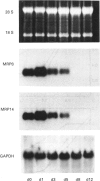
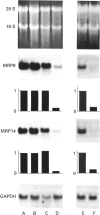
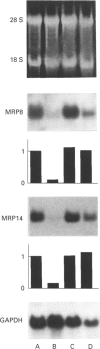
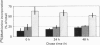Abstract
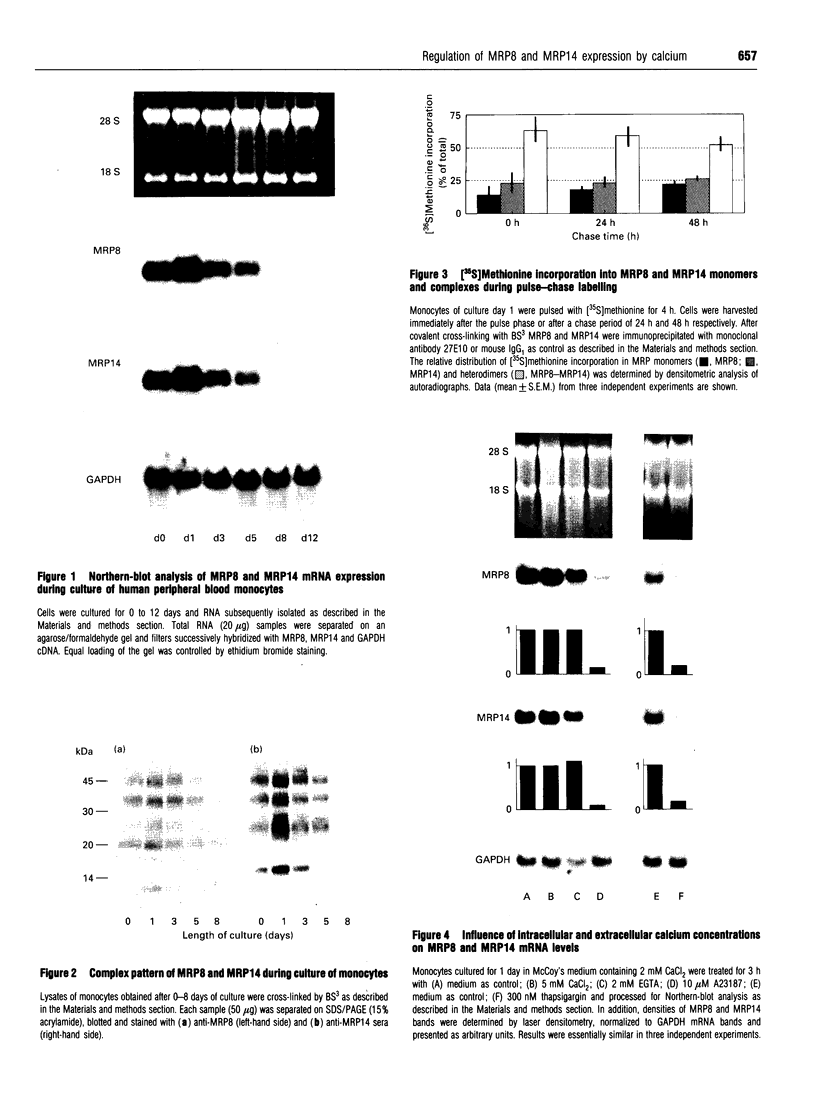
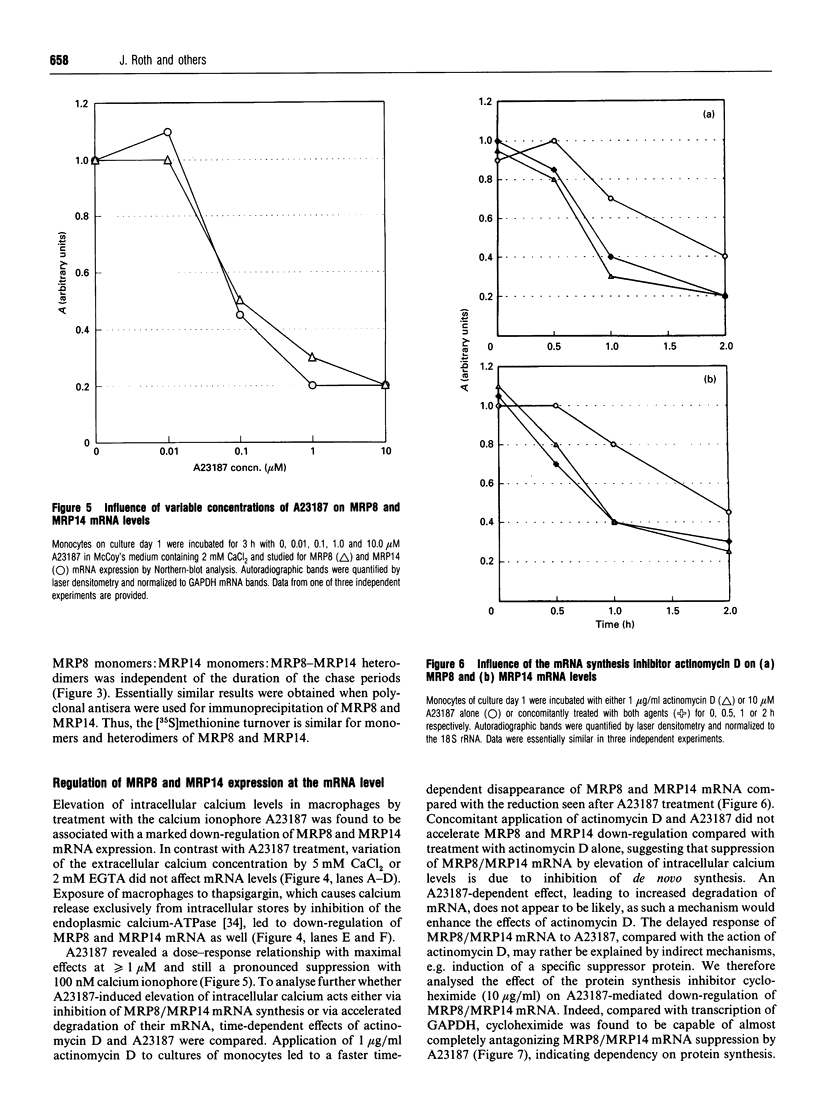
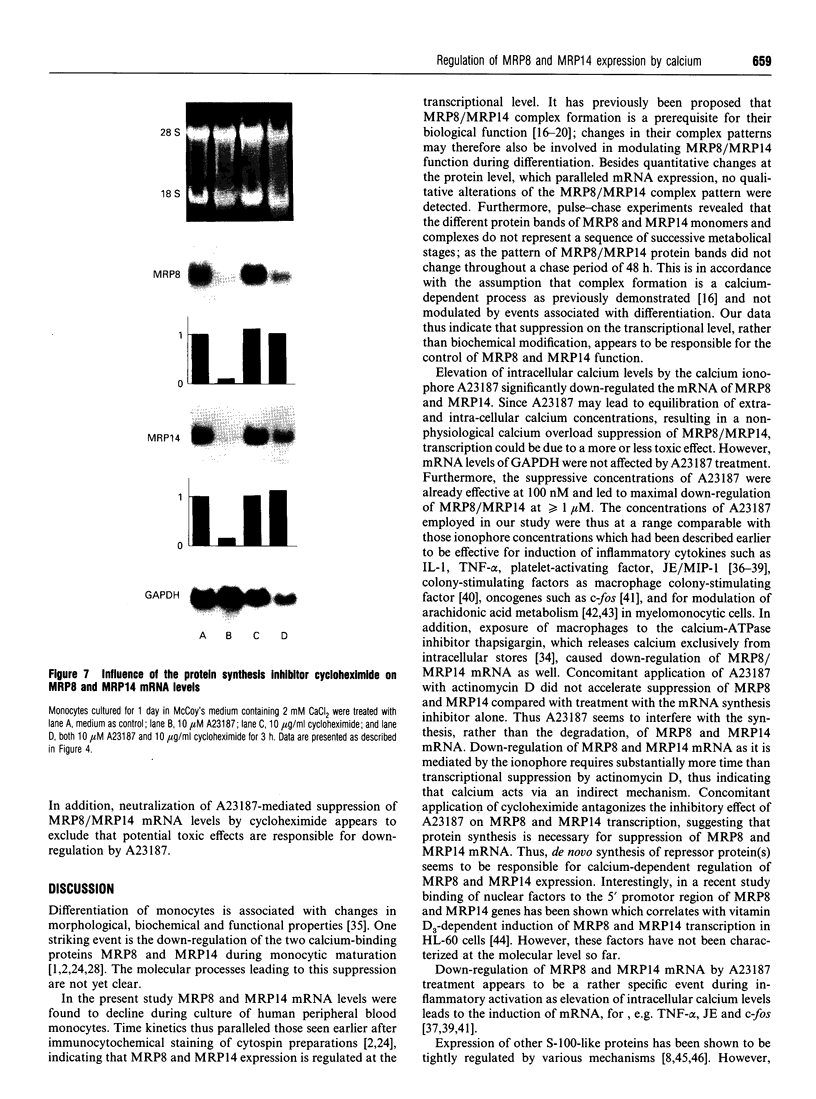
MRP8 and MRP14 are two calcium-binding proteins of the S-100 family the expression of which is restricted to distinct stages of monocytic differentiation. Heteromeric MRP8/MRP14 complexes have been shown to represent their biologically active forms. However, it is not as yet clear whether biochemical modification of complexes, or regulation on the transcriptional level, are responsible for the control of MRP8/MRP14 expression. Employing Western-blot analysis and metabolic labelling we have demonstrated that patterns and metabolism of MRP8/MRP14 complexes do not change during up- or down-regulation of MRP8/MRP14. By Northern-blot analysis it was shown that MRP8/MRP14 are regulated at the transcriptional level rather than by biochemical modification of the complexes. Elevation of intracellular calcium levels by A23187, as well as by thapsigargin, was found to lead to specific down-regulation of MRP8/MRP14 mRNA which is in contrast with data reported for inflammatory factors such as interleukin-1 or tumour necrosis factor alpha. Concomitant application of actinomycin D and calcium ionophore indicated that this suppressive effect is mediated by decreased synthesis rather than increased degradation of MRP8/MRP14 mRNA. Finally, we demonstrated that calcium-mediated down-regulation of MRP8-MRP14 can be antagonized by cycloheximide, suggesting that a calcium-induced repressor protein is responsible for suppression of MRP8-MRP14 at the transcriptional level. Our data indicate that the function of MRP8-MRP14 is restricted to events associated with early stages of myelomonocytic activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K. B., Sletten K., Berntzen H. B., Fagerhol M. K., Dale I., Brandtzaeg P., Jellum E. Leukocyte L1 protein and the cystic fibrosis antigen. Nature. 1988 Apr 21;332(6166):688–688. doi: 10.1038/332688a0. [DOI] [PubMed] [Google Scholar]
- Barraclough R., Savin J., Dube S. K., Rudland P. S. Molecular cloning and sequence of the gene for p9Ka. A cultured myoepithelial cell protein with strong homology to S-100, a calcium-binding protein. J Mol Biol. 1987 Nov 5;198(1):13–20. doi: 10.1016/0022-2836(87)90453-0. [DOI] [PubMed] [Google Scholar]
- Baudier J., Cole R. D. Interactions between the microtubule-associated tau proteins and S100b regulate tau phosphorylation by the Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1988 Apr 25;263(12):5876–5883. [PubMed] [Google Scholar]
- Bethea J. R., Gillespie G. Y., Benveniste E. N. Interleukin-1 beta induction of TNF-alpha gene expression: involvement of protein kinase C. J Cell Physiol. 1992 Aug;152(2):264–273. doi: 10.1002/jcp.1041520207. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R. S., Zotz C., Zwadlo-Klarwasser G., Roth J., Goebeler M., Mahnke K., Falk M., Meinardus-Hager G., Sorg C. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol. 1992 Jul;22(7):1891–1897. doi: 10.1002/eji.1830220732. [DOI] [PubMed] [Google Scholar]
- Brüggen J., Tarcsay L., Cerletti N., Odink K., Rutishauser M., Holländer G., Sorg C. The molecular nature of the cystic fibrosis antigen. Nature. 1988 Feb 18;331(6157):570–570. doi: 10.1038/331570a0. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Battini R., Kaczmarek L., de Riel J. K., Baserga R. Molecular cloning of the cDNA for a growth factor-inducible gene with strong homology to S-100, a calcium-binding protein. J Biol Chem. 1986 Sep 25;261(27):12628–12632. [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Salvidio G., Baglioni C. Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet-activating factor. J Exp Med. 1987 Nov 1;166(5):1390–1404. doi: 10.1084/jem.166.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dale I., Brandtzaeg P., Fagerhol M. K., Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985 Jul;84(1):24–34. doi: 10.1093/ajcp/84.1.24. [DOI] [PubMed] [Google Scholar]
- Dorin J. R., Novak M., Hill R. E., Brock D. J., Secher D. S., van Heyningen V. A clue to the basic defect in cystic fibrosis from cloning the CF antigen gene. Nature. 1987 Apr 9;326(6113):614–617. doi: 10.1038/326614a0. [DOI] [PubMed] [Google Scholar]
- Edgeworth J., Gorman M., Bennett R., Freemont P., Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991 Apr 25;266(12):7706–7713. [PubMed] [Google Scholar]
- Freeman M. R., Sueoka N. Induction and segregation of glial intermediate filament expression in the RT4 family of peripheral nervous system cell lines. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5808–5812. doi: 10.1073/pnas.84.16.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebeler M., Roth J., Henseleit U., Sunderkötter C., Sorg C. Expression and complex assembly of calcium-binding proteins MRP8 and MRP14 during differentiation of murine myelomonocytic cells. J Leukoc Biol. 1993 Jan;53(1):11–18. doi: 10.1002/jlb.53.1.11. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Hessian P. A., Edgeworth J., Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993 Feb;53(2):197–204. [PubMed] [Google Scholar]
- Hogg N., Allen C., Edgeworth J. Monoclonal antibody 5.5 reacts with p8,14, a myeloid molecule associated with some vascular endothelium. Eur J Immunol. 1989 Jun;19(6):1053–1061. doi: 10.1002/eji.1830190615. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Kligman D., Hilt D. C. The S100 protein family. Trends Biochem Sci. 1988 Nov;13(11):437–443. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Kuwayama A., Kuruto R., Horie N., Takeishi K., Nozawa R. Appearance of nuclear factors that interact with genes for myeloid calcium binding proteins (MRP-8 and MRP-14) in differentiated HL-60 cells. Blood. 1993 Jun 1;81(11):3116–3121. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagasse E., Weissman I. L. Mouse MRP8 and MRP14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992 Apr 15;79(8):1907–1915. [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Swisshelm K., Keyomarsi K., Sager R. Down-regulation of a member of the S100 gene family in mammary carcinoma cells and reexpression by azadeoxycytidine treatment. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2504–2508. doi: 10.1073/pnas.89.6.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarchand P., Vaglio M., Mauël J., Markert M. Translocation of a small cytosolic calcium-binding protein (MRP-8) to plasma membrane correlates with human neutrophil activation. J Biol Chem. 1992 Sep 25;267(27):19379–19382. [PubMed] [Google Scholar]
- Mackenzie R., Coles G. A., Topley N., Powell W. S., Williams J. D. Down-regulation of cyclooxygenase product generation by human peritoneal macrophages. Immunology. 1992 Aug;76(4):648–654. [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Calcium ionophore (A23187) increases interleukin 1 (IL-1) production by human peripheral blood monocytes and interacts synergistically with IL-1 to augment concanavalin A stimulated thymocyte proliferation. Cell Immunol. 1985 Jan;90(1):226–233. doi: 10.1016/0008-8749(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Murao S., Collart F. R., Huberman E. A protein containing the cystic fibrosis antigen is an inhibitor of protein kinases. J Biol Chem. 1989 May 15;264(14):8356–8360. [PubMed] [Google Scholar]
- Murao S., Collart F., Huberman E. A protein complex expressed during terminal differentiation of monomyelocytic cells is an inhibitor of cell growth. Cell Growth Differ. 1990 Oct;1(10):447–454. [PubMed] [Google Scholar]
- Odink K., Cerletti N., Brüggen J., Clerc R. G., Tarcsay L., Zwadlo G., Gerhards G., Schlegel R., Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987 Nov 5;330(6143):80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- Radzioch D., Varesio L. c-fos mRNA expression in macrophages is downregulated by interferon-gamma at the posttranscriptional level. Mol Cell Biol. 1991 May;11(5):2718–2722. doi: 10.1128/mcb.11.5.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Burwinkel F., van den Bos C., Goebeler M., Vollmer E., Sorg C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood. 1993 Sep 15;82(6):1875–1883. [PubMed] [Google Scholar]
- Roth J., Sunderkötter C., Goebeler M., Gutwald J., Sorg C. Expression of the calcium-binding proteins MRP8 and MRP14 by early infiltrating cells in experimental contact dermatitis. Int Arch Allergy Immunol. 1992;98(2):140–145. doi: 10.1159/000236177. [DOI] [PubMed] [Google Scholar]
- Roth J., Teigelkamp S., Wilke M., Grün L., Tümmler B., Sorg C. Complex pattern of the myelo-monocytic differentiation antigens MRP8 and MRP14 during chronic airway inflammation. Immunobiology. 1992 Nov;186(3-4):304–314. doi: 10.1016/S0171-2985(11)80259-7. [DOI] [PubMed] [Google Scholar]
- Sater R. A., Cripe L. D., Taylor J. D., Niedel J. E. Complex regulation of macrophage colony-stimulating factor in the HL-60 promyelocytic cell line. J Immunol. 1991 Jul 15;147(2):633–637. [PubMed] [Google Scholar]
- Shibata Y., McCaffrey P. G., Minowada J., Volkman A., Oghiso Y. Regulation of phospholipase A2 activation and arachidonic acid metabolism in an interleukin-3-dependent macrophage-like cell line. J Leukoc Biol. 1992 Jan;51(1):32–38. doi: 10.1002/jlb.51.1.32. [DOI] [PubMed] [Google Scholar]
- Staros J. V. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry. 1982 Aug 17;21(17):3950–3955. doi: 10.1021/bi00260a008. [DOI] [PubMed] [Google Scholar]
- Steinbakk M., Naess-Andresen C. F., Lingaas E., Dale I., Brandtzaeg P., Fagerhol M. K. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990 Sep 29;336(8718):763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- Sunderkötter C., Beil W., Roth J., Sorg C. Cellular events associated with inflammatory angiogenesis in the mouse cornea. Am J Pathol. 1991 Apr;138(4):931–939. [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C. S., Hamilton T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989 Feb 15;142(4):1274–1280. [PubMed] [Google Scholar]
- Teigelkamp S., Bhardwaj R. S., Roth J., Meinardus-Hager G., Karas M., Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem. 1991 Jul 15;266(20):13462–13467. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M. M., Busuttil A., Hayward C., Brock D. J., Dorin J. R., Van Heyningen V. Expression pattern of two related cystic fibrosis-associated calcium-binding proteins in normal and abnormal tissues. J Cell Sci. 1988 Oct;91(Pt 2):221–230. doi: 10.1242/jcs.91.2.221. [DOI] [PubMed] [Google Scholar]
- Zokas L., Glenney J. R., Jr The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987 Nov;105(5):2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwadlo G., Brüggen J., Gerhards G., Schlegel R., Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988 Jun;72(3):510–515. [PMC free article] [PubMed] [Google Scholar]
- Zwadlo G., Schlegel R., Sorg C. A monoclonal antibody to a subset of human monocytes found only in the peripheral blood and inflammatory tissues. J Immunol. 1986 Jul 15;137(2):512–518. [PubMed] [Google Scholar]