with electrocorticography (ECoG)5–11, stereoencephalography (sEEG)12, and intracortical microelectrode arrays13,14. Decoders trained on overt speech of able speakers could synthesize unintelligible speech during miming, whispering or imaging speaking tasks online8,15 and offline16. Recently, intermittently intelligible speech was synthesized seconds after a user with ALS spoke overtly (and intelligibly)17 from a six-word vocabulary. While the aforementioned studies were done with able speakers, the study by3 with a participant with anarthria adapted a text decoding approach to decode discrete speech units acausally at the end of the sentence to synthesize speech from 1,024 words vocabulary. However, this is still very different from healthy speech, where people immediately hear what they are saying and can use this to accomplish communication goals such as interjecting in a conversation. In this work, we sought to synthesize voice continuously and with low latency from neural activity as the user attempted to speak, which we refer to as “instantaneous” voice synthesis to contrast it with earlier work demonstrating acausal delayed synthesis.
Here, we report an instantaneous brain-to-voice BCI using 256 microelectrodes chronically placed in the precentral gyrus of a man with severe dysarthria due to amyotrophic lateral sclerosis (ALS). Despite lacking ground-truth voice data from this participant, we were able to train a deep learning model that synthesized his intended voice in real-time by decoding his neural activity causally within 10 ms. To overcome this challenge of lacking ground truth speech, we generated synthetic target speech waveforms from the prompt text and time-aligned these with neural activity to estimate the participant’s intended speech. The resulting synthesized voice was often (but not consistently) intelligible and human listeners were able to identify the words with high accuracy. This flexible brain-to-voice framework – which maps neural activity to acoustic features without an intermediary such as discrete speech tokens or limited vocabulary – could convert participant’s neural activity to a realistic representation of his pre-ALS voice, demonstrating voice personalization, and it enabled the participant to speak out-of-dictionary pseudo-words and make interjections.
We also found that in addition to previously-documented phonemic information1,2, there is substantial paralinguistic information in the intracortical signals recorded from ventral precentral gyrus. These features were causally decoded to enable the participant to modulate his BCI voice to change intonation in order to ask a question or emphasize specific words in a sentence, and to sing a melody with different pitch targets. Finally, we investigated the dynamics of the neural ensemble activity, which revealed that putatively output-null neural dimensions are highly active well before each word is vocalized, with greater output-null activity present when there were more upcoming words planned and when the upcoming word needed to be modulated.
Results:
Continuous speech synthesis from intracortical neural activity with immediate auditory feedback
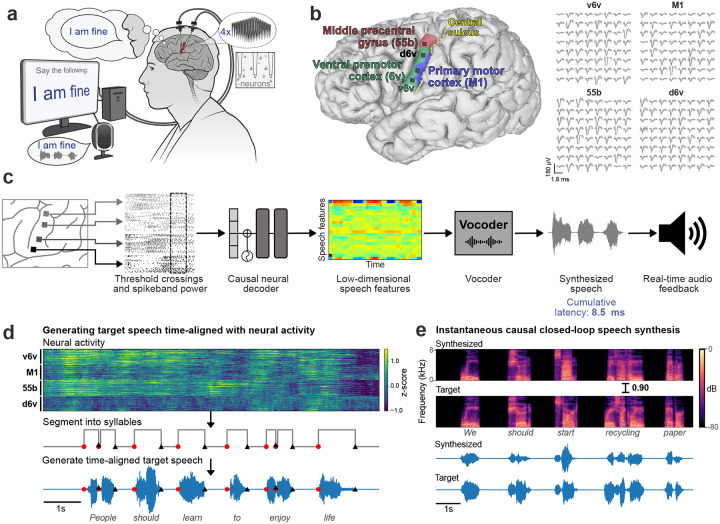
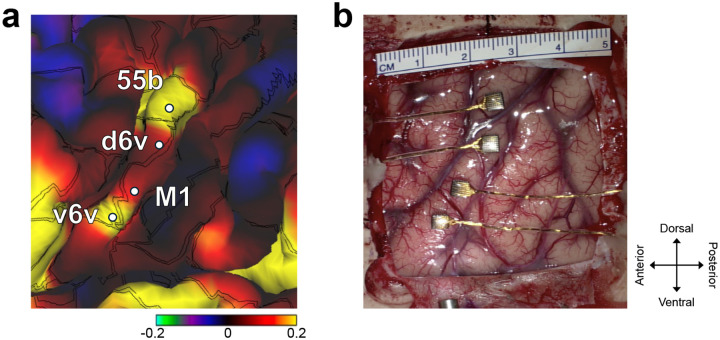
We recorded neural activity from four microelectrode arrays with a total of 256 electrodes implanted in the ventral premotor cortex (6v), primary motor cortex (M1) and middle precentral gyrus (55b) (see Fig. 1a, b) as estimated using the Human Connectome Project pipeline1,18 in BrainGate2 clinical trial participant ‘T15’ (Extended Fig. 1). T15 was a 45-year-old man with ALS and severe dysarthria. He retained some orofacial movement and an ability to vocalize but was unable to produce intelligible speech (Video 1).
Fig. 1. Closed-loop voice synthesis from intracortical neural activity in a participant with ALS.
a. Schematic of the brain-to-voice neuroprosthesis. Neural features extracted from four chronically implanted microelectrode arrays are decoded in real-time and used to directly synthesize his voice. b. Array locations on the left hemisphere and typical neuronal spikes from each microelectrode recorded over 1s. Color overlays are estimated from a Human Connectome Project cortical parcellation. c. Closed-loop causal voice synthesis pipeline: voltages are sampled at 30 kHz; threshold-crossings and spike-band power features are extracted from 1ms segments; these features are binned into 10 ms non-overlapping bins, normalized and smoothed. The Transformer model maps these neural features to a low-dimensional representation of speech involving Bark-frequency cepstral coefficients, pitch, and voicing, which are used as input to a vocoder. d. Lacking T15’s ground truth speech, we first generated synthetic speech from the known text cue in the training data using text-to-speech, and then used the neural activity itself to time-align the synthetic speech on a syllable level with the neural data time-series to obtain a target speech waveform. e. Representative example causally synthesized neural data, which matches the target speech with high fidelity.
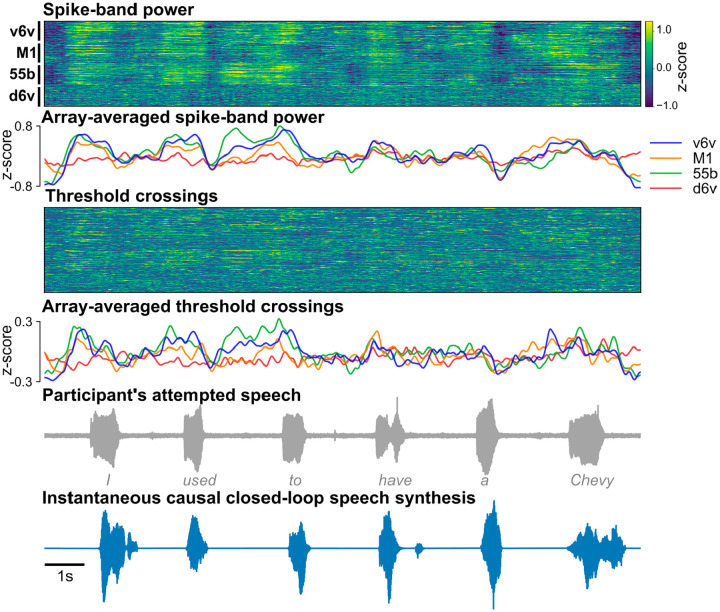
We developed a real-time neural decoding pipeline (Fig. 1c) to synthesize T15’s voice instantaneously from intracortical neural activity, with continuous audio feedback, as he attempted to speak a sentence cued on a screen at his own pace. Since the participant could not speak intelligibly, we did not have the ground truth for how and when he attempted to speak. Therefore, to generate aligned neural and voice data for training the decoder, we developed an algorithm to identify putative syllable boundaries directly from neural activity. This allowed us to generate target speech that was time-aligned to neural recordings as a proxy to T15’s intended speech (Fig. 1d).
We trained a multilayered Transformer-based19 model to causally predict spectral and pitch features of the target speech every 10 ms using the preceding binned threshold crossings and spike-band power. The base Transformer model architecture was augmented to compensate for session-to-session neural signal nonstationarities20 and to lower the inference time for instantaneous voice synthesis. The entire signal processing from signal acquisition to synthesis of speech samples occurred within 10 ms, enabling nearly-instantaneous speech synthesis. The resulting audio was synthesized into voice samples by a vocoder21 and continuously played back to T15 through a speaker (Fig. 1e).
Flexible and accurate closed-loop voice synthesis
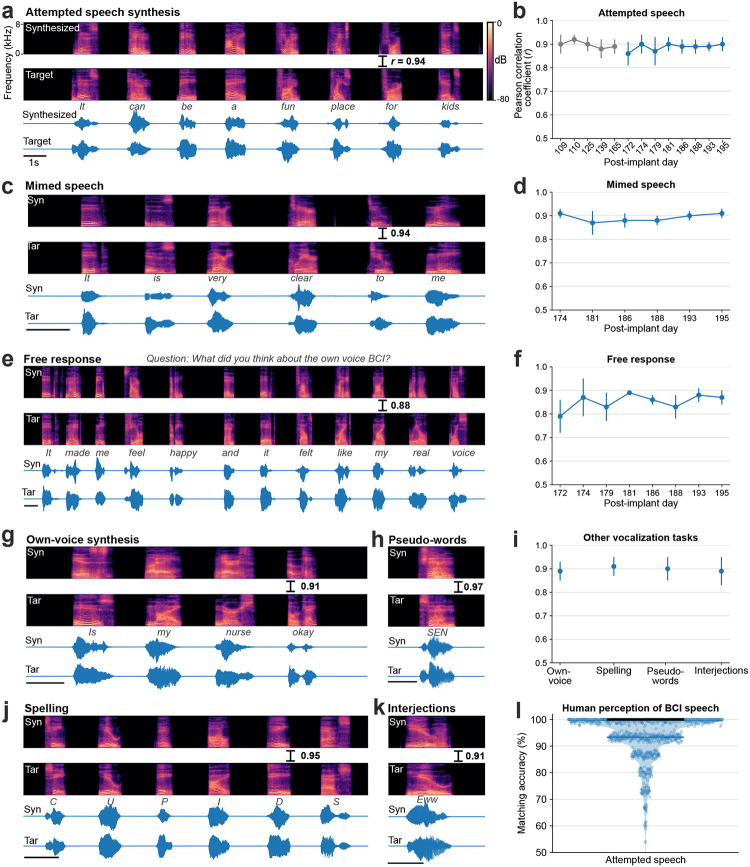
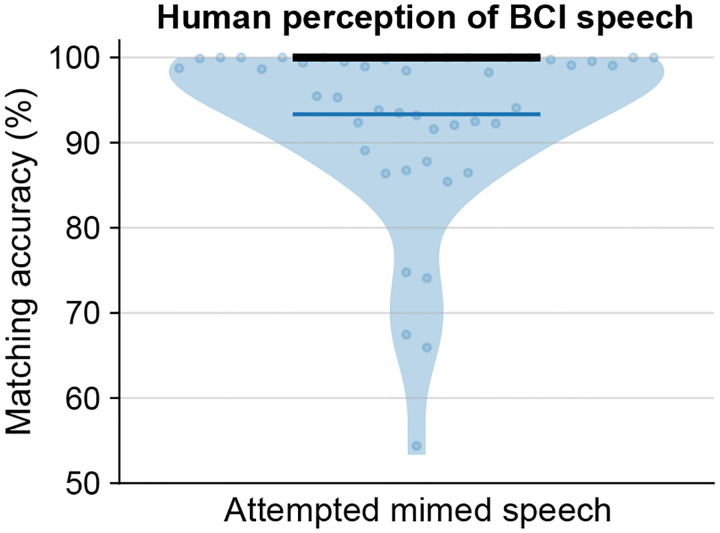
We first tested the brain-to-voice BCI’s ability to causally synthesize voice from neural activity while T15 attempted to speak cued sentences (Fig. 2a and Video 2). Each trial consisted of a unique sentence which was never repeated in the training or evaluation trials. The synthesized voice was similar to the target speech (Fig. 2b), with a Pearson correlation coefficient of 0.89±0.04 across 40 Mel-frequency bands (Extended Fig. 2 reports Mel-cepstral distortion). We quantified intelligibility by asking 15 human listeners to match each of the 933 evaluation sentences with the correct transcript (choosing from 6 possible sentences of the same length). The mean and median accuracies were 94.23% and 100%, respectively (Fig. 2i). The instantaneous voice synthesis accurately tracked T15’s pace of attempted speech (Extended Fig. 3), which – due to his ALS – meant slowly speaking one word at a time. These results demonstrate that the real-time synthesized speech recapitulates the intended speech to a high degree, and can be identified by non-expert listeners. We also demonstrated that this brain-to-voice speech neuroprosthesis could be paired with our previously-reported high accuracy brain-to-text decoder1, which essentially acted as closed-captioning (Video 3).
Fig. 2. Voice neuroprosthesis allows a wide range of vocalizations.
a. Spectrogram and waveform of an example trial showing closed-loop synthesis during attempted speech of a cued sentence and the target speech. The Pearson correlation coefficient (r) is computed across 40 Mel-frequency bands between the synthesized and target speech. b. Pearson correlation coefficients (mean ± s.d) for attempted speech of cued sentences across research sessions. Sessions in blue were predetermined as evaluation sessions and all performance summaries are reported over these sessions. c. An example mimed speech trial where the participant attempted to speak without vocalizing and d. mimed speech Pearson correlations across sessions. e. An example trial of self-guided attempted speech in response to an open-ended question and f. self-guided speech Pearson correlations across sessions. g. An example personalized own-voice synthesis trial. h, j, k. Example trials where the participant said pseudo-words, spelled out words letter by letter, and said interjections, respectively. The decoder was not trained on these words. i. Pearson correlation coefficients of own-voice synthesis, spelling, pseudo-words and interjections synthesis. l. Human perception accuracy of synthesized speech where 15 naive listeners for each of the 979 evaluation sentences selected the correct transcript from 6 possible sentences of the same length. Individual points on the violin plot show the average matching accuracy of each evaluation sentence (random vertical jitter added for visual clarity). The bold black line shows median accuracy (which was 100%) and the thin blue line shows the (bottom) 25th percentile.
All four arrays showed significant speech-related modulation and contributed to voice synthesis, with the most speech-related modulation on the v6v and 55b arrays (Extended Fig. 3). Thanks to this high neural information content, the brain-to-voice decoder could be trained even with limited data, as shown by an online demonstration using a limited 50-word vocabulary on the first day of neuroprosthesis use (Video 14). Lastly, we compared this instantaneous voice synthesis method to an acausal method3 that decoded a sequence of discrete speech units at the end of each sentence (Audio 1). As expected, acausal synthesis – which benefits from integrating over the entire utterance – generated high quality voice (MCD 2.4±0.03); this result illustrates that instantaneous voice synthesis is a substantially more challenging problem.
People with neurodegenerative diseases may eventually lose their ability to vocalize all together, or may find vocalizing tiring. We therefore tested the brain-to-voice BCI during silent “mimed” speech where the participant was instructed to attempt to mouth the sentence without vocalizing. Although the decoder was only trained on attempted vocalized speech, it generalized well to mimed speech: the Pearson correlation coefficient was 0.89±0.03, which was not statistically different from voice synthesis during vocalized attempted speech (Fig. 2c, d and Video 4). Extended Fig. 4 shows human perception accuracy of synthesized speech during miming. T15 reported that he found attempting mimed speech less tiring.
The aforementioned demonstrations involved T15 attempting to speak cued sentences. Next, we tested if the brain-to-voice BCI could synthesize unprompted self-initiated speech, more akin to how a neuroprosthesis would be used for real-world conversation. We presented T15 with questions on the screen (including asking for his feedback about the voice synthesis), which he responded to using the brain-to-voice BCI (Fig. 2e). We also asked him to say whatever he wanted (Video 5). The accuracy of his free response synthesis was slightly lower than that of cued speech (Pearson correlation coefficient 0.84±0.1, Fig. 2f, Wilcoxon rank-sum, p=10−6, n1=57, n2=933). We speculate that this reflected him using a different attempted speech strategy (with less attention to enunciating each phoneme) that he commonly used for his personal use with the brain-to-text BCI1.
This brain-to-voice decoder directly predicts acoustic speech features and is therefore not restricted to a vocabulary or a language model. This approach allows the user to produce a variety of expressive sounds, including non-word sounds and interjections, which are not possible with language- and vocabulary-dependent speech BCIs. To demonstrate this flexibility, we instructed T15 to use the brain-to-voice BCI to say made-up pseudo-words and interjections (e.g., “aah”, “eww”, “ooh”, “hmm”, “shoo”) (Fig. 2h, k and Videos 7, 8). The neuroprosthesis also enabled T15 to spell out words one letter at a time (Fig. 2j and Video 9). The brain-to-voice decoder was not trained on pseudo-words, spellings or interjections tasks but was able synthesize these sounds with a Pearson correlation coefficient of 0.90±0.01 (Fig. 2i).
Voice is an important element of people’s identities, and synthesizing a user’s own voice could further improve the restorative aspect of a speech neuroprosthesis. We therefore demonstrated that the instantaneous brain-to-voice framework was personalizable and could approximate T15’s pre-ALS voice (Fig. 2g and Video 6). To achieve this, we trained the brain-to-voice decoder on target speech produced by a voice cloning text-to-speech algorithm22 that sounded like T15. The participant used the speech synthesis BCI to report that listening to his own voice “made me feel happy and it felt like my real voice” (Fig. 2e). The accuracy of the own-voice synthesis was similar to the previously described default voice synthesis (Pearson correlation coefficient of 0.87±0.04, Fig. 2i).
Through these varied speech tasks, we demonstrated that the brain-to-voice BCI framework is flexible and generalizable, enabling the participant to synthesize a wide variety of vocalizations.
Online decoding of paralinguistic features from neural activity
Paralinguistic features such as pitch, cadence, and volume play an important role in human speech, allowing us to be more expressive. Changing the stress on different words can change the semantic meaning of a sentence; modulating intonations can convey a question, surprise or other emotions; and modulating pitch allows us to sing. Incorporating these paralinguistic features into BCI-synthesized voice is an important step towards restoring naturalistic speech. We investigated whether these paralinguistic features are encoded in the neural activity in the ventral precentral gyrus and developed algorithms to decode and modulate these speech features during closed-loop voice synthesis.
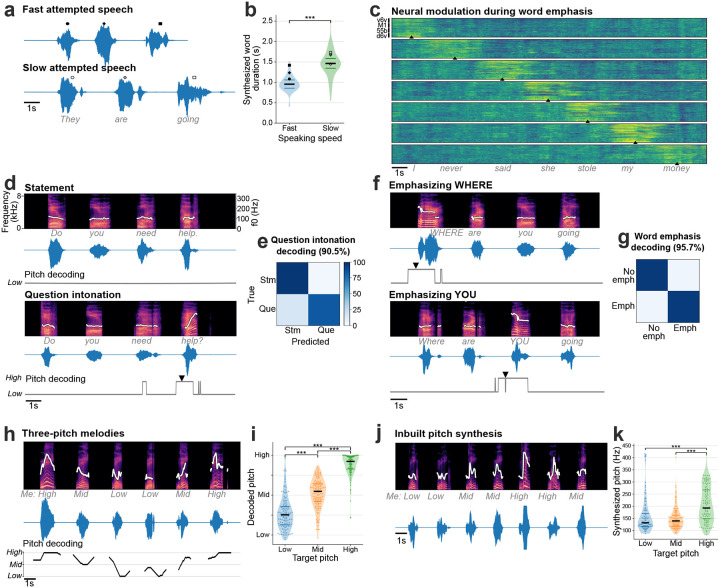
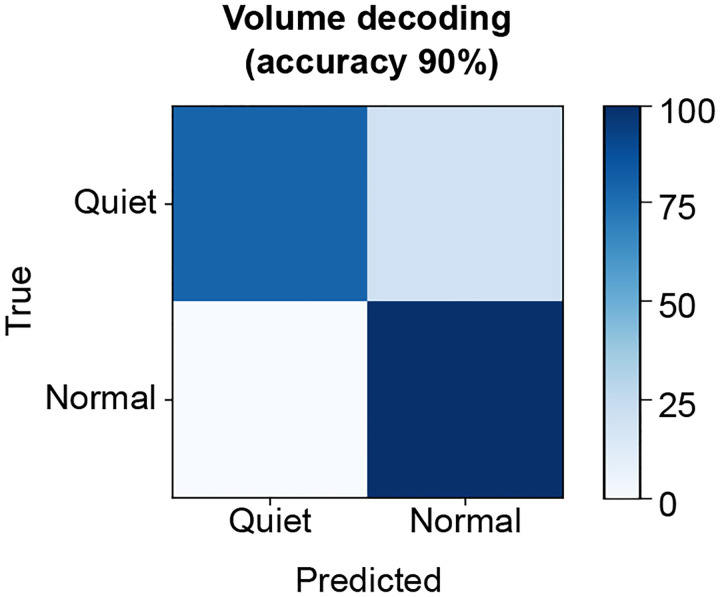
Since the brain-to-voice decoder causally and immediately synthesizes voice, it inherently captures the natural pace of T15’s speech. To quantify this, T15 was asked to speak sentences at either a faster or slower speed. The voice synthesized by the neuroprosthesis reflected his intended speaking speed (Fig. 3a). Fig. 3b shows the differing distributions of durations of synthesized words attempted at fast (average speed of 0.97±0.19 s per word) and slow (average speed of 1.46±0.31 s per word) speeds. Additionally, we were able to decode quiet or loud attempted speech volume from the neural features with 90% accuracy (Extended Fig. 5).
Fig. 3. Modulating paralinguistic features in synthesized voice.
a. Two example synthesized trials are shown where the same sentence was spoken at faster and slower speeds. b. Violin plots showing significantly different durations of words instructed to be spoken fast and slowly (Wilcoxon rank-sum, p=10−14, n1=72, n2=57). The bold black horizontal line shows the median value of the synthesized word duration and thin colored horizontal lines show the range between 25th and 75th percentiles. c. Trial-averaged normalized spike-band power (each row in a panel is one electrode) during trials where the participant emphasized each word in the sentence “I never said she stole my money”, grouped by the emphasized word. Trials were aligned using dynamic time warping and the mean activity across all trials was subtracted to better show the increased neural activity around the emphasized word. The emphasized word’s onset is indicated by the arrowhead at the bottom of each condition. d. Spectrograms and waveforms of two synthesized voice trials where the participant says the same sentence as a statement and as a question. The intonation decoder output is shown below each trial. An arrowhead marks the onset of causal pitch modulation in synthesized voice. The white trace overlaid on the spectrograms shows the synthesized pitch contour, which is constant for a statement and increases during the last word of the sentence for questions. e. Confusion matrix showing accuracies for closed-loop intonation modulation during real-time voice synthesis. f. Spectrograms and waveforms of two synthesized voice trials where different words of the same sentence are emphasized, with pitch contours overlaid. Emphasis decoder output is shown below. Arrowheads show onset of emphasis modulation. g. Confusion matrix showing accuracies for closed-loop word emphasis during real-time voice synthesis. h. Example trial of singing a melody with three pitch targets. The pitch decoder output that was used to modulate pitch during closed-loop voice synthesis is shown below. The pitch contour of the synthesized voice shows different pitch levels synthesized accurately for the target melody. i. Violin plots showing significantly different decoded pitch levels for low, medium and high pitch target words (Wilcoxon rank-sum, p=10−14 with correction for multiple comparisons, n1=122, n2=132, n3=122). Each point indicates a single trial. j. Example three-pitch melody singing synthesized by a unified brain-to-voice model. The pitch contour of the synthesized voice shows the pitch levels tracked the target melody. k. Violin plot showing peak synthesized pitch frequency achieved by the inbuilt pitch synthesis model for low, medium and high pitch targets. Synthesized high pitch was significantly different from low and middle pitch (Wilcoxon rank-sum, p=10−3, n1=106, n2=113, n3=105). Each point shows an individual trial.
Next, we decoded the intent to modulate intonation to ask a question or to emphasize a specific word. We recorded neural activity while T15 attempted to speak the same set of sentences as either statements (no extra modulation in pitch) or as questions (with increasing pitch at the end of the sentence). This revealed increased neural activity recorded on all four arrays towards the end of the questions (Extended Fig. 6). To study the effect of attempted word emphasis on neural activity, on different trials we asked T15 to emphasize one of the seven words in the sentence “I never said she stole my money” by increasing that word’s pitch. This sentence, modeled after23, changes its semantic meaning for each condition whilst keeping the phonemic content the same. Similar to the effect observed during the question intonation task, we observed increased neural activity around the emphasized word (Fig. 3c) on all four arrays (Extended Fig. 7) starting ~350 ms prior to the onset of the word.
As a proof-of-principle that these paralinguistic features could be captured by a speech neuroprosthesis, we trained two separate binary decoders to identify the change in intonation during these question intonation and word emphasis tasks. We then applied these intonation decoders in parallel to the brain-to-voice decoder to modulate the pitch and amplitude of synthesized voice in closed loop, enabling T15 to ask a question or emphasize a word (Extended Fig. 8). Fig. 3d shows two example closed-loop voice synthesis trials, including their pitch contours, where T15 spoke a sentence as a statement and as a question. The synthesized speech pitch increased at the end of the sentence during question intonation (Video 10). Fig. 3f shows two example synthesized trials of the same sentence where different words were emphasized (Video 11). Across all closed-loop evaluation trials, we decoded and modulated question intonation with 90.5% accuracy (Fig. 3e) and word emphasis with 95.7% accuracy (Fig. 3g).
After providing the aforementioned binary intonation control for questions or word emphasis, we investigated decoding multiple pitch levels from neural activity. We designed a three-pitch melody task where T15 attempted to “sing” different melodies consisting of 6 to 7 notes of low, medium and high pitch (e.g., low-mid-high-high-mid-low). These data were used to train a two-stage Transformer-based pitch decoder. During closed-loop voice synthesis, this pitch decoder ran simultaneously with the brain-to-voice decoder to modulate its pitch output; visual feedback of the decoded pitch level was also provided on-screen (Video 12). T15 was able to control the synthesized melody’s pitch levels (Fig. 3h). Fig. 3i shows three distinct distributions of pitch levels decoded from neural activity across all singing task evaluation trials, demonstrating that the pitch and phonemic content of speech could be simultaneously decoded from neural activity in real-time.
In the preceding experiments, we used a data-efficient separate discrete pitch decoder to modulate the synthesized voice because the vast majority of our training data consisted of neutral sentences without explicit instructions to modulate intonation or pitch. However, a more generalizable approach would be to develop a unified (single) brain-to-voice decoder that takes into account these paralinguistic features. We demonstrated the feasibility of such an approach by training our regular brain-to-voice decoder model architecture with the time-aligned target speech consisting of data from the three-pitch singing task. This enabled the decoder to implicitly learn the mapping between neural features and the desired pitch level in addition to learning the mapping from neural activity to phonemic content (as before). During continuous closed-loop voice synthesis evaluation, this unified “pitch-enhanced” brain-to-voice decoder was able to synthesize different pitch levels as T15 attempted to sing different melodies (Video 13 and Fig. 3j, k). This demonstrates that the brain-to-voice BCI framework has an inherent capability to synthesize paralinguistic features if provided with training data where the participant attempts the desired range of vocal properties (in this case, pitch).
Rich output-null neural dynamics during speech production
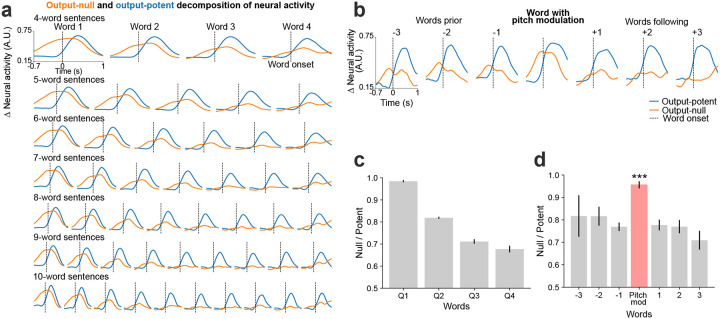
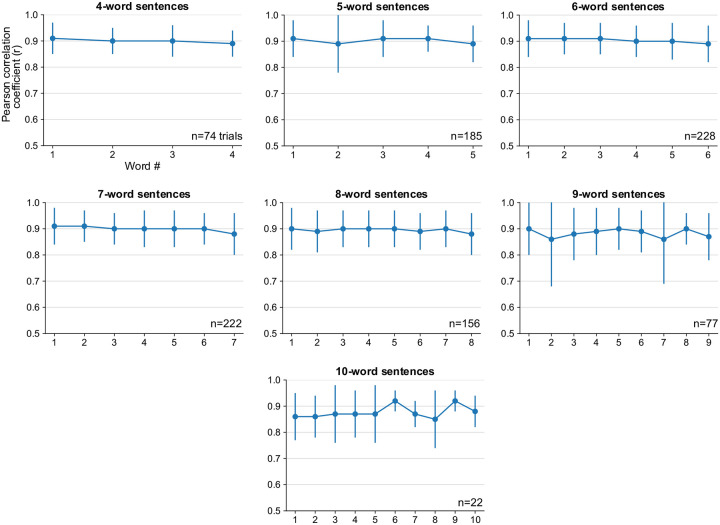
Instantaneous brain-to-voice synthesis provides a unique view into neural dynamics with high temporal precision. We noticed that neural activity increased prior to and during the utterance of each word in a cued sentence, but that the aggregate neural activity decreased over the course of the sentence (Fig. 1d, Extended Fig. 3). Yet despite this broad activity decrease, the synthesis quality remained consistent throughout the sentence (Extended Fig. 9). This seeming mismatch between overall neural activity and voice output suggested that the “extra” activity – which preceded voice onset for each word and also gradually diminished towards the end of a sentence – could be a form of output-null neural subspace activity previously implicated in movement preparation24, feedback processing25, and other computational support roles26. We estimated the output-null and output-potent neural dimensions by linearly decomposing the population activity into a subspace that best predicted the speech features (output-potent dimensions, which putatively most directly relate to behavioral output) and its orthogonal complement (output-null dimensions, which putatively have less direct effect on the behavioral output). Fig. 4a shows output-null and output-potent components of neural activity around the onset of each word in sentences of different lengths. A clear decrease in output-null activity can be seen over the course of a sentence regardless of its length, whereas the output-potent activity remains consistent (Fig. 4c). An exception to this was the very last word, which tended to have an increase in output-null activity, especially as the last word was being finished. We do not know why the end of the sentence exhibited this effect but speculate that it is related to an end-of-trial cognitive change (e.g., the participant assessing his performance).
Fig. 4. Output-null and output-potent neural dynamics during speech production.
a. Average approximated output-null (orange) and output-potent (blue) components of neural activity during attempted speech of cued sentences of different lengths. Output-null activity gradually decayed over the course of the sentence, whereas the output-potent activity remained consistent irrespective of the length of the sentence. b. Average output-null and output-potent activity during pitch modulation (intonation or emphasis); data are trial-averaged aligned to the emphasized word (center) and the words preceding or following that word in the sentence. The output-null activity increased during pitch modulation as compared to the words preceding or following it. c. Average null/potent activity ratios for words in the first-quarter, second-quarter, third-quarter, and fourth-quarter of a sentence are summarized (mean ± s.e.). d. Average null/potent activity ratios of the pitch modulated word (red) and the words preceding or following it (gray) (mean ± s.e.). The null/potent ratios of modulated words were significantly different from that of non-modulated words (Wilcoxon rank-sum, p= 10−21, n1=460, n2=922).
We also examined the putatively output-null and output-potent activity when the participant volitionally modulated his intonation. We found that the output-null activity increased significantly (p=10−21) for the word that was modulated (Fig. 4b, d) as compared to the words that preceded or followed it, explaining the previously noted increase in overall neural activity preceding intonation-emphasized words (Fig. 3c, Extended Fig. 6).
Discussion:
This study demonstrated a “brain-to-voice” neuroprosthesis that directly mapped the neural activity recorded from four microelectrode arrays spanning ventral precentral gyrus into voice features. A man with severe dysarthria due to ALS used the system to synthesize his voice in real-time as he attempted to speak in both highly structured and open-ended conversation. The resulting voice was often intelligible. The decoding models were trained for a participant who could no longer speak intelligibly (and thus could not provide a ground truth speech target), and could be adjusted to emulate his pre-ALS voice. Unlike prior studies3,17, this brain-to-voice neuroprosthesis output sounds as soon as the participant tried to speak, without being restricted to a small number of words17 and without a constrained intermediate representation of discrete speech units that were generated after completion of each sentence3. To demonstrate the flexibility conferred by this direct voice synthesis BCI, the participant used it to synthesize various vocalizations including unseen words, interjections, and made-up words.
Furthermore, this study demonstrates that a brain-to-voice neuroprosthesis can restore additional communication capabilities over existing brain-to-text BCIs1–3,27. Neuronal activity in precentral gyrus encoded both phonemic and paralinguistic features simultaneously. Beyond providing a more immediate way to say words, this system could decode the neural correlates of volume, pitch, and intonation. In online demonstrations, the neuroprosthesis enabled the participant to control a variety of aspects of his instantaneous digital vocalization, including the duration of words, emphasizing specific words in a sentence, ending a sentence as either a statement or a question, and singing three-pitch melodies. This represents a step towards restoring the ability of people living with speech paralysis to regain the full range of expression provided by the human voice.
We note that participant T15, who had been severely dysarthric for several years at time of this study, reported that he found it difficult to try to precisely modulate the tone, pitch, and amplitude of his attempted speech. Thus, we propose that using discrete classifiers to generate real-time modulated voice (which provides feedback to the participant that helps them mentally “hone in” on how to modulate their voice) can provide an intermediate set of training data useful for training a single unified decoder capable of continuous control of phonemic and paralinguistic vocal features. We demonstrated a proof of concept of this unified approach by training a single core decoder to intrinsically synthesize voice with different pitch levels, which the participant used for singing melodies.
A functional neuroanatomy result observed in this study that would not be predicted from prior ECoG23,28–30 and microstimulation studies31,32 is that the neural activity is correlated with paralinguistic features across all four microelectrode arrays, from ventral-most precentral gyrus to the middle precentral gyrus. We also observed that cortical activity across all four arrays increased well before attempted speech. We hypothesize that this reflects output-null preparatory activity24,26, and note that its presence is particularly fortuitous for the goal of causally decoding voice features because it gives the decoder a “sneak peak” shortly before intended vocalization. A particularly interesting observation was that this output-null activity seems to decrease over the course of a sentence. This may indicate that the speech motor cortex has a “buffer” for the whole sentence, which is gradually emptied out as the sentence approaches completion. We also observed an increase in output-null activity preceding words that were emphasized or modulated, which we speculate may be a signature of the additional neural computations involved in changing how that word is said. These results hint at considerable richness in speech-related motor cortical ensemble activity, beyond just the activity that is directly linked to driving the articulators. These phenomena represent an opportunity for future study, including leveraging the computation through dynamics framework and neural network modeling which have helped explain the complexity of motor cortical activity for preparing and producing arm and hand movements (reviewed in26).
Limitations
This study was limited to a single participant with ALS. It remains to be seen whether similar brain-to-voice performance will be replicated in additional participants, including those with other etiologies of speech loss. The participant’s ALS should also be considered when interpreting the study’s scientific results. Encouragingly, however, prior studies have found that neural coding observations related to hand movements have generalized across people with ALS and able-bodied animal models33 and across a variety of etiologies of BCI clinical trial participants34,35. Furthermore, the phonemic and paralinguistic tuning reported here at cellular resolution has parallels in meso-scale ECoG measurements over sensorimotor cortex in able speakers being treated for epilepsy23,28.
Although the performance demonstrated compares favorably with prior studies, the synthesized words were still not consistently intelligible. We also anecdotally observed that the participant’s energy level and engagement on a given block, as well as whether he attempted to enunciate the words clearly and fully, influenced synthesis quality. Brain-to-voice evaluations performed during the research sessions provided limited opportunity for practice-based improvement (i.e., sensorimotor learning). It remains an open question whether consistent long-term use will result in improved accuracy due to additional training data and/or learning. Separately, we predict that accuracy improvement is possible with further algorithm refinement and increasing the number of electrodes, which was previously shown to improve brain-to-text decoding accuracy1,2.
Methods:
Participant
A participant with amyotrophic lateral sclerosis (ALS) and severe dysarthria (referred to as ‘T15’), who gave informed consent, was enrolled in the BrainGate2 Neural Interface System clinical trial (ClinicalTrials.gov Identifier: NCT00912041). This pilot clinical trial was approved under an Investigational Device Exemption (IDE) by the US Food and Drug Administration (Investigational Device Exemption #G090003). Permission was also granted by the Institutional Review Boards at the University of California, Davis (protocol #1843264) and Mass General Brigham (#2009P000505). T15 consented to publication of photographs and videos containing his likeness. This manuscript does not report any clinical trial-related outcomes; instead, it describes scientific and engineering discoveries that were made using data collecting in the context of the ongoing clinical trial.
T15 was a left-handed 45-year-old man. T15’s ALS symptoms began five years before enrolment into this study. At the time of enrolment, he was non-ambulatory, had no functional use of his upper and lower extremities, and was dependent on others for activities of daily living (e.g., moving his wheelchair, dressing, eating, hygiene). T15 had mixed upper and lower-motor neuron dysarthria and an ALS Functional Rating Scale Revised (ALSFRS-R) score of 23 (range 0 to 48 with higher scores indicating better function). He retained some neck and eye movements but had limited orofacial movement. T15 could vocalize but was unable to produce intelligible speech (see Video 1). He could be interpreted by expert listeners in his care team, which was his primary mode of communication.
Four 64-electrode, 1.5 mm-length silicon microelectrode arrays coated with sputtered iridium oxide (Utah array, Blackrock Microsystems, Salt Lake City, Utah) were placed in T15’s left precentral gyrus (putatively in the ventral premotor cortex, dorsal premotor cortex, primary motor cortex and middle precentral gyrus) (Fig. 1b). The array placement locations were identified based on pre-operative scans using the Human Connectome Project pipeline1,18. Neural recordings from the arrays were transmitted to a percutaneous connection pedestal. An external receiver (Neuroplex-E) connected to the pedestal sent information to a series of computers used for neural decoding. Data reported here are from post-implant days 25–342.
Real-time neural feature extraction and signal-processing
Raw neural signals (filtered between 0.3 to 7.5 kHz and sampled at 30 kHz with 250nV resolution) were recorded from 256 electrodes and sent to the processing computers in 1 ms packets. We developed the real-time signal processing and neural decoding pipeline using the custom-made BRAND platform36, where each processing step was conducted in a separate “node” running asynchronously.
We extracted neural features of threshold crossings and spike-band power from each 1 ms incoming signal packet within 1 ms to minimize upstream delays. First, each packet was band pass filtered between 250 to 5000 Hz (4th order zero-phase non-causal Butterworth filter) by padding on both sides to minimize discontinuities at edges and denoised using Linear Regression Referencing (LRR)37. Then, threshold crossing was detected when the voltage was above −4.5 times the root mean squared (RMS) value for each channel. Spike-band power was computed by squaring and averaging the samples in the filtered window for each channel and was clipped at 50k μV2 to avoid outliers.
Neural features were binned into 10 ms non-overlapping bins (counting threshold crossings and averaging spike-band power across 10 consecutive feature windows). Each bin was first log transformed, then normalized using rolling means and s.d. from past 10 s and causally smoothed using a sigmoid kernel of length 1.5 s of the past activity. Thus, a vector of 1×512 binned neural features was sent to the brain-to-voice decoder every 10 ms. After each “block” of neural recording, we computed RMS thresholds and LLR weights to be used in the next block, which helped in minimizing non-stationaries in the neural signal.
Experimental paradigm
This study comprises various closed-loop speech tasks to develop and evaluate voice synthesis neuroprosthesis. Research sessions were structured in blocks of ~50 trials of a specific task. Each trial began with a “delay” period of 1.5–4 s in which a text cue was shown on the screen (indicated by a red square) and the participant read the cue. This was followed by a “go” period (indicated by a green square) where the participant was instructed to attempt to speak the cued text at his own pace after which, he ended the trial using an eye tracker. Closed-loop instantaneous voice synthesis was done during the “go” period. There was a short 1–1.5 s interval before the start of the next trial.
We conducted the following speech tasks with the above trial structure: attempting to speak cued sentences, mime (without vocalizing) cued sentences, respond to open-ended questions in his own words or say anything he wanted, spell out words letter by letter, attempt to speak made-up pseudo-words, say interjections, modulate intonation to say a sentence as a statement or as a question, emphasize certain words in a sentence, and sing melodies with different pitch level targets (this task had a reference audio cue for the melody which was played during the delay period).
After the initial eight research sessions with a mix of open-loop and closed-loop blocks, all sentence trials in the rest of the sessions were conducted with either closed-loop voice synthesis, text decoding1 or both to improve participant’s engagement in the task. All other types of tasks had closed-loop voice synthesis feedback. In a typical research session, we recorded ~150–350 structured trials.
Closed-loop continuous voice synthesis
Target speech generation for decoder training
Since T15 was unable to produce intelligible speech, we did not have a ground-truth reference of his speech to match with the neural activity required for training a decoder to causally synthesize voice. Hence, we generated a “target” speech waveform aligned with neural activity as an approximation of T15’s speech.
We first generated synthetic speech waveforms from the known text cues in the training data using text-to-speech (native TTS on Mac). Next, we identified putative syllable boundaries of T15’s attempted speech from the corresponding neural activity and aligned the synthetic speech by dynamically time stretching it to match these syllable boundaries. Thus, we obtained the time-aligned target speech. The target speech was aligned on syllable level because syllables are the fundamental units of prosody in human speech38. During our first research session, there was no prior neural data available, so we used coregistered microphone recordings of T15’s attempted (unintelligible) speech to segment word boundaries and generate time-aligned target speech. In subsequent sessions, we relied solely on neural data to estimate syllable boundaries – we used a brain-to-voice model trained on past neural data to synthesize speech and used its envelope to segment syllable boundaries39 and align target speech with manual oversight. Thus, we used the previous brain-to-voice model to generate target speech for the current session iteratively from neural data alone.
Brain-to-voice decoder architecture
The core brain-to-voice model was adapted from Transformer architecture19. The model had two main components: an input embedding network and a base Transformer. Separate input embedding networks consisting of 2 fully connected dense layers (512 and 128 units respectively, ReLU activation) were used for each week of neural data recording to compensate for week-to-week nonstationarities. The output from input embedding network was passed into the base Transformer model consisting of 8 Transformer encoder blocks (head size 128, number of heads 4, a dropout of 0.5 after multi-head attention layer and each of the two feed-forward layers with 256 and 128 units respectively, and normalization layer at the beginning and the end). Positional encoding was added before the first Transformer block. Additionally, we included residual connections between each Transformer block (separate from the residual connections within each block). The output sequence from Transformer blocks was pooled by averaging and passed to two dense layers (1024, 512 units, ReLU activation) and finally through a dense layer of size 20 to output 20-dimensional predicted speech features.
At each step, an input to the brain-to-voice decoder was a 600 ms window of binned neural features (threshold crossings and spike-band power) of shape 60×512 (60 bins of 10 ms with 256 channels × 2 features). First layer of the model averaged two adjacent bins of the input sequence to sequence length. The output of the decoder was a vector of 20 predicted speech features (which were then sent to a vocoder to generate synthesized speech samples in closed-loop blocks). The decoder ran every 10 ms to produce a single 10 ms frame of voice samples. All the model hyperparameters were tuned manually with special consideration given to minimize the inference time for instantaneous closed-loop voice synthesis.
Decoder training
We trained a new decoder for each session using all trials (which were unique) from all previous research sessions. To train the decoder robustly, we used 4–20 augmented copies of each trial. Neural features were augmented using three strategies: adding white noise (mean 0, s.d. 1.2) to all timepoints of all channels independently, a constant offset (mean 0, s.d. 0.6) to all spike-band channels independently and its scaled version (x0.67) to threshold crossings, and same cumulative noise (mean 0, s.d. 0.02) to all channels along the time course of the trial. We extracted 600 ms sliding windows shifted by 10 ms from continuous neural features and its corresponding 10 ms frame (20-dimensional normalized and smoothed vector) of output target speech features as a single training sample. 20-dimensional speech features (18 Bark cepstral coefficients, pitch period and pitch strength) for every 10 ms of target speech waveform was extracted using the encoder for the pretrained LPCNet vocoder21.
The model was trained for ~15–20 epochs with a batch size of 1024, a constant learning rate of 5×10−4, Adam optimizer (β1=0.9, β2=0.98, ε=1e-9) and Hubert loss (δ=1.35) which affords the advantage of both L1 and L2 losses and is less sensitive to outliers. The training took between 20–40 hrs on three NVIDIA GeForce RTX 3090 GPUs depending on the amount of data used for training.
On the first session of neural recording, we collected 190 open-loop trials of attempted speech from a 50-word vocabulary to train the decoder and were able to synthesize voice in closed-loop with audio feedback on the same day. Although the closed-loop synthesis on this data was less intelligible due to the model not being optimized on the first day, we later demonstrated offline that with an optimized model, we could get highly intelligible synthesis with this small amount of neural data and limited vocabulary (Video 14).
In subsequent sessions, we collected more attempted speech trials with large open vocabulary and iteratively optimized our brain-to-voice decoder architecture to improve the synthesis quality. Here, we report the performance of closed-loop voice synthesis from neural activity using the stable final brain-to-voice decoder for predetermined evaluation sessions. For each of these sessions, the decoder was trained on all the data collected up to one week prior (total of ~5500–8900 trials).
For training the personalized-own voice synthesis model, we first generated time-aligned target speech that sounded like T15’s pre-ALS voice using the StyleTT2 text-to-speech model22 fine-tuned on T15’s voice samples prior to developing ALS. Rest of the process for decoder architecture and training was the same as above.
Closed-loop voice synthesis
During closed-loop real-time voice synthesis, we first extracted neural features every 1 ms which were binned, log transformed, causally normalized and smoothed and aggregated into 600 ms causal sliding windows. This neural feature sequence was decoded by the brain-to-voice model into 20 acoustic speech features at each time step. The predicted speech features were rescaled to normal range and 16 linear predictive coding (LPC) features were reconstructed from the 18 predicted cepstral features. This 36-feature vector was synthesized into a single 10 ms frame of speech waveform (sampled at 16 kHz) using the pretrained LPCNet vocoder every 10 ms. The entire pipeline from neural signal acquisition to reconstruction of speech samples of a single frame took less than 10 ms. These samples were then sent to the audio playback computer as they were generated and played through a speaker continuously providing closed-loop audio feedback. All results reported in this study are for closed-loop voice synthesis.
Evaluation of synthesized speech
We evaluated synthesis speech by measuring the Pearson correlation coefficient between the synthesized and target (fully intelligible) speech. We computed average Pearson correlation across 40 Mel-frequency bands of audio sampled at 16 kHz. The Mel-spectrogram with 40 Mel-frequency bands was computed using sliding (Hanning) windows of 50 ms with 10 ms overlap and converted to decibel units. We also computed Mel-cepstral distortion between the synthesized speech and the target speech using the method described in27.
To evaluate human perception of BCI-synthesized speech, we asked fifteen naïve listeners to listen to each synthesized speech trial and identify the transcript that matched the audio from six possible sentences of the same length. We used crowd analytics platform Amazon Mechanical Turk to evaluate 979 synthesized sentences (vocalized and mimed trials) each by fifteen individuals. To test if the crowd workers actually listened to the audio, we included a fully intelligible control audio clip with each synthesized audio. We rejected the trials with wrong answers for the control audio and resubmitted these trials for evaluation.
Decoding paralinguistic features for modulating synthesized voice
Decoding intonation from neural activity
We collected neural data from blocks where T15 was instructed to modulate his attempted speech intonation to say cued sentences as statements (no change in pitch) or as questions (by changing the pitch from low to high towards the end of the sentence) and to emphasize capitalized words in cued sentences (by increasing pitch with slight increase in volume for emphasis). We analyzed question and word emphasis tasks separately but followed the same decoding procedure. We did not have the ground truth of when T15 modulated his intonations to train the intonation decoders. Hence, neural trials were grouped by the cue sentence and aligned using dynamic time warping40. The average of aligned trials was subtracted from each trial to reveal increased neural activity (example, Fig. 3c), which was used to label the portions of neural trial as intonation modulation (1) or no modulation (0). For intonation decoding, we only used the spike-band power feature due to its higher signal-to-noise ratio. Sliding windows of 600 ms shifted by 10 ms were derived from binned neural activity to generate samples for decoder training. For each window, adjacent bins were averaged, and sequence of features was flattened to obtain a single feature vector as input to the decoder. A binary logistic regression decoder was trained to classify the neural feature vectors into ‘no change in intonation’ or ‘change in intonation’. During closed-loop trials, this decoder was used to classify features from preceding 600 ms of neural activity, every 10 ms leading to continuous decoding of intonation. Separate binary decoders were trained to detect intonation modulation for asking questions and for word emphasis. These intonation decoders ran simultaneously with the main brain-to-voice decoder. Same procedure was followed to train a volume decoder to classify quiet volume and normal volume from neural activity.
Intonation modulation in synthesized voice
One of the speech features predicted by the brain-to-voice decoder characterizes the pitch component which is used by the LPCNet vocoder to synthesize speech waveform. We leveraged this feature and modified its value upon detection of change in intonation from neural activity. The binary intonation decoder upon robustly detecting the change during question intonation, sent a trigger to modulate the pitch feature predicted by the brain-to-voice decoder according to a predefined pitch profile for asking a question (gradually increasing the pitch of the word from low to high) which was then synthesized by the vocoder as usual (see Extended Fig. 8).
Similarly, for emphasizing certain words in a sentence in closed-loop, the binary emphasis decoder sent a trigger to modulate predicted pitch features by the brain-to-voice decoder according to a predefined pitch profile for word emphasis – modulating the pitch from high to low and increasing the volume of synthesized speech by 20%.
We computed the accuracy of closed-loop intonation modulation by evaluating whether individual words in a sentence were modulated appropriately. Interestingly, both the binary decoders were able to detect change in intonation for question or word emphasis prior to the onset of the word being modulated, which allowed causal modulation of the resulting synthesized voice.
Pitch decoding for singing melodies
We collected neural data while T15 attempted to sing the three-pitch melodies comprised of 6–7 notes of three pitch levels (e.g., low-mid-high-high-mid-low). An audio cue of the melody was played during the delay period for reference. However, we did not define the exact pitch targets for these three different pitch levels as it was difficult for T15 to precisely modulate his pitch; rather we let T15 self-determine how he wanted to attempt to sing low, middle and high pitch notes.
We used a two-stage pitch decoding approach to decode pitch produced by T15 from his spike-band power. We built a first Transformer-based decoder (same architecture as above, but with only two Transformer blocks and no input embedding network) to identify his intention to speak (before attempted speech onset) and a second Transformer-based decoder to decode his intended pitch level (1-low, 2-mid, 3-high) if intention to speak was detected. Both decoders were trained using the categorical cross entropy loss. Since results of the previous intonation modulation task showed that changes in paralinguistic features can be detected in advance of speech onset, labels for each pitch level were assigned to the neural data from 600 ms prior to the word onset to the end of the word attempted at that pitch.
During the closed-loop singing task, the two-staged pitch decoder ran simultaneously with the core brain-to-voice decoder. The output of the pitch decoder was smoothed with moving average and then used to continuously modulate the predicted brain-to-voice pitch feature in real-time, which was then vocoded as usual (Extended Fig. 8). Thus, the participant was able to sing melodies through his synthesized voice. Additionally, we provided a closed-loop visual feedback on the screen by showing the decoded pitch level and interactive target cues for the note in the melody that T15 was singing (Video 13).
Inbuilt pitch synthesis for singing melodies
In the previous intonation and pitch modulation tasks, we used a separate decoder to detect changes in the paralinguistic features and modulate the synthesized voice. Here we developed a unified brain-to-voice decoder that is inherently capable of synthesizing pitch in the melody singing task. To achieve this, we used the regular brain-to-voice decoder and trained it using the target speech waveform with the desired pitch levels. The brain-to-voice decoder was generalizable and was able to inherently map the neural activity to the pitch levels in the target waveform, which flexibly enabled T15 to sing melodies.
Output-null and output-potent analysis of neural activity
To study the underlying neural dynamics of speech production, we decomposed the neural activity into two orthogonal output-null and output-potent components. To achieve this, we adopted a simplified linear decoding approach. We trained a linear decoder y = Wx, where x is a vector of neural features, y is a 20-dimensional vector of speech features and W is the linear decoder. We trained a separate linear decoder for each session to account for session-to-session nonstationarities. The linear decoder matrix W was decomposed into orthogonal null- and row-subspaces. The neural activity x was projected onto the null space and row space. The delta neural activity for null and row space projections for each trial was obtained by computing the Euclidean distance of the projections from the baseline (first 500 ms of the trial) and normalizing it between 0 and 1 to get output-null and output-potent components respectively.
Trial averaged output-null and output-potent components were obtained for all sentences of the same length between −700 ms to +1s from the onset of each word in a sentence (Fig. 4). Output-null and output-potent analysis was also done on intonation modulation for questions and word emphasis tasks and the output was compared with that of the regular cued attempted speech task.
Statistical testing
We used a two-sided Wilcoxon rank-sum test to compare two groups of data. The p-values were corrected for multiple comparisons using Bonferroni correction where necessary. We used a non-parametric test because datasets being compared were of different size and normal distribution was not assumed because the actual underlying distribution was unknown.
Extended Data
Extended Fig. 1: Microelectrode array placement.
a. The estimated resting state language network from Human Connectome Project data overlaid on T15’s brain anatomy. b. Intraoperative photograph showing the four microelectrode arrays placed on the surface of T15’s precentral gyrus.
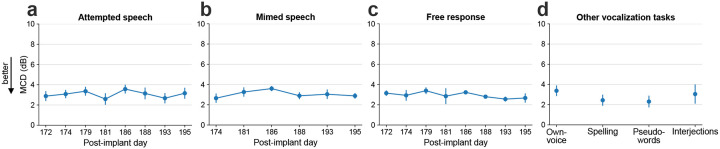
Extended Fig. 2: Mel-cepstral distortion of synthesized speech.
a. Mel-cepstral distortion (MCD) is computed across 25 Mel-frequency bands between the closed-loop synthesized speech and the target speech. MCDs (mean ± s.d) for attempted speech of cued sentences for eight evaluation research sessions are shown. b. MCDs between the synthesized and target speech during mimed speech trials. c. MCDs between the synthesized and target speech during free response trials where the participant responded to the open-ended questions. d. MCDs between the synthesized and target speech during own-voice synthesis, spelling words letter by letter, saying made-up pseudo-words, and interjections.
Extended Fig. 3: Example closed-loop speech synthesis trial.
Spike-band power and threshold crossing spikes from each electrode are shown for one example sentence. These neural features are binned and causally normalized and smoothed on a rolling basis before being used to synthesize speech. The mean spike-band power and threshold crossing activity for each individual array are also shown. Speech-related modulation was observed on all arrays, with the highest modulation recorded in v6v and 55b. The synthesized speech is shown in the bottom-most row. The gray trace above it shows the participant’s attempted (unintelligible) speech as recorded with a microphone.
Extended Fig. 4: Human perception assessment of voice synthesized during mimed speech.
Human perception accuracy of synthesized speech miming trials where 15 naïve listeners for each of the 58 evaluation sentences selected the correct transcript from 6 possible sentences of the same length. Individual points on the violin plot show the average matching accuracy of each evaluation sentence (random vertical jitter added for visual clarity). The bold black line shows median accuracy (which was 100%) and the thin blue line shows the (bottom) 25th percentile.
Extended Fig. 5: Volume decoding from neural activity.
Confusion matrix showing offline accuracies for classifying the volume of attempted speech from neural activity using a binary decoder while the participant was instructed to speak either quietly or in his normal volume.
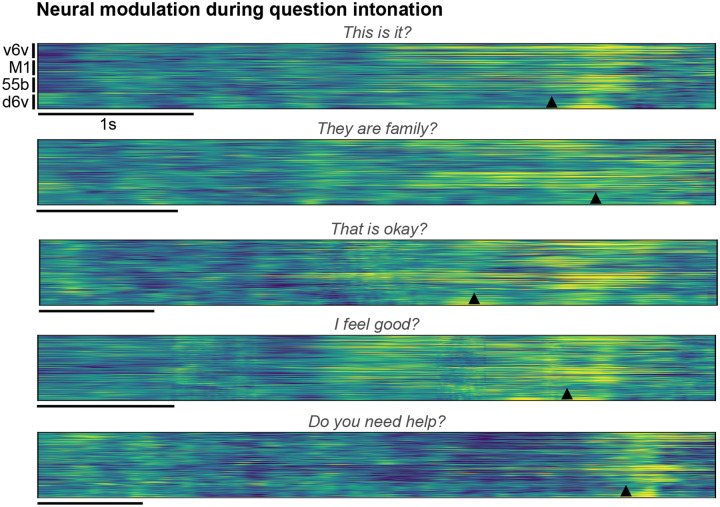
Extended Fig. 6: Neural modulation during question intonation.
Trial-averaged normalized spike-band power (each row in a group is one electrode) during trials where the participant modulated his intonation to say the cued sentence as a question. Trials with the same cue sentence (n=16) were aligned using dynamic time warping and the mean activity across trials spoken as statements was subtracted to better show the increased neural activity around the intonation-modulated word. The modulated word’s onset is indicated by the arrowhead at the bottom of each example.
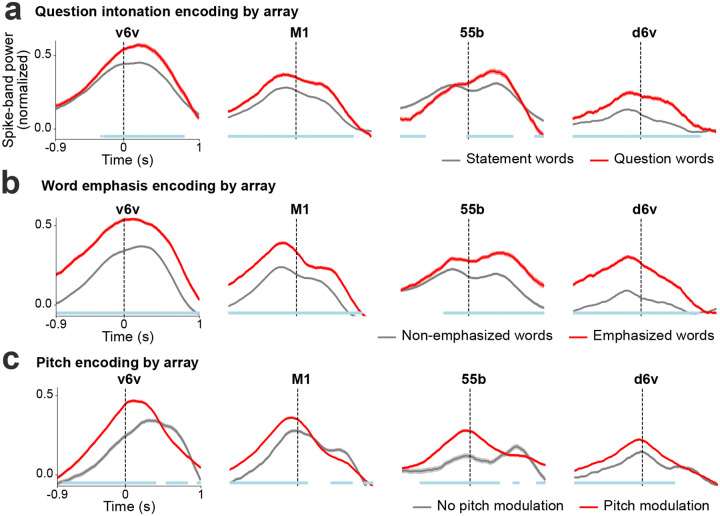
Extended Fig. 7: Paralinguistic features encoding recorded from individual arrays.
a. Trial-averaged spike-band power (mean ± s.e.), averaged across all electrodes within each array, for words spoken as statements and as questions. At every time point, the spike-band power for statement words and question words were compared using the Wilcoxon rank-sum test. The blue line at the bottom indicates the time points where the spike-band power in statement words and question words were significantly different (p<0.001, n1=970, n2=184). b. Trial averaged spike-band power across each array for non-emphasized and emphasized words. The spike-band power was significantly different between non-emphasized words and emphasized words at time points shown in blue (p<0.001, n1=1269, n2=333). c. Trial-averaged spike-band power across each array for words without pitch modulation and words with pitch modulation (from the three-pitch melodies singing task). Words with low and high pitch targets are grouped together as the ‘pitch modulation’ category, we excluded middle pitch target words where the participant used his normal pitch. The spike-band power was significantly different between no pitch modulation and pitch modulation at time points shown in blue (p<0.001, n1=486, n2=916).
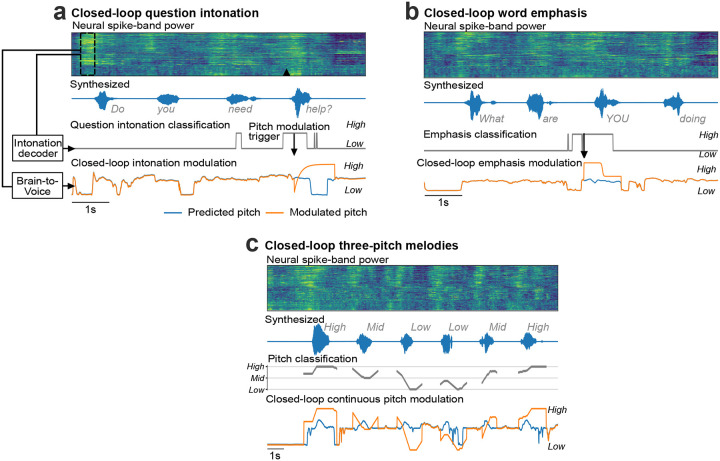
Extended Fig. 8: Closed-loop pitch modulation.
a. An example trial of closed-loop intonation modulation for speaking a sentence as a question. A separate binary decoder identified the change in intonation and sent a trigger to modulate the pitch feature output of the regular brain-to-voice decoder according to a predefined pitch profile for asking a question (low pitch to high pitch). Neural activity of an example trial with its synthesized voice output is shown along with the intonation decoder output, time of modulation trigger, originally predicted pitch feature and the modulated pitch feature used for voice synthesis. b. An example trial of closed-loop word emphasis where the word “YOU” from “What are YOU doing” was emphasized. To emphasize a word, we applied a predefined pitch profile (high pitch to low pitch) along with a 20% increase in the volume to the predicted speech features. c. An example trial of closed-loop pitch modulation for singing a melody with three pitch levels. The pitch classifier output was used to continuously modulate the predicted pitch feature output from the brain-to-voice decoder.
Extended Fig. 9: Pearson correlation coefficients over the course of a sentence.
Pearson correlation coefficient (r) of individual words in sentences of different lengths (mean ± s.d.). The correlation between target and synthesized speech remained consistent throughout the length of sentence, indicating that the quality of synthesized voice was consistent throughout the sentence. Note that there were fewer longer evaluation sentences.
Supplementary Material
Acknowledgements:
We thank participant T15 and his family and care partners for their immense contributions to this research.
Funding:
Funded by: the Office of the Assistant Secretary of Defense for Health Affairs through the Amyotrophic Lateral Sclerosis Research Program under award number AL220043; a New Innovator Award (DP2) from the NIH Office of the Director and managed by NIDCD (1DP2DC021055); a Seed Grant from the ALS Association (23-SGP-652); A. P. Giannini Postdoctoral Fellowship (Card); Searle Scholars Program; a Pilot Award from the Simons Collaboration for the Global Brain (AN-NC-GB-Pilot Extension-00002343-01); NIHNIDCD (U01DC017844); VA RR&D (A2295-R); Stavisky holds a Career Award at the Scientific Interface from BWF.
Competing Interests:
Stavisky is an inventor on intellectual property owned by Stanford University that has been licensed to Blackrock Neurotech and Neuralink Corp. Wairagkar, Stavisky, and Brandman have patent applications related to speech BCI owned by the Regents of the University of California. Brandman is a surgical consultant to Paradromics Inc. Stavisky is a scientific advisor to Sonera and ALVI Labs. The MGH Translational Research Center has a clinical research support agreement with Neuralink, Synchron, Axoft, Precision Neuro, and Reach Neuro, for which LRH provides consultative input. Mass General Brigham (MGB) is convening the Implantable Brain-Computer Interface Collaborative Community (iBCI-CC); charitable gift agreements to MGB, including those received to date from Paradromics, Synchron, Precision Neuro, Neuralink, and Blackrock Neurotech, support the iBCI-CC, for which LRH provides effort.
Funding Statement
Funded by: the Office of the Assistant Secretary of Defense for Health Affairs through the Amyotrophic Lateral Sclerosis Research Program under award number AL220043; a New Innovator Award (DP2) from the NIH Office of the Director and managed by NIDCD (1DP2DC021055); a Seed Grant from the ALS Association (23-SGP-652); A. P. Giannini Postdoctoral Fellowship (Card); Searle Scholars Program; a Pilot Award from the Simons Collaboration for the Global Brain (AN-NC-GB-Pilot Extension-00002343-01); NIHNIDCD (U01DC017844); VA RR&D (A2295-R); Stavisky holds a Career Award at the Scientific Interface from BWF.
Data and materials availability:
Data and code that can recreate the analyses and figures will be released with the peer-reviewed published paper.
References:
- 1.Card N. S. et al. An accurate and rapidly calibrating speech neuroprosthesis. 2023.12.26.23300110 Preprint at 10.1101/2023.12.26.23300110 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willett F. R. et al. A high-performance speech neuroprosthesis. Nature 620, 1031–1036 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzger S. L. et al. A high-performance neuroprosthesis for speech decoding and avatar control. Nature 620, 1037–1046 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva A. B., Littlejohn K. T., Liu J. R., Moses D. A. & Chang E. F. The speech neuroprosthesis. Nat. Rev. Neurosci. 25, 473–492 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herff C. et al. Generating Natural, Intelligible Speech From Brain Activity in Motor, Premotor, and Inferior Frontal Cortices. Front. Neurosci. 13, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angrick M. et al. Speech synthesis from ECoG using densely connected 3D convolutional neural networks. J. Neural Eng. 16, 036019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anumanchipalli G. K., Chartier J. & Chang E. F. Speech synthesis from neural decoding of spoken sentences. Nature 568, 493–498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng K. et al. Continuous synthesis of artificial speech sounds from human cortical surface recordings during silent speech production. J. Neural Eng. 20, 046019 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Le Godais G. et al. Overt speech decoding from cortical activity: a comparison of different linear methods. (2023) doi: 10.3389/fnhum.2023.1124065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y. et al. Decoding and synthesizing tonal language speech from brain activity. Sci. Adv. 9, eadh0478 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berezutskaya J. et al. Direct speech reconstruction from sensorimotor brain activity with optimized deep learning models. J. Neural Eng. 20, 056010 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X. et al. A neural speech decoding framework leveraging deep learning and speech synthesis. Nat. Mach. Intell. 6, 467–480 (2024). [Google Scholar]
- 13.Wilson G. H. et al. Decoding spoken English from intracortical electrode arrays in dorsal precentral gyrus. J. Neural Eng. 17, 066007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wairagkar M., Hochberg L. R., Brandman D. M. & Stavisky S. D. Synthesizing Speech by Decoding Intracortical Neural Activity from Dorsal Motor Cortex. in 2023 11th International IEEE/EMBS Conference on Neural Engineering (NER) 1–4 (2023). doi: 10.1109/NER52421.2023.10123880. [DOI] [Google Scholar]
- 15.Angrick M. et al. Real-time synthesis of imagined speech processes from minimally invasive recordings of neural activity. Commun. Biol. 4, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X., Wellington S., Fu Z. & Zhang D. Speech decoding from stereo-electroencephalography (sEEG) signals using advanced deep learning methods. J. Neural Eng. 21, 036055 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Angrick M. et al. Online speech synthesis using a chronically implanted brain–computer interface in an individual with ALS. Sci. Rep. 14, 9617 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glasser M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaswani A. et al. Attention Is All You Need. Preprint at 10.48550/arXiv.1706.03762 (2023). [DOI] [Google Scholar]
- 20.Downey J. E., Schwed N., Chase S. M., Schwartz A. B. & Collinger J. L. Intracortical recording stability in human brain–computer interface users. J. Neural Eng. 15, 046016 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Valin J.-M. & Skoglund J. LPCNET: Improving Neural Speech Synthesis through Linear Prediction. in ICASSP 2019 – 2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) 5891–5895 (2019). doi: 10.1109/ICASSP.2019.8682804. [DOI] [Google Scholar]
- 22.Li Y. A., Han C., Raghavan V. S., Mischler G. & Mesgarani N. StyleTTS 2: Towards Human-Level Text-to-Speech through Style Diffusion and Adversarial Training with Large Speech Language Models. Preprint at 10.48550/arXiv.2306.07691 (2023). [DOI] [Google Scholar]
- 23.Dichter B. K., Breshears J. D., Leonard M. K. & Chang E. F. The Control of Vocal Pitch in Human Laryngeal Motor Cortex. Cell 174, 21–31.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman M. T., Churchland M. M., Ryu S. I. & Shenoy K. V. Cortical activity in the null space: permitting preparation without movement. Nat. Neurosci. 17, 440–448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stavisky S. D., Kao J. C., Ryu S. I. & Shenoy K. V. Motor Cortical Visuomotor Feedback Activity Is Initially Isolated from Downstream Targets in Output-Null Neural State Space Dimensions. Neuron 95, 195–208.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchland M. M. & Shenoy K. V. Preparatory activity and the expansive null-space. Nat. Rev. Neurosci. 25, 213–236 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Moses D. A. et al. Neuroprosthesis for Decoding Speech in a Paralyzed Person with Anarthria. N. Engl. J. Med. 385, 217–227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchard K. E., Mesgarani N., Johnson K. & Chang E. F. Functional organization of human sensorimotor cortex for speech articulation. Nature 495, 327–332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chartier J., Anumanchipalli G. K., Johnson K. & Chang E. F. Encoding of Articulatory Kinematic Trajectories in Human Speech Sensorimotor Cortex. Neuron 98, 1042–1054.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J. et al. Neural control of lexical tone production in human laryngeal motor cortex. Nat. Commun. 14, 6917 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breshears J. D., Molinaro A. M. & Chang E. F. A probabilistic map of the human ventral sensorimotor cortex using electrical stimulation. (2015) doi: 10.3171/2014.11.JNS14889. [DOI] [PubMed] [Google Scholar]
- 32.Ammanuel S. G. et al. Intraoperative cortical stimulation mapping with laryngeal electromyography for the localization of human laryngeal motor cortex. (2024) doi: 10.3171/2023.10.JNS231023. [DOI] [PubMed] [Google Scholar]
- 33.Pandarinath C. et al. Neural population dynamics in human motor cortex during movements in people with ALS. eLife 4, e07436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stavisky S. D. et al. Neural ensemble dynamics in dorsal motor cortex during speech in people with paralysis. eLife 8, e46015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willett F. R. et al. Hand Knob Area of Premotor Cortex Represents the Whole Body in a Compositional Way. Cell 181, 396–409.e26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali Y. H. et al. BRAND: a platform for closed-loop experiments with deep network models. J. Neural Eng. 21, 026046 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young D. et al. Signal processing methods for reducing artifacts in microelectrode brain recordings caused by functional electrical stimulation. J. Neural Eng. 15, 026014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levelt W. J., Roelofs A. & Meyer A. S. A theory of lexical access in speech production. Behav. Brain Sci. 22, 1–38; discussion 38–75 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Räsänen O., Doyle G. & Frank M. C. Unsupervised word discovery from speech using automatic segmentation into syllable-like units. in 3204–3208 (2015). doi: 10.21437/Interspeech.2015-645. [DOI] [Google Scholar]
- 40.Williams A. H. et al. Discovering Precise Temporal Patterns in Large-Scale Neural Recordings through Robust and Interpretable Time Warping. Neuron 105, 246–259.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code that can recreate the analyses and figures will be released with the peer-reviewed published paper.