Abstract
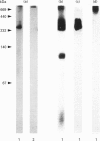
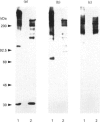
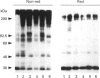
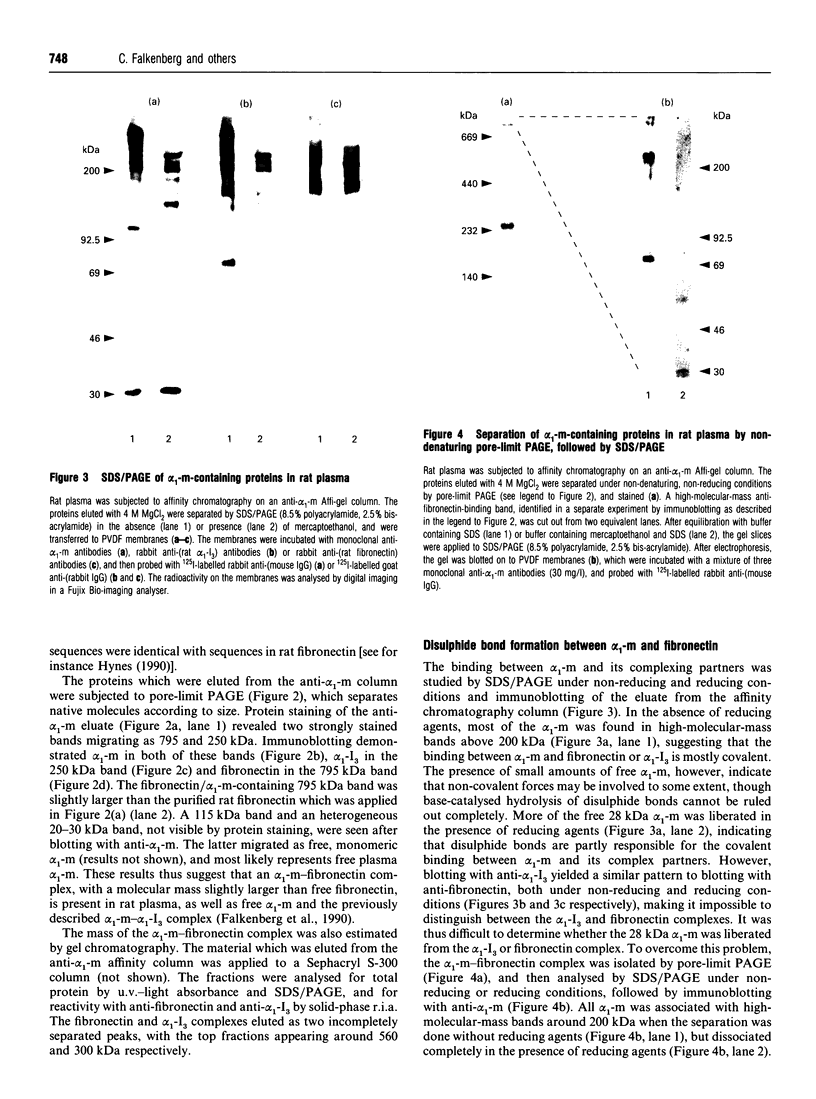
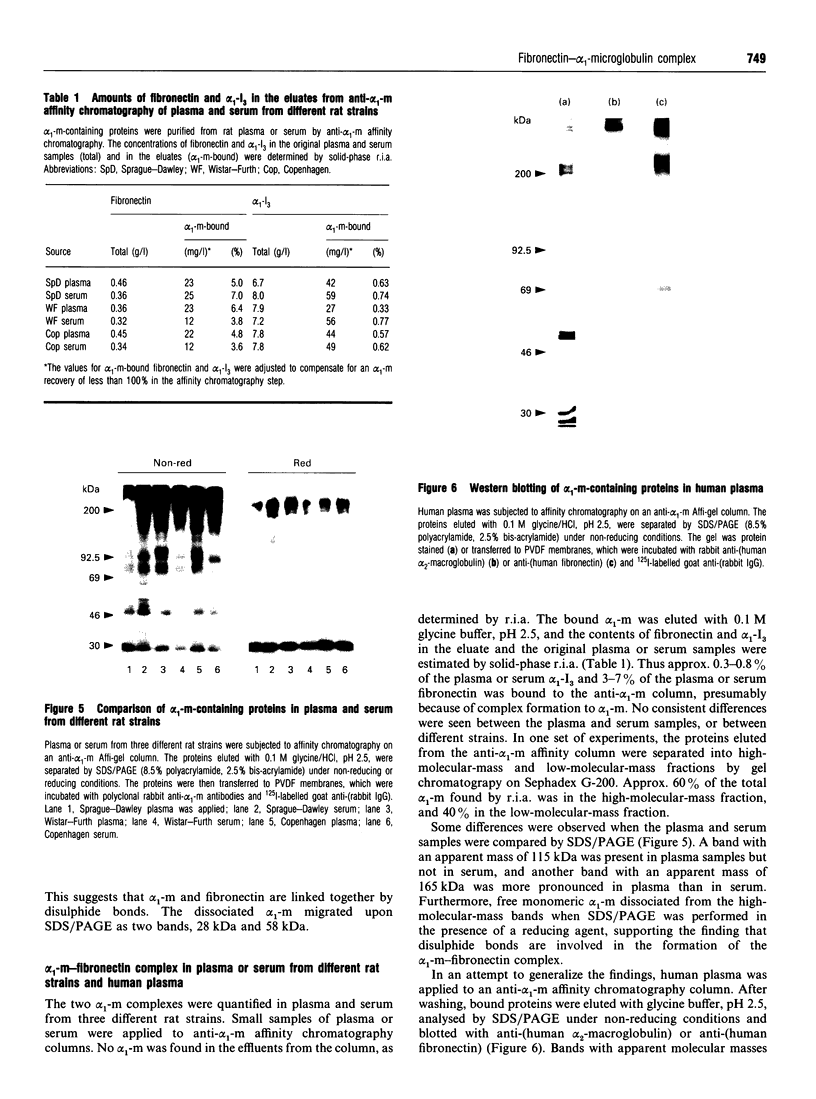
Molecules containing the 28 kDa immunoregulatory protein alpha 1-microglobulin (alpha 1-m), also known as protein HC, were isolated from rat plasma or serum by immunoaffinity chromatography. Three molecular species were distinguished on the basis of nondenaturing PAGE. Two of these have been described previously: uncomplexed alpha 1-m, and the complex of alpha 1-m with alpha 1-inhibitor-3. The third species was analysed by denaturing PAGE, immunoblotting, proteinase digestion and N-terminal-sequence analyses, and shown to consist of a complex between alpha 1-m and fibronectin. This complex, with a mass of about 560 kDa, was resistant to dissociation in the presence of denaturants, but not in the presence of reducing agents in combination with denaturants, and we conclude that the two components are linked by disulphide bonds. About 60% of the total detectable plasma alpha 1-m exists as high-molecular-mass complexes distributed approximately evenly between fibronectin and alpha 1-inhibitor-3. Immunochemical analyses were used to determine the proportion of the total plasma pools of fibronectin and alpha 1-inhibitor-3 that circulate in complex with alpha 1-m. About 3-7% of the total plasma fibronectin from three different rat strains contained alpha 1-m, whereas 0.3-0.8% of the total plasma alpha 1-inhibitor-3 contained alpha 1-m. Complexes were found at similar levels in plasma and serum, indicating that coagulation is not responsible for complex formation. Moreover, immunochemical analyses of human plasma revealed small amounts of alpha 1-m in complex with fibronectin and alpha 2-macroglobulin (an alpha 1-inhibitor-3 homologue). The existence of a complex between alpha 1-m and fibronectin in rats and humans suggests a mechanism for the incorporation of the immunoregulatory molecule alpha 1-m into the extracellular matrix.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B. Immunological analysis of alpha 1-microglobulin in different mammalian and chicken serum. alpha 1-Microglobulin is 5-8 kilodaltons larger in primates. J Biol Chem. 1985 Apr 25;260(8):4839–4844. [PubMed] [Google Scholar]
- Akerström B., Lögdberg L. An intriguing member of the lipocalin protein family: alpha 1-microglobulin. Trends Biochem Sci. 1990 Jun;15(6):240–243. doi: 10.1016/0968-0004(90)90037-c. [DOI] [PubMed] [Google Scholar]
- Akerström B. Role of alpha 1-microglobulin in immune response and inflammation. Folia Histochem Cytobiol. 1992;30(4):183–186. [PubMed] [Google Scholar]
- Babiker-Mohamed H., Akerström B., Lögdberg L. Mitogenic effect of alpha 1-microglobulin on mouse lymphocytes. Evidence of T- and B-cell cooperation, B-cell proliferation, and a low-affinity receptor on mononuclear cells. Scand J Immunol. 1990 Jul;32(1):37–44. doi: 10.1111/j.1365-3083.1990.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Babiker-Mohamed H., Forsberg M., Olsson M. L., Winquist O., Nilson B. H., Lögdberg L., Akerström B. Characterization of monoclonal anti-alpha 1-microglobulin antibodies: binding strength, binding sites, and inhibition of lymphocyte stimulation. Scand J Immunol. 1991 Nov;34(5):655–666. doi: 10.1111/j.1365-3083.1991.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Bratt T., Olsson H., Sjöberg E. M., Jergil B., Akerström B. Cleavage of the alpha 1-microglobulin-bikunin precursor is localized to the Golgi apparatus of rat liver cells. Biochim Biophys Acta. 1993 Jun 11;1157(2):147–154. doi: 10.1016/0304-4165(93)90058-g. [DOI] [PubMed] [Google Scholar]
- Diarra-Mehrpour M., Bourguignon J., Sesboü R., Salier J. P., Léveillard T., Martin J. P. Structural analysis of the human inter-alpha-trypsin inhibitor light-chain gene. Eur J Biochem. 1990 Jul 20;191(1):131–139. doi: 10.1111/j.1432-1033.1990.tb19102.x. [DOI] [PubMed] [Google Scholar]
- Ekström B., Berggård I. Human alpha1-microglobulin. Purification procedure, chemical and physiochemical properties. J Biol Chem. 1977 Nov 25;252(22):8048–8057. [PubMed] [Google Scholar]
- Ekström B., Peterson P. A., Berggård I. A urinary and plasma alpha1-glycoprotein of low molecular weight: isolation and some properties. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1427–1433. doi: 10.1016/s0006-291x(75)80388-3. [DOI] [PubMed] [Google Scholar]
- Enghild J. J., Salvesen G., Thøgersen I. B., Valnickova Z., Pizzo S. V., Hefta S. A. Presence of the protein-glycosaminoglycan-protein covalent cross-link in the inter-alpha-inhibitor-related proteinase inhibitor heavy chain 2/bikunin. J Biol Chem. 1993 Apr 25;268(12):8711–8716. [PubMed] [Google Scholar]
- Enghild J. J., Thøgersen I. B., Pizzo S. V., Salvesen G. Analysis of inter-alpha-trypsin inhibitor and a novel trypsin inhibitor, pre-alpha-trypsin inhibitor, from human plasma. Polypeptide chain stoichiometry and assembly by glycan. J Biol Chem. 1989 Sep 25;264(27):15975–15981. [PubMed] [Google Scholar]
- Escribano J., Grubb A., Calero M., Méndez E. The protein HC chromophore is linked to the cysteine residue at position 34 of the polypeptide chain by a reduction-resistant bond and causes the charge heterogeneity of protein HC. J Biol Chem. 1991 Aug 25;266(24):15758–15763. [PubMed] [Google Scholar]
- Falkenberg C., Björck L., Akerström B. Localization of the binding site for streptococcal protein G on human serum albumin. Identification of a 5.5-kilodalton protein G binding albumin fragment. Biochemistry. 1992 Feb 11;31(5):1451–1457. doi: 10.1021/bi00120a023. [DOI] [PubMed] [Google Scholar]
- Falkenberg C., Grubb A., Akerström B. Isolation of rat serum alpha 1-microglobulin. Identification of a complex with alpha 1-inhibitor-3, a rat alpha 2-macroglobulin homologue. J Biol Chem. 1990 Sep 25;265(27):16150–16157. [PubMed] [Google Scholar]
- Fernandez-Luna J. L., Leyva-Cobian F., Mollinedo F. Identification of the protein HC receptor. FEBS Lett. 1988 Aug 29;236(2):471–474. doi: 10.1016/0014-5793(88)80079-6. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pardo A., Pearlstein E., Frangione B. Primary structure of human plasma fibronectin. The 29,000-dalton NH2-terminal domain. J Biol Chem. 1983 Oct 25;258(20):12670–12674. [PubMed] [Google Scholar]
- Grubb A., Méndez E., Fernandez-Luna J. L., López C., Mihaesco E., Vaerman J. P. The molecular organization of the protein HC-IgA complex (HC-IgA). J Biol Chem. 1986 Oct 25;261(30):14313–14320. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kaumeyer J. F., Polazzi J. O., Kotick M. P. The mRNA for a proteinase inhibitor related to the HI-30 domain of inter-alpha-trypsin inhibitor also encodes alpha-1-microglobulin (protein HC). Nucleic Acids Res. 1986 Oct 24;14(20):7839–7850. doi: 10.1093/nar/14.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindqvist A., Bratt T., Altieri M., Kastern W., Akerström B. Rat alpha 1-microglobulin: co-expression in liver with the light chain of inter-alpha-trypsin inhibitor. Biochim Biophys Acta. 1992 Feb 28;1130(1):63–67. doi: 10.1016/0167-4781(92)90462-9. [DOI] [PubMed] [Google Scholar]
- Lögdberg L., Akerström B. Immunosuppressive properties of alpha 1-microglobulin. Scand J Immunol. 1981;13(4):383–390. doi: 10.1111/j.1365-3083.1981.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Manwell C. A simplified electrophoretic system for determining molecular weights of proteins. Biochem J. 1977 Sep 1;165(3):487–495. doi: 10.1042/bj1650487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Méndez E., Fernández-Luna J. L., Grubb A., Leyva-Cobián F. Human protein HC and its IgA complex are inhibitors of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1472–1475. doi: 10.1073/pnas.83.5.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson B., Björck L., Akerström B. Detection and purification of rat and goat immunoglobulin G antibodies using protein G-based solid-phase radioimmunoassays. J Immunol Methods. 1986 Jul 24;91(2):275–281. doi: 10.1016/0022-1759(86)90490-4. [DOI] [PubMed] [Google Scholar]
- Pierzchalski P., Rokita H., Koj A., Fries E., Akerström B. Synthesis of alpha 1-microglobulin in cultured rat hepatocytes is stimulated by interleukin-6, leukemia inhibitory factor, dexamethasone and retinoic acid. FEBS Lett. 1992 Feb 24;298(2-3):165–168. doi: 10.1016/0014-5793(92)80047-k. [DOI] [PubMed] [Google Scholar]
- Rouet P., Daveau M., Salier J. P. Electrophoretic pattern of the inter-alpha-inhibitor family proteins in human serum, characterized by chain-specific antibodies. Biol Chem Hoppe Seyler. 1992 Oct;373(10):1019–1024. doi: 10.1515/bchm3.1992.373.2.1019. [DOI] [PubMed] [Google Scholar]
- Salier J. P. Inter-alpha-trypsin inhibitor: emergence of a family within the Kunitz-type protease inhibitor superfamily. Trends Biochem Sci. 1990 Nov;15(11):435–439. doi: 10.1016/0968-0004(90)90282-g. [DOI] [PubMed] [Google Scholar]
- Sochorová L., Deyl Z., Michl J. Changes in serum and tissue fibronectin during ontogeny. Physiol Bohemoslov. 1983;32(6):481–485. [PubMed] [Google Scholar]
- Tamkun J. W., Hynes R. O. Plasma fibronectin is synthesized and secreted by hepatocytes. J Biol Chem. 1983 Apr 10;258(7):4641–4647. [PubMed] [Google Scholar]
- Tejler L., Grubb A. O. A complex-forming glycoprotein heterogeneous in charge and present in human plasma, urine, and cerebrospinal fluid. Biochim Biophys Acta. 1976 Jul 19;439(1):82–94. doi: 10.1016/0005-2795(76)90164-1. [DOI] [PubMed] [Google Scholar]
- Vetr H., Gebhard W. Structure of the human alpha 1-microglobulin-bikunin gene. Biol Chem Hoppe Seyler. 1990 Dec;371(12):1185–1196. doi: 10.1515/bchm3.1990.371.2.1185. [DOI] [PubMed] [Google Scholar]