Abstract
Herpes simplex virus 1 (HSV-1) gD interaction with the host cell receptor nectin-1 triggers the membrane fusion cascade during viral entry. Potent neutralizing antibodies to gD prevent receptor-binding or prevent gD interaction with gH/gL critical for fusion. HSV has many strategies to evade host immune responses. We investigated the ability of virion envelope gC to protect envelope gD from antibody neutralization. HSV-1 lacking gC was more sensitive to neutralization by anti-gD monoclonal antibodies than a wild type rescuant virus. gD in the HSV-1 gC-null viral envelope had enhanced reactivity to anti-gD antibodies compared to wild type. HSV-1 ΔgC binding to the nectin-1 receptor was more readily inhibited by a neutralizing anti-gD monoclonal antibody. HSV-1 ΔgC was also more sensitive to inhibition by soluble nectin-1 receptor. The viral membrane protein composition of HSV-1 ΔgC was equivalent to that of wild type, suggesting that the lack of gC is responsible for the increased reactivity of gD-specific antibodies and the consequent increased susceptibility to neutralization by those antibodies. Together, the results suggest that gC in the HSV-1 envelope shields both receptor-binding domains and gH/gL-interacting domains of gD from neutralizing antibodies, facilitating HSV cell entry.
Introduction
Herpes simplex virus 1 (HSV-1) is a ubiquitous pathogen that is estimated to affect 90% of adults worldwide (1). Typical symptoms include recurrent oral or genital lesions. Infection is lifelong and there is no vaccine (2). Grave outcomes of HSV infection include encephalitis, blindness, and disseminated infections of the immunocompromised (3, 4). The high prevalence and persistence of HSV is partly due to immune evasion strategies employed by the virus.
HSV-1 glycoprotein C (gC) is a multifunctional 511 amino acid, type 1 membrane glycoprotein present in the virion envelope and on the surface of infected cells (5). gC is specific to the alphaherpesviruses. Virion envelope gC functions in viral entry into host cells (6–9). gC also plays roles in immune evasion and has been a focus of HSV vaccine strategies (10–18). Virion gC protects gB from antibody-mediated neutralization (13, 15). Here we investigate the ability of gC to shield the HSV receptor-binding protein gD.
HSV-1 glycoprotein D (gD) is a 369 amino acid type I envelope glycoprotein (19). Host cell receptors nectin-1 and HVEM bind to the same face of gD, near the C-terminus of the gD ectodomain (Fig. 1), but at distinct sites (20–29). Binding of gD to a cognate receptor triggers the movement of the C-terminal extension, revealing receptor contact sites on the core. The receptor-triggered structural change in gD is thought to initiate the membrane fusion cascade by promoting interaction with gH/gL (30–36). HSV-1 gD is the major target of neutralizing antibodies and is a prime target for vaccine development (37, 38). MAbs to gD can block HSV entry by preventing binding to host cell receptors or can block fusion with no effect on receptor binding.
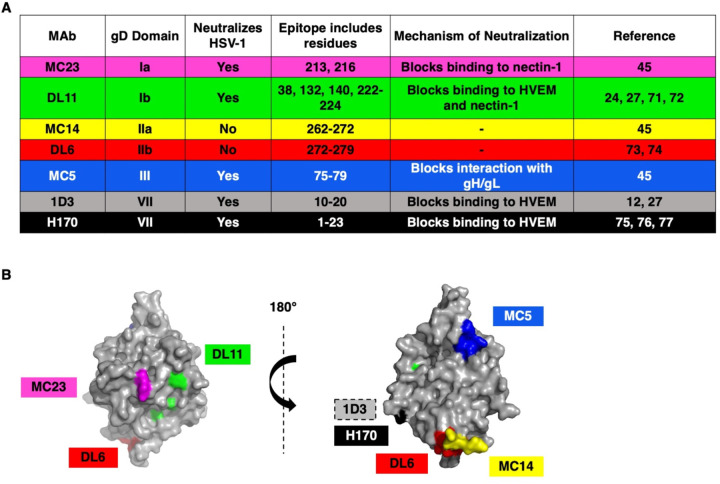
Fig. 1.
(A) Monoclonal antibodies to HSV-1 gD used in this study. (B) Structure of HSV-1 gD ectodomain (PDB accession number 2C36) (25) with MAb epitopes indicated. The receptor binding face of gD is on the left. MAb 1D3 binds to gD residues near the N-terminus that are not resolved in this structure.
In this study, we provide evidence that gC protects gD from antibody recognition of neutralizing epitopes. The envelope glycoproteins of several viruses protect themselves from antibody neutralization (39–42). The results support a unique viral immune protection mechanism whereby HSV-1 gC shields distinct neighboring glycoproteins from entry-blocking antibodies.
The absence of gC renders HSV-1 more sensitive to neutralization by gD antibodies on two distinct cell types.
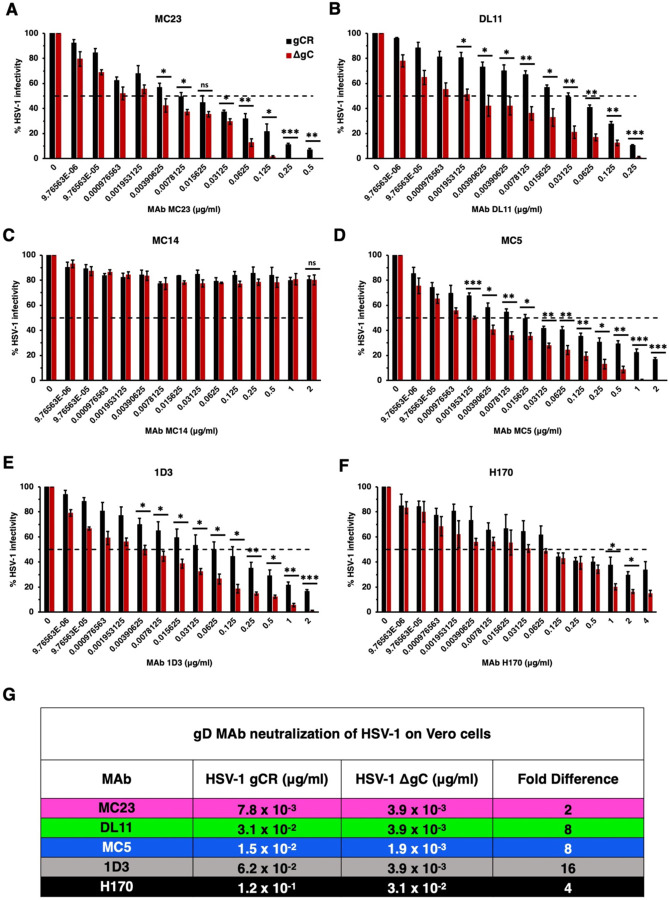
To determine the impact of virion gC on HSV-1 infectivity in the presence of neutralizing MAbs we employed a panel of mouse anti-gD MAbs against multiple epitopes and functions of gD (Fig. 1). We tested two-fold dilutions of these MAbs ranging from 2 μg/mL to 9.76 x 10−6 μg/mL on Vero cells. HSV-1 neutralization was defined as a reduction in infectivity of >50% in the presence of anti-gD MAb. Importantly, HSV-1 gCR and ΔgC contain similar levels of viral proteins gB, gD, gH, and VP5 (data not shown) (9, 15). Thus, differences detected between the two viruses may be attributed to the lack of gC in the gC-null virus. HSV-1 ΔgC was more sensitive to MAb neutralization ranging from 2–16-fold more sensitive compared to HSV-1 gCR (Fig. 2). The negative control MAb MC14 failed to neutralize either virus, as expected (Fig. 2). MAb 1D3, which blocks gD from interacting with HVEM, neutralized ΔgC at a concentration of 3.9 x 10−3 μg/mL. 1D3 neutralized gCR at 0.125 μg/mL, which was 16-fold higher than the concentration required to neutralize ΔgC (Fig. 2E). MAb MC5, which blocks gD from interacting with gH/gL, neutralized HSV-1 ΔgC at 3.9 x 10−3 μg/mL and gCR at a concentration of 1.5 x 10−2 μg/mL on Vero cells (Fig. 2D). This was a 4-fold difference in MAb MC5 concentration. MAb MC23, which blocks gD interaction with nectin-1, required a 2-fold higher concentration to neutralize HSV-1 gCR. MAb DL11, which blocks gD interactions with both nectin-1 and HVEM, required an 8-fold higher concentration to neutralize gCR (Fig. 2A and B). In summary, 2- to 16-fold more antibody was required to neutralize HSV-1 when gC was present (Fig. 2G).
Fig. 2.
Neutralization of gC-null mutant HSV-1 infection of Vero cells by antibodies to gD. HSV-1 gCR (black) or HSV-1 ΔgC (red) (100 PFU) was treated with monoclonal antibodies MC23 (A), DL11 (B), MC14 (C), MC5 (D), 1D3 (E), or H170 (F) for 1 h at 37°C. Infectivity was determined by plaque formation on Vero cells. Each experiment was performed with triplicate samples. Values are the means and standard errors of results from three independent experiments. Statistical significance was determined via Student’s t-test where *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. (G) Antibody concentration at which > 50% of virus was neutralized. Fold difference was calculated by dividing the concentration of MAb required to neutralize HSV-1 gCR by the concentration of MAb required to neutralize ΔgC.
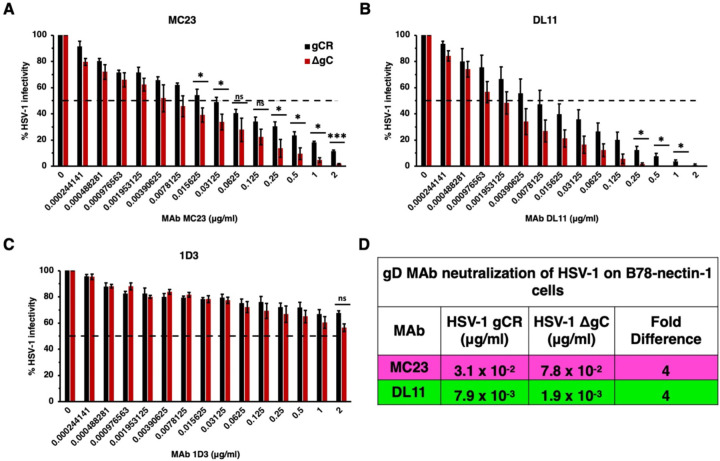
Next, we investigated whether virion gC impacts the ability of nectin-1 blocking antibodies to neutralize HSV-1 infection specifically mediated by nectin-1. Mouse melanoma B78 cells are resistant to HSV entry and must be supplied with a gD-receptor to render them permissive to HSV entry and infection (43). On B78-nectin-1 cells, MAb DL11 neutralized HSV-1 ΔgC at a concentration of 1.9 x10−3 μg/mL. Four-fold more DL11 (7.8 x 10−3 μg/mL) was required to neutralize gCR (Fig. 3B). MAb MC23 neutralized HSV-1 ΔgC at 7.8 x10−3 μg/ml and gCR at 0.3125 μg/ml. Thus, 4-fold more antibody was required to neutralize gCR (Fig. 3A). MAb 1D3 failed to neutralize HSV-1 infection mediated by nectin-1, as expected (Fig. 3C). In summary, on B78-nectin-1 cells, 4-fold more MAb was required to neutralize HSV-1 gCR compared to ΔgC (Fig. 3D). HSV-1 that lacks gC had enhanced sensitivity to anti-gD MAbs across two cell types and across all domains of gD tested. It was previously shown that MAb DL6 neutralized HSV-1 ΔgC at a dilution of 1:2000 on Vero cells and failed to neutralize gCR (15). These data demonstrate that the absence of gC renders HSV-1 more sensitive to neutralization by gD MAbs.
Fig. 3.
Neutralization of gC-null mutant HSV-1 infection mediated specifically by the nectin-1 receptor. HSV-1 gCR (black) or HSV-1 ΔgC (red) was treated with gD monoclonal antibodies MC23 (A), DL11 (B), or 1D3 (C) for 1 h at 37°C. Infectivity was determined by plaque formation on B78-nectin-1 cells. Values are the means and standard errors from three independent experiments. Statistical significance was determined via Student’s t-test where *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. (D) Antibody concentration at which > 50% of virus was neutralized. Fold difference was calculated by dividing the concentration of MAb required to neutralize HSV-1 gCR by the concentration of MAb required to neutralize ΔgC.
The absence of virion gC enhances HSV-1 reactivity to gD antibodies.
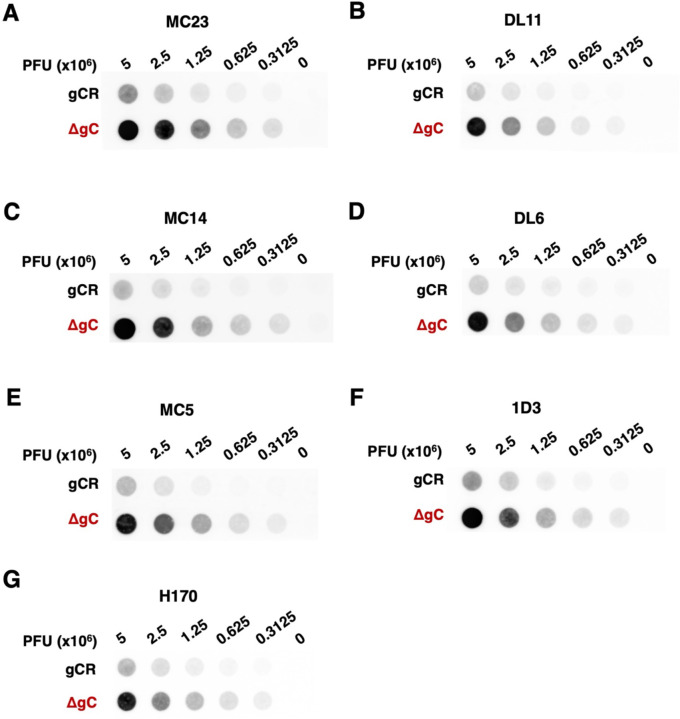
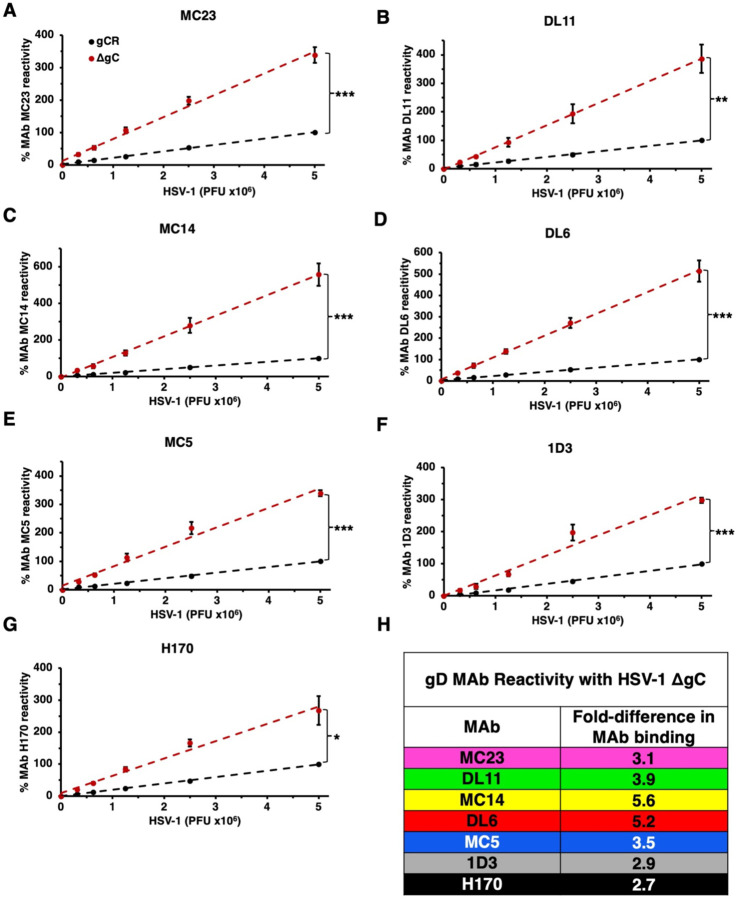
To interrogate the mechanism by which HSV-1 ΔgC is more sensitive to neutralization, we assessed the antigenic reactivity of gD MAbs with both HSV-1 gCR and ΔgC. The binding of the panel of gD antibodies to HSV-1 ΔgC and gCR was compared by dot blot immunoassay. Virus was blotted directly onto nitrocellulose membrane under native conditions. The membrane was probed with anti-gD Mabs, and binding was determined via fluorescence imaging (Fig. 4) followed by densitometry (Fig. 5). HSV-1 ΔgC was more sensitive to gD MAb binding compared to gCR, ranging from 2.7- to 5.6-fold more sensitive. MAb MC23, which blocks gD from interacting with nectin-1, bound to HSV-1 ΔgC 3.1-fold more intensely than to gCR (Fig. 4A and 5A). MAb MC14, which is non-neutralizing, bound to ΔgC 5.6-fold more intensely than to gCR (Fig. 4C and 5C). This trend remained constant across all domains of gD tested, with every antibody being more reactive with HSV-1 ΔgC. For ΔgC, there was an enhanced reactivity of 3.9-fold with DL11, 5.2-fold with DL6, 3.5-fold with MC5, 2.9-fold with 1D3, and 2.7-fold with 1D3 (Fig. 4 and 5). In summary, HSV-1 ΔgC was more sensitive to gD MAb binding regardless of the MAb’s epitope or function (Fig. 5H).
Fig. 4.
Reactivity of HSV-1 ΔgC with MAbs to gD. Equivalent infectious particles of HSV-1 ΔgC or gCR were serially diluted and blotted directly onto nitrocellulose membranes and probed with gD MAbs MC23 (A), DL11 (B), MC14 (C), DL6 (D), MC5 (E), 1D3 (F), or H170 (G). MAb reactivity was determined via densitometry with ImageJ (Fig. 5).
Fig. 5.
Reactivity of HSV-1 ΔgC with MAbs to gD. (A-G) HSV-1 ΔgC (red) or gCR (black) was blotted onto a nitrocellulose membrane and probed with antibodies against gD. Antibody reactivity was determined via densitometry with ImageJ. Results are the mean and standard error of three independent experiments. Representative blots are shown in Figure 4. Statistical significance was determined via Student’s t-test where *, p < 0.05; **, p < 0.01; ***, p < 0.001. (H) Differences in HSV-1 ΔgC and gCR reactivity were determined by comparing slopes of the best fit lines in panels A-G.
The presence of gC in HSV-1 reduces the ability of a gD monoclonal antibody to block receptor binding.
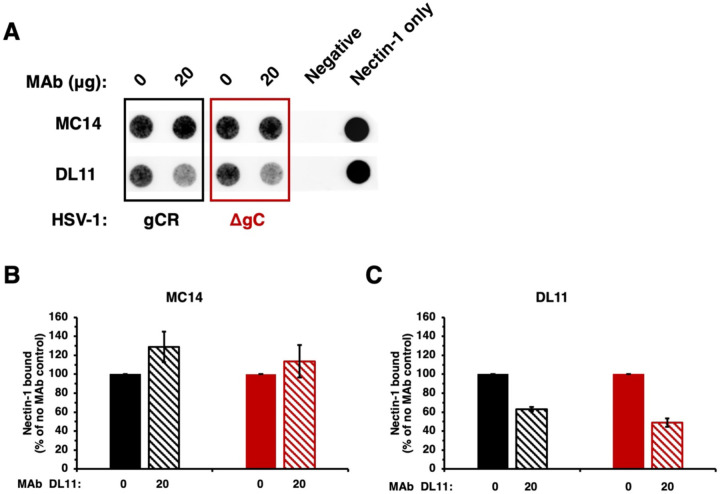
We next assessed the effect of gC on the ability of anti-gD antibody to inhibit HSV-1 binding to receptor. Soluble ectodomain forms of gD-receptors bind directly to HSV particles and block entry and infection (24, 27, 44). We tested the ability of HSV-1 ΔgC to bind to soluble nectin-1 in the presence of nectin-1 blocking gD MAb DL11 (24, 27). HSV-1 ΔgC or gCR was pre-incubated with gD MAb, and then soluble nectin-1 was added. Samples were layered onto a sucrose gradient and separated by ultracentrifugation. The virion fraction was recovered and blotted directly onto a nitrocellulose membrane, and then probed for the presence of soluble nectin-1 (Fig. 6A).
Fig. 6.
Inhibition of HSV-1 ΔgC binding to nectin-1 by gD antibodies. (A) HSV-1 ΔgC (red) or gCR (black) was treated with gD MAb DL11 or MC14 at 37°C for 1 h. Soluble nectin-1 was added at 4°C for 2 h. Samples were separated on a sucrose gradient, and the HSV-1-containing fraction was blotted onto a nitrocellulose membranes and probed with anti-6x-HIS tag MAb to detect nectin-1. (B, C) Nectin-1 binding was determined via densitometry with ImageJ. Results are the mean and standard error of three independent experiments.
Following pre-incubation with 20 μg MC14, a non-neutralizing gD MAb, soluble nectin-1 binding to HSV-1 ΔgC and gCR was not inhibited, as expected (Fig. 6A and B). MAb MC14 enhanced nectin-1 reactivity with both HSV-1 gCR and HSV-1 ΔgC, as previously reported (45). MAb MC14’s impact on ΔgC binding to nectin-1 was similar to gCR (Fig. 6B). gD MAb DL11 inhibited nectin-1 binding to both viruses (Fig. 6A). MAb DL11 inhibited 51% of soluble nectin-1 binding to HSV-1 ΔgC and inhibited 37% of soluble nectin-1 binding to gCR (Fig. 6C). This is consistent with findings from the dot blot assay (Fig. 4 and 5) and neutralization assay (Fig. 2 and 3).
The absence of gC renders HSV-1 more sensitive to inhibition by soluble nectin-1.
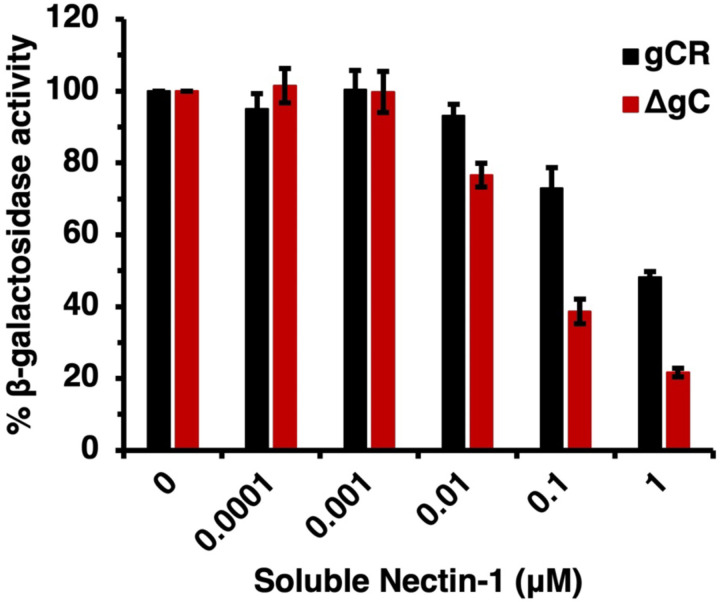
We investigated the ability of a recombinant nectin-1 ectodomain to block entry of HSV-1 in the absence of gC. Soluble nectin-1 receptor inhibits HSV-1 entry and infection by competing with receptors on target cells (44). To evaluate the ability of soluble nectin-1 to inhibit HSV-1 ΔgC entry, we conducted a β-galactosidase reporter assay. B78-nectin-1 cells contain the E. coli lacZ gene under the control of the HSV-1 ICP4 gene promoter (43). HSV-1 ΔgC or gCR was incubated with soluble nectin-1 for 2 h at 4°C and then added to B78-nectin-1 cells. At 6 h p.i., β-galactosidase activity was determined. Soluble nectin-1 inhibited entry of both HSV-1 ΔgC and gCR in a concentration-dependent manner starting at 0.01 μM (Fig. 7). However, soluble nectin-1 hampered HSV-1 ΔgC entry more robustly than HSV-1 gCR. Following pretreatment with 1 μM soluble nectin-1, HSV-1 ΔgC entry was reduced to 21% vs. 48% for HSV-1 gCR entry (Fig. 7). Together, the results suggest that virion gC renders HSV-1 less sensitive to inhibition by both gD antibodies and soluble receptor.
Fig. 7.
Inhibition of HSV-1 ΔgC entry by soluble nectin-1. HSV-1 ΔgC (red) or gCR (black) (2 x 105 PFU) was treated with soluble nectin-1 at 4°C for 2 h and then added to B78-nectin-1 cells (MOI 5). At 6 h p.i., β-galactosidase activity was detected as an indicator of entry and infection. Results are the mean and standard error of three independent experiments.
Discussion
HSV-1 gC has multiple functions during the viral infectious cycle, including entry, egress, and immune evasion. The current study demonstrates the ability of virion gC to shield the essential receptor-binding protein gD. We present several lines of evidence suggesting that gC in the HSV particle protects against neutralizing antibody to gD and against inhibition by soluble gD-receptors. We propose that gC is broadly shielding the entire neighboring gD molecule, including important functional domains for fusion and entry.
HSV-1 harbors many immune protective features that contribute to persistence in the host. HSV-1 gE, an envelope glycoprotein that is non-essential for entry, forms a high affinity Fc receptor with its partner gI. gE/gI binds to the Fc region of immunoglobulin G (IgG) antibodies to prevent epitope recognition (46, 47). gC prevents complement activation by binding and sequestering complement protein C3b (12, 18). Antibodies to gC can block this function (48). The increased sensitivity of gC-null HSV-1 to antibody-mediated neutralization (Fig. 2 and 3) can be explained at least in part by enhanced binding of antibodies to the virus in the absence of virion gC (Fig. 4 and 5). Neutralizing and non-neutralizing antibodies bound better to HSV that lacks gC. gC also shields gB and gH/gL from monoclonal antibody binding and neutralization (13, 15). This protective role is specific to gC. The absence of gE from the HSV-1 particle had little to no effect on MAb-mediated neutralization of HSV-1 (15).
For several viruses including influenza, HIV, and Nipah virus, the N-linked glycans of the viral fusion protein shield its own epitopes from neutralization (39–42, 49). The N-linked glycans of HSV-1 fusion protein gB provide self-protection against antibody-mediated neutralization and antibody-dependent cytotoxicity (50). gC is not a fusion protein, but it contains a heavily glycosylated N-terminal domain. Future research will determine whether N-glycans on gC shield neighboring glycoproteins. Whether N-glycans on gC block the binding of anti-gC antibodies also remains to be determined. This would be a unique feature for a non-fusion glycoprotein. Importantly, gC is in close enough proximity to gD to be chemically crosslinked in HSV particles (51). However, direct interaction between gC and gD has not been detected. Low-affinity or transient interactions may be difficult to capture. Physical interactions between and among HSV-1 gD, gH/gL and gB have also been difficult to capture, despite demonstrations of functional interactions (33, 52–56). The specifics of how gC protects neighboring glycoproteins from antibody-mediated neutralization is the subject of ongoing work.
Initial attachment of HSV-1 to the host cell is mediated by gC interaction with cell surface proteoglycans, principally heparan sulfate (6, 7). Alphaherpesviruses utilize low pH endosomal entry pathways in a cell-specific manner (57–62). The fusion protein gB undergoes well-documented antigenic changes upon exposure to mildly acidic pH, such as that present in the host cell endosomes (63–68). During endosomal entry into epithelial cells, gC undergoes pH-triggered changes and is thought to regulate the conformational change and function of the fusion protein gB (8, 9). gC also enhances virion release from infected cells (69).
This study highlights gC as an immune protective molecule that shields neighboring entry glycoproteins from neutralizing antibody binding and activity. Antibodies against gC can block immune evasion functions (14). Several vaccine candidates for HSV-1 and HSV-2 contain two or more different surface glycoprotein immunogens, including gC (10, 11, 14, 16, 17). Inclusion of gC in an HSV vaccine may block intrinsic protective properties of HSV.
Materials and Methods
Cells and viruses
Vero cells (American Type Culture collection; ATCC; Rockville, MD) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Corporation, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA) and penicillin, streptomycin, and glutamine (PSG; Life Technologies Corporation). B78 murine melanoma cells expressing nectin-1 (B78-nectin-1) (43), gifted by G. Cohen and R. Eisenberg (University of Pennsylvania), were cultured with the same medium. B78-nectin-1 cells were selected every third passage in culture medium supplemented with 250 μg/mL geneticin (G418; Sigma-Aldrich, St. Louis, MO) and 6 μg/ml puromycin (Sigma-Aldrich). HSV-1 KOS strain with the gC gene deleted, HSV-1ΔgC2-3 (ΔgC), and its rescuant, HSV-1gC2-3R (gCR) (70) were gifts from C.R. Brandt (University of Wisconsin, Madison).
Antibodies
Anti-HSV-1 gD mouse monoclonal antibodies MC23 (domain Ia) (45), DL11 (domain Ib) (24, 27, 71, 72), MC14 (domain IIa) (45), DL6 (domain IIb) (73, 74), MC5 (domain III) (45), and 1D3 (domain VII) (12, 27) were gifts from G. Cohen and R. Eisenberg (University of Pennsylvania). H170 (domain VII) (75–77) was purchased from Virusys (Milford, MA).
Plaque inhibition (neutralization) assay
Antibodies to gD were diluted two-fold in complete DMEM to achieve final concentrations ranging from 2 μg/ml to 2.4 x 10−4 μg/ml. HSV-1 ΔgC or gCR (100 PFU) was added to the antibody dilutions and incubated at 37°C for 1 h. The antibody-virus mixture was added to subconfluent Vero cells or B78-nectin-1 cells grown in 24-well plates. At 1 h p.i., the antibody-virus mixture was removed and replaced with fresh culture medium. At 18 to 24 h p.i., cells were fixed with an ice-cold 1:2 methanol-acetone solution. Plaque formation was determined by immunoperoxidase staining. Anti-HSV polyclonal antibody HR50 (Fitzgerald Industries International Inc., Acton, MA) was added to cells overnight at room temperature. Pro A-horseradish peroxidase (Invitrogen, Rockford, IL) secondary antibody was added for 2 h at room temperature. 4-chloro-1-napthol substrate (Sigma-Aldrich) was added for 15 min at room temperature. A MAb was considered neutralizing if there was a >50% reduction in plaque formation (infectivity).
Dot blot assay
Serial dilutions of cell-free HSV-1 ΔgC or gCR, were prepared in Dulbecco’s phosphate buffered saline (PBS)(Life Technologies Limited, Paisley, UK). Samples were blotted onto a nitrocellulose membrane using a Minifold dot blot system (78) (Whatman, Kent, UK). Five percent milk in 0.2% PBS-Tween 20 blocking buffer was added, and the membrane was gently rocked for 30 min. Primary anti-HSV-1 gD antibody was prepared in blocking buffer and added to the membrane overnight at 4°C. Goat-anti-mouse polyclonal antibody conjugated with Alexa Fluor 647 (Invitrogen) was prepared in blocking buffer and added to the membrane at room temperature for 30 min. The membrane was imaged with an Azure Biosystems c400 fluorescent western blot imager and quantified via densitometry (ImageJ).
Receptor binding assay
VP5 equivalents of HSV-1 ΔgC or gCR were incubated with 20 μg anti-gD-MAbs MC14 or DL11 in 10% BSA in PBS for 1 h at 37°C. 15 μg of a soluble ectodomain form of nectin-1 (containing amino acids Gln 31 – Thr 334) truncated prior to the transmembrane region and containing a C-terminal 6 x His tag (ACRO Biosystems, Newark, DE) was added. The mixture was incubated at 4°C for 2 h. Samples were added to the top of a 60%-30%-10% sucrose/PBS gradient and centrifuged at 16,000 x g for 4.5 h at 4°C with an SW32 Ti rotor (Beckman, Brea, CA). Virus bands at the 60%-30% sucrose interface were collected via tube side puncture. Virus bands were then blotted onto nitrocellulose membrane. Membranes were incubated in blocking buffer as described above. To detect nectin-1, a 6x-HIS antibody conjugated with CoraLite Plus 647 (Proteintech Group, Rosemont, IL) was added for 1.5 h at RT. The membrane was imaged with an Azure Biosystems c400 fluorescent western blot imager and quantified via densitometry (ImageJ)
β-galactosidase reporter assay for HSV-1 entry
HSV-1 gCR or ΔgC was incubated with 1 x 10−4 μM to 1 μM soluble nectin-1 in cell culture medium for 2 h at 4°C. B78-nectin-1 cells were infected with the virus-nectin-1 mixture in quadruplicate for 6 h at 37°C. Cells were lysed with 1% IGEPAL C-630 (Sigma-Aldrich) and frozen at −80°C overnight. Lysates were thawed and chlorophenol red-beta-d-galactopyranoside (Roche Diagnostics, Indianapolis, IN) substrate was added. β-galactosidase activity was read at 595 nm with an ELx808 microtiter plate reader (BioTek Instruments, Winooski, VT).
Importance.
HSV-1 causes lifelong infections. There is no vaccine and no cure. Understanding HSV immune evasion strategies is an important goal. HSV-1 gC is a multi-functional envelope glycoprotein. This study suggests that virion gC physically shields neighboring gD from antibodies, including neutralizing monoclonal antibodies. This mechanism may allow HSV to escape immune detection, promoting HSV infection in the host.
Acknowledgments
We thank Curtis Brandt, Gary Cohen, and Roselyn Eisenberg for the gifts of reagents. This work was supported by National Institutes of Health grant R56AI119159 (A.V. N.).
References
- 1.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One 10:e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitley RJ, Johnston C. 2022. Herpes Simplex Viruses: Pathogenesis and Clinical Insights, p 297–323. In Knipe DM, Howley PM (ed), Fields Virology, 7th edition, vol 2. Wolters Kluwer. [Google Scholar]
- 3.Kennedy PG, Steiner I. 2013. Recent issues in herpes simplex encephalitis. J Neurovirol 19:346–50. [DOI] [PubMed] [Google Scholar]
- 4.Liesegang TJ. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13. [DOI] [PubMed] [Google Scholar]
- 5.Heine JW, Honess RW, Cassai E, Roizman B. 1974. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol 14:640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold BC, WuDunn D, Soltys N, Spear PG. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol 65:1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WuDunn D, Spear PG. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol 63:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianopulos KA, Komala Sari T, Weed DJ, Pritchard SM, Nicola AV. 2022. Conformational Changes in Herpes Simplex Virus Glycoprotein C. J Virol 96:e0016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komala Sari T, Gianopulos KA, Weed DJ, Schneider SM, Pritchard SM, Nicola AV. 2020. Herpes Simplex Virus Glycoprotein C Regulates Low-pH Entry. mSphere 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2011. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J Virol 85:10472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awasthi S, Hook LM, Pardi N, Wang F, Myles A, Cancro MP, Cohen GH, Weissman D, Friedman HM. 2019. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633–5. [DOI] [PubMed] [Google Scholar]
- 13.Hook LM, Huang J, Jiang M, Hodinka R, Friedman HM. 2008. Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E. J Virol 82:6935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hook LM, Awasthi S, Dubin J, Flechtner J, Long D, Friedman HM. 2019. A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine 37:664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komala Sari T, Gianopulos KA, Nicola AV. 2020. Glycoprotein C of Herpes Simplex Virus 1 Shields Glycoprotein B from Antibody Neutralization. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel CD, Taylor SA, Mehrbach J, Awasthi S, Friedman HM, Leib DA. 2020. Trivalent Glycoprotein Subunit Vaccine Prevents Neonatal Herpes Simplex Virus Mortality and Morbidity. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weir JP, Bennett M, Allen EM, Elkins KL, Martin S, Rouse BT. 1989. Recombinant vaccinia virus expressing the herpes simplex virus type 1 glycoprotein C protects mice against herpes simplex virus challenge. J Gen Virol 70 (Pt 10):2587–94. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg RJ, Ponce de Leon M, Friedman HM, Fries LF, Frank MM, Hastings JC, Cohen GH. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog 3:423–35. [DOI] [PubMed] [Google Scholar]
- 19.Watson RJ, Weis JH, Salstrom JS, Enquist LW. 1982. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science 218:381–4. [DOI] [PubMed] [Google Scholar]
- 20.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8:169–79. [DOI] [PubMed] [Google Scholar]
- 21.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J Virol 77:8127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly SA, Landsburg DJ, Carfi A, Whitbeck JC, Zuo Y, Wiley DC, Cohen GH, Eisenberg RJ. 2005. Potential nectin-1 binding site on herpes simplex virus glycoprotein d. J Virol 79:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7:e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol 72:7064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J 24:4144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazear E, Carfi A, Whitbeck JC, Cairns TM, Krummenacher C, Cohen GH, Eisenberg RJ. 2008. Engineered disulfide bonds in herpes simplex virus type 1 gD separate receptor binding from fusion initiation and viral entry. J Virol 82:700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol 72:3595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon M, Zago A, Shukla D, Spear PG. 2003. Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1. J Virol 77:9221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon M, Spear PG. 2004. Random mutagenesis of the gene encoding a viral ligand for multiple cell entry receptors to obtain viral mutants altered for receptor usage. Proc Natl Acad Sci U S A 101:17252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol 84:12292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atanasiu D, Saw WT, Lazear E, Whitbeck JC, Cairns TM, Lou H, Eisenberg RJ, Cohen GH. 2018. Using Antibodies and Mutants To Localize the Presumptive gH/gL Binding Site on Herpes Simplex Virus gD. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atanasiu D, Saw WT, Cairns TM, Eisenberg RJ, Cohen GH. 2021. Using Split Luciferase Assay and anti-HSV Glycoprotein Monoclonal Antibodies to Predict a Functional Binding Site Between gD and gH/gL. J Virol 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atanasiu D, Saw WT, Cairns TM, Friedman HM, Eisenberg RJ, Cohen GH. 2023. Receptor Binding-Induced Conformational Changes in Herpes Simplex Virus Glycoprotein D Permit Interaction with the gH/gL Complex to Activate Fusion. Viruses 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cairns TM, Huang ZY, Whitbeck JC, Ponce de Leon M, Lou H, Wald A, Krummenacher C, Eisenberg RJ, Cohen GH. 2014. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J Virol 88:12612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairns TM, Ditto NT, Atanasiu D, Lou H, Brooks BD, Saw WT, Eisenberg RJ, Cohen GH. 2019. Surface Plasmon Resonance Reveals Direct Binding of Herpes Simplex Virus Glycoproteins gH/gL to gD and Locates a gH/gL Binding Site on gD. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cairns TM, Atanasiu D, Saw WT, Lou H, Whitbeck JC, Ditto NT, Bruun B, Browne H, Bennett L, Wu C, Krummenacher C, Brooks BD, Eisenberg RJ, Cohen GH. 2020. Localization of the Interaction Site of Herpes Simplex Virus Glycoprotein D (gD) on the Membrane Fusion Regulator, gH/gL. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cairns TM, Huang ZY, Gallagher JR, Lin Y, Lou H, Whitbeck JC, Wald A, Cohen GH, Eisenberg RJ. 2015. Patient-Specific Neutralizing Antibody Responses to Herpes Simplex Virus Are Attributed to Epitopes on gD, gB, or Both and Can Be Type Specific. J Virol 89:9213–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Para MF, Parish ML, Noble AG, Spear PG. 1985. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol 55:483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skehel JJ, Stevens DJ, Daniels RS, Douglas AR, Knossow M, Wilson IA, Wiley DC. 1984. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A 81:1779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–12. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar HC, Matreyek KA, Filone CM, Hashimi ST, Levroney EL, Negrete OA, Bertolotti-Ciarlet A, Choi DY, McHardy I, Fulcher JA, Su SV, Wolf MC, Kohatsu L, Baum LG, Lee B. 2006. N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J Virol 80:4878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vu HL, Kwon B, Yoon KJ, Laegreid WW, Pattnaik AK, Osorio FA. 2011. Immune evasion of porcine reproductive and respiratory syndrome virus through glycan shielding involves both glycoprotein 5 as well as glycoprotein 3. J Virol 85:5555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller CG, Krummenacher C, Eisenberg RJ, Cohen GH, Fraser NW. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther 3:160–8. [DOI] [PubMed] [Google Scholar]
- 44.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–20. [DOI] [PubMed] [Google Scholar]
- 45.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol 86:1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubin G, Socolof E, Frank I, Friedman HM. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol 65:7046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frank I, Friedman HM. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol 63:4479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman HM, Glorioso JC, Cohen GH, Hastings JC, Harris SL, Eisenberg RJ. 1986. Binding of complement component C3b to glycoprotein gC of herpes simplex virus type 1: mapping of gC-binding sites and demonstration of conserved C3b binding in low-passage clinical isolates. J Virol 60:470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe Y, Raghwani J, Allen JD, Seabright GE, Li S, Moser F, Huiskonen JT, Strecker T, Bowden TA, Crispin M. 2018. Structure of the Lassa virus glycan shield provides a model for immunological resistance. Proc Natl Acad Sci U S A 115:7320–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukui A, Maruzuru Y, Ohno S, Nobe M, Iwata S, Takeshima K, Koyanagi N, Kato A, Kitazume S, Yamaguchi Y, Kawaguchi Y. 2023. Dual impacts of a glycan shield on the envelope glycoprotein B of HSV-1: evasion from human antibodies and neurovirulence. Mbio 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Handler CG, Cohen GH, Eisenberg RJ. 1996. Cross-linking of glycoprotein oligomers during herpes simplex virus type 1 entry. J Virol 70:6076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci U S A 104:18718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ. 2010. Bimolecular complementation defines functional regions of Herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J Virol 84:3825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avitabile E, Forghieri C, Campadelli-Fiume G. 2007. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J Virol 81:11532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avitabile E, Forghieri C, Campadelli-Fiume G. 2009. Cross talk among the glycoproteins involved in herpes simplex virus entry and fusion: the interaction between gB and gH/gL does not necessarily require gD. J Virol 83:10752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanarsdall AL, Howard PW, Wisner TW, Johnson DC. 2016. Human Cytomegalovirus gH/gL Forms a Stable Complex with the Fusion Protein gB in Virions. PLoS Pathog 12:e1005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicola AV, McEvoy AM, Straus SE. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol 77:5324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frampton AR Jr., Stolz DB, Uchida H, Goins WF, Cohen JB, Glorioso JC. 2007. Equine herpesvirus 1 enters cells by two different pathways, and infection requires the activation of the cellular kinase ROCK1. J Virol 81:10879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller JL, Weed DJ, Lee BH, Pritchard SM, Nicola AV. 2019. Low-pH Endocytic Entry of the Porcine Alphaherpesvirus Pseudorabies Virus. J Virol 93:e01849–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tebaldi G, Pritchard SM, Nicola AV. 2020. Herpes simplex virus entry by a nonconventional endocytic pathway. J Virol 94:e01910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pastenkos G, Lee B, Pritchard SM, Nicola AV. 2018. Bovine Herpesvirus 1 Entry by a Low-pH Endosomal Pathway. J Virol 92:e00839–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Synowiec A, Dabrowska A, Pachota M, Baouche M, Owczarek K, Nizanski W, Pyrc K. 2023. Feline herpesvirus 1 (FHV-1) enters the cell by receptor-mediated endocytosis. J Virol 97:e0068123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dollery SJ, Delboy MG, Nicola AV. 2010. Low pH-induced conformational change in herpes simplex virus glycoprotein B. J Virol 84:3759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dollery SJ, Lane KD, Delboy MG, Roller DG, Nicola AV. 2010. Role of the UL45 protein in herpes simplex virus entry via low pH-dependent endocytosis and its relationship to the conformation and function of glycoprotein B. Virus Res 149:115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicola AV. 2016. Herpesvirus Entry into Host Cells Mediated by Endosomal Low pH. Traffic 17:965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siekavizza-Robles CR, Dollery SJ, Nicola AV. 2010. Reversible conformational change in herpes simplex virus glycoprotein B with fusion-from-without activity is triggered by mildly acidic pH. Virol J 7:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weed DJ, Pritchard SM, Gonzalez F, Aguilar HC, Nicola AV. 2017. Mildly Acidic pH Triggers an Irreversible Conformational Change in the Fusion Domain of Herpes Simplex Virus 1 Glycoprotein B and Inactivation of Viral Entry. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weed DJ, Dollery SJ, Komala Sari T, Nicola AV. 2018. Acidic pH Mediates Changes in Antigenic and Oligomeric Conformation of Herpes Simplex Virus gB and Is a Determinant of Cell-Specific Entry. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frost TC, Salnikov M, Rice SA. 2024. Enhancement of HSV-1 cell-free virion release by the envelope protein gC. Virology 596:110120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herold BC, Visalli RJ, Susmarski N, Brandt CR, Spear PG. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol 75 (Pt 6):1211–22. [DOI] [PubMed] [Google Scholar]
- 71.Cohen GH, Isola VJ, Kuhns J, Berman PW, Eisenberg RJ. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol 60:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muggeridge MI, Isola VJ, Byrn RA, Tucker TJ, Minson AC, Glorioso JC, Cohen GH, Eisenberg RJ. 1988. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol 62:3274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eisenberg RJ, Long D, Ponce de Leon M, Matthews JT, Spear PG, Gibson MG, Lasky LA, Berman P, Golub E, Cohen GH. 1985. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol 53:634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Isola VJ, Eisenberg RJ, Siebert GR, Heilman CJ, Wilcox WC, Cohen GH. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol 63:2325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisenberg RJ, Long D, Pereira L, Hampar B, Zweig M, Cohen GH. 1982. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol 41:478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira L, Dondero DV, Gallo D, Devlin V, Woodie JD. 1982. Serological analysis of herpes simplex virus types 1 and 2 with monoclonal antibodies. Infect Immun 35:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dietzschold B, Eisenberg RJ, Ponce de Leon M, Golub E, Hudecz F, Varrichio A, Cohen GH. 1984. Fine structure analysis of type-specific and type-common antigenic sites of herpes simplex virus glycoprotein D. J Virol 52:431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Komala Sari T. 2020. Conformational Change in Herpes Simplex Virus Entry Glycoproteins Detected by Dot Blot, p 319–326. In Diefenbach R, Fraefel C (ed), Herpes Simplex Virus: Methods and Protocols, 2nd Edition, 2nd ed, vol 2060. Humana Press. [DOI] [PMC free article] [PubMed] [Google Scholar]