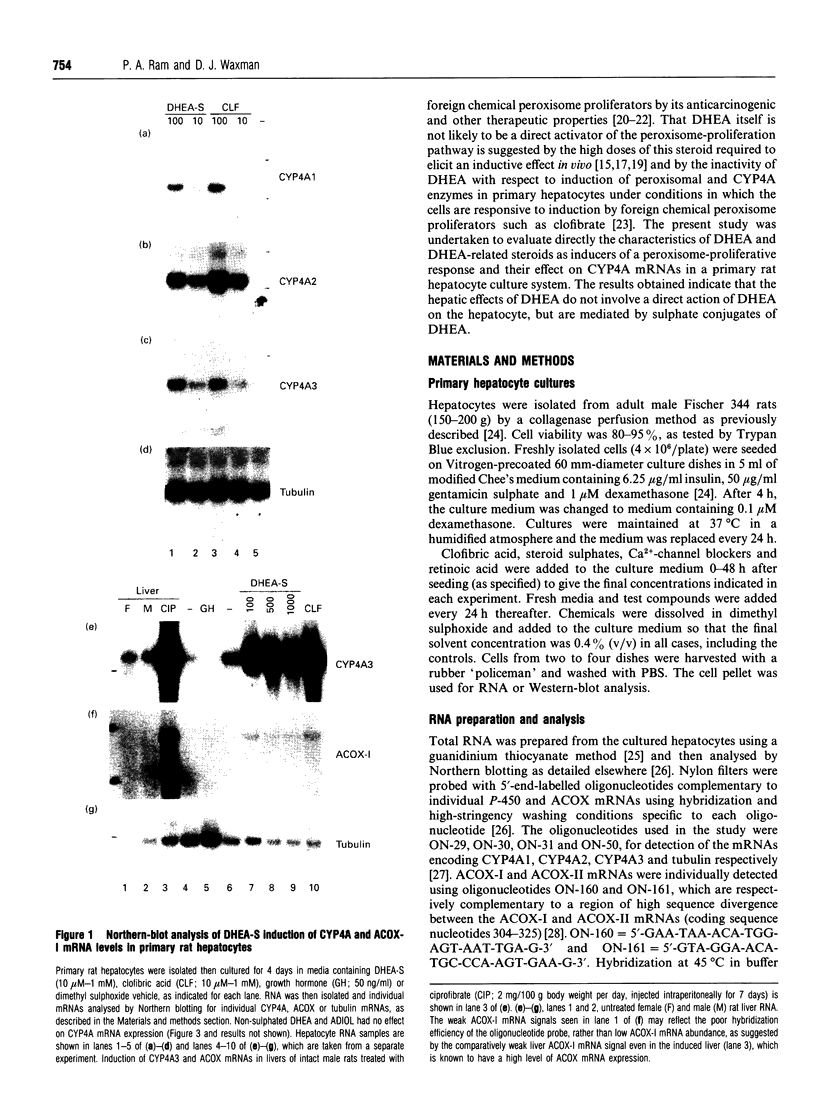
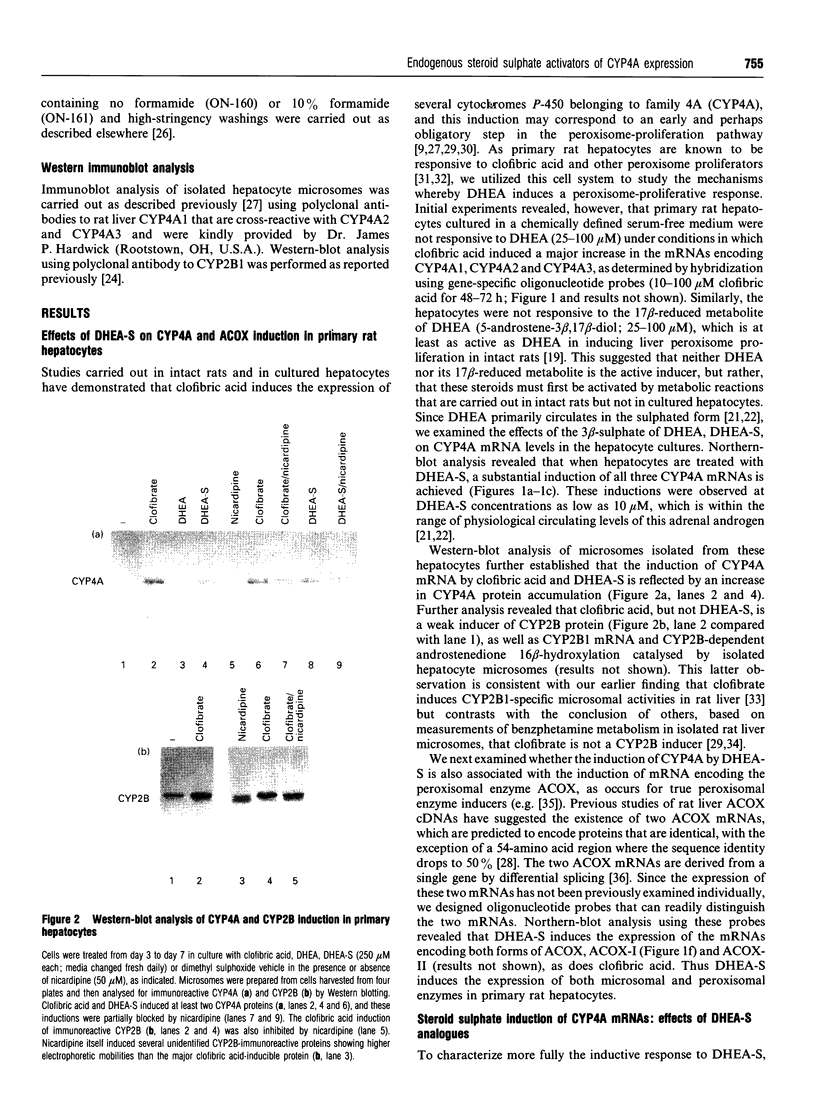
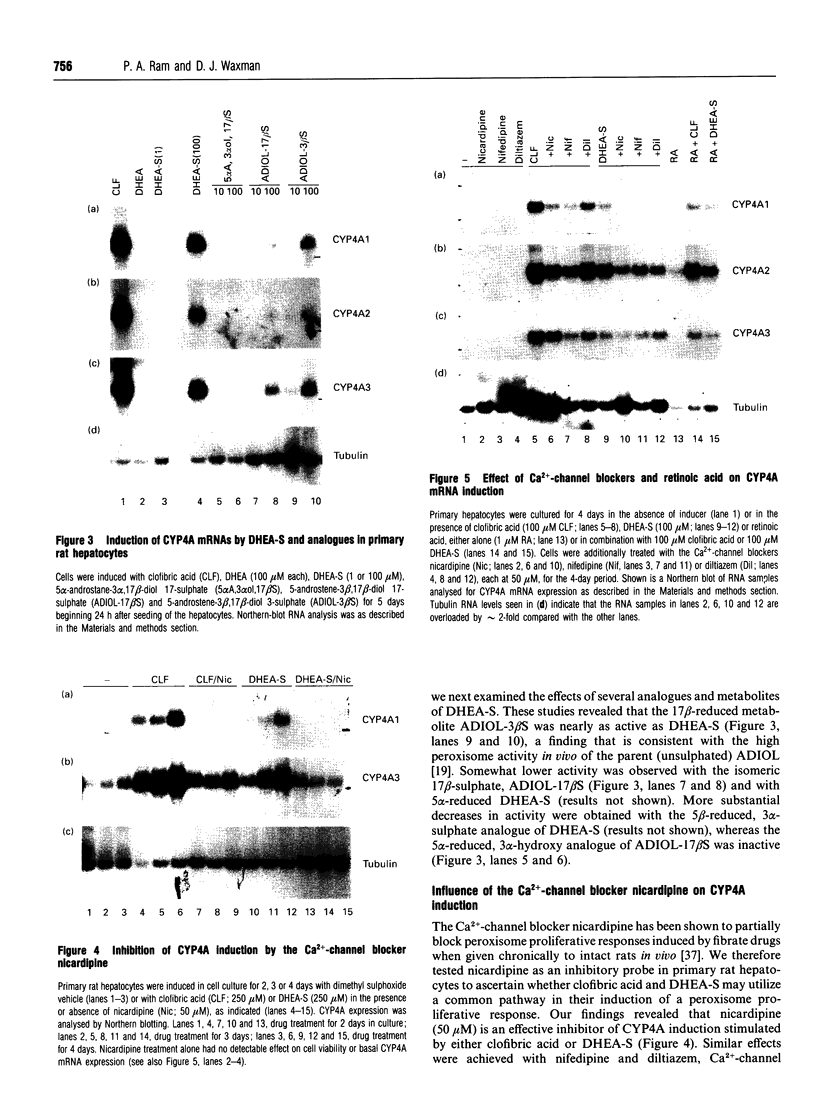
Abstract
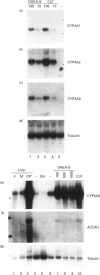
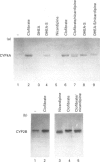
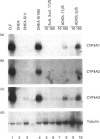
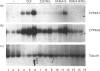
The role of steroids related to the adrenal androgen dehydroepiandrosterone (5-androstene-3 beta-ol-17-one; DHEA) in regulating the expression of peroxisomal and cytochrome P-450 4A (CYP4A) enzymes active in fatty acid metabolism was assessed using a primary rat hepatocyte culture system. Exposure of hepatocytes to the peroxisome proliferator, clofibric acid (10-250 microM), for 48-96 h led to substantial increases in CYP4A protein, CYP4A1, CYP4A2 and CYP4A3 mRNAs, and the mRNAs encoding both forms of peroxisomal acyl-CoA oxidase (ACOX-I and ACOX-II), as judged by Northern-blot analysis using gene-specific oligonucleotide probes. Although DHEA treatment in vivo is effective in inducing these mRNAs in rat liver, it had no effect in the cultured hepatocytes. In contrast, treatment of the cells with DHEA 3 beta-sulphate (DHEA-S; 10-250 microM) stimulated major increases in CYP4A and ACOX mRNA levels. Examination of several analogues indicated a preference for 3 beta-sulphate over 17 beta-sulphated steroids and the inactivity of a 3 alpha-hydroxy-17 beta-sulphate derivative (DHEA-S > 5-androstene-3 beta,17 beta-diol 3-sulphate approximately 5 alpha-androstene-3 beta-ol-17-one 3-sulphate > 5-androstene-3 beta, 17 beta,17 beta-diol 17-sulphate approximately 5 beta-androstane-3 alpha-ol-17-one 3-sulphate >> 5 alpha-androstane-3 alpha, 17 beta-diol 17-sulphate). Induction of CYP4A mRNAs by either DHEA-S or clofibric acid was partially blocked by structurally diverse Ca(2+)-channel antagonists (nicardipine, nifedipine and diltiazem; 50 microM), suggesting that both the steroidal and fibrate classes of CYP4A inducers stimulate peroxisomal-proliferative responses via a Ca(2+)-dependent pathway. Retinoic acid alone slightly induced CYP4A mRNAs but did not enhance the induction by clofibrate or DHEA-S. As DHEA-S corresponds to a physiologically important major circulating androgen, these findings suggest that it may serve as an endogenous regulator of hepatic peroxisome enzyme levels. They further suggest that Ca(2+)-channel blockers may be useful pharmacological tools for the further study of the underlying cellular mechanism whereby endogenous steroids and fibrate drugs induce peroxisome proliferation, and the relationship of these events to activation of the peroxisome proliferator-activated receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. R., Elcombe C. R. Induction of acyl-CoA oxidase and cytochrome P450IVA1 RNA in rat primary hepatocyte culture by peroxisome proliferators. Biochem J. 1991 Nov 15;280(Pt 1):249–253. doi: 10.1042/bj2800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. M., Williams G. M. Alteration of rat liver endoplasmic reticulum Ca(2+)-ATPase thiol integrity by ciprofibrate, a peroxisome proliferator. Biochem Pharmacol. 1993 May 25;45(10):2093–2098. doi: 10.1016/0006-2952(93)90021-n. [DOI] [PubMed] [Google Scholar]
- Bennett A. M., Williams G. M. Reduction of rat liver endoplasmic reticulum Ca(2+)-ATPase activity and mobilization of hepatic intracellular calcium by ciprofibrate, a peroxisome proliferator. Biochem Pharmacol. 1992 Feb 4;43(3):595–605. doi: 10.1016/0006-2952(92)90583-5. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992 Jun;13(6):256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke L., Waxman D. J. Oxidative metabolism of cyclophosphamide: identification of the hepatic monooxygenase catalysts of drug activation. Cancer Res. 1989 May 1;49(9):2344–2350. [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992 Mar 6;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel R. A., Slaughter C. A., Orth K., Moomaw C. R., Hicks S. H., Snyder J. M., Bennett M., Prough R. A., Putnam R. S., Milewich L. Peroxisome proliferation and induction of peroxisomal enzymes in mouse and rat liver by dehydroepiandrosterone feeding. J Steroid Biochem. 1990 Feb;35(2):333–342. doi: 10.1016/0022-4731(90)90293-2. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Helzlsouer K. J., Comstock G. W. Serum levels of dehydroepiandrosterone and its sulfate and the risk of developing bladder cancer. Cancer Res. 1991 Mar 1;51(5):1366–1369. [PubMed] [Google Scholar]
- Gordon G. B., Shantz L. M., Talalay P. Modulation of growth, differentiation and carcinogenesis by dehydroepiandrosterone. Adv Enzyme Regul. 1987;26:355–382. doi: 10.1016/0065-2571(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Gray T. J., Lake B. G., Beamand J. A., Foster J. R., Gangolli S. D. Peroxisome proliferation in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol. 1983 Jan;67(1):15–25. doi: 10.1016/0041-008x(83)90240-5. [DOI] [PubMed] [Google Scholar]
- Green S. Receptor-mediated mechanisms of peroxisome proliferators. Biochem Pharmacol. 1992 Feb 4;43(3):393–401. doi: 10.1016/0006-2952(92)90554-v. [DOI] [PubMed] [Google Scholar]
- Göttlicher M., Widmark E., Li Q., Gustafsson J. A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. P., Song B. J., Huberman E., Gonzalez F. J. Isolation, complementary DNA sequence, and regulation of rat hepatic lauric acid omega-hydroxylase (cytochrome P-450LA omega). Identification of a new cytochrome P-450 gene family. J Biol Chem. 1987 Jan 15;262(2):801–810. [PubMed] [Google Scholar]
- Hertz R., Aurbach R., Hashimoto T., Bar-Tana J. Thyromimetic effect of peroxisomal proliferators in rat liver. Biochem J. 1991 Mar 15;274(Pt 3):745–751. doi: 10.1042/bj2740745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz R., Bar-Tana J. Induction of peroxisomal beta-oxidation genes by retinoic acid in cultured rat hepatocytes. Biochem J. 1992 Jan 1;281(Pt 1):41–43. doi: 10.1042/bj2810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Itoga H., Tamura H., Watanabe T., Suga T. Characteristics of the suppressive effect of nicardipine on peroxisome induction in rat liver. Biochim Biophys Acta. 1990 Jan 23;1051(1):21–28. doi: 10.1016/0167-4889(90)90169-e. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Uy-Yu N., Kozuka H. Sex-related difference in the inductions by perfluoro-octanoic acid of peroxisomal beta-oxidation, microsomal 1-acylglycerophosphocholine acyltransferase and cytosolic long-chain acyl-CoA hydrolase in rat liver. Biochem J. 1989 Jul 15;261(2):595–600. doi: 10.1042/bj2610595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Hanioka N., Matsunaga E., Gonzalez F. J. The rat clofibrate-inducible CYP4A gene subfamily. I. Complete intron and exon sequence of the CYP4A1 and CYP4A2 genes, unique exon organization, and identification of a conserved 19-bp upstream element. DNA. 1989 Sep;8(7):503–516. doi: 10.1089/dna.1.1989.8.503. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992 Aug 27;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lock E. A., Mitchell A. M., Elcombe C. R. Biochemical mechanisms of induction of hepatic peroxisome proliferation. Annu Rev Pharmacol Toxicol. 1989;29:145–163. doi: 10.1146/annurev.pa.29.040189.001045. [DOI] [PubMed] [Google Scholar]
- Makowska J. M., Anders C., Goldfarb P. S., Bonner F., Gibson G. G. Characterization of the hepatic responses to the short-term administration of ciprofibrate in several rat strain. Co-induction of microsomal cytochrome P-450 IVA1 and peroxisome proliferation. Biochem Pharmacol. 1990 Sep 1;40(5):1083–1093. doi: 10.1016/0006-2952(90)90497-9. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Miyata K. S., Zhang B., Subramani S., Rachubinski R. A., Capone J. P. Diverse peroxisome proliferator-activated receptors bind to the peroxisome proliferator-responsive elements of the rat hydratase/dehydrogenase and fatty acyl-CoA oxidase genes but differentially induce expression. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5723–5727. doi: 10.1073/pnas.90.12.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Hayashi H., Hijikata M., Ishii N., Furuta S., Kagamiyama H., Osumi T., Hashimoto T. Complete nucleotide sequence of cDNA and predicted amino acid sequence of rat acyl-CoA oxidase. J Biol Chem. 1987 Jun 15;262(17):8131–8137. [PubMed] [Google Scholar]
- Moudgil V. K. Phosphorylation of steroid hormone receptors. Biochim Biophys Acta. 1990 Dec 10;1055(3):243–258. doi: 10.1016/0167-4889(90)90040-k. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Nemali M. R., Usuda N., Reddy M. K., Oyasu K., Hashimoto T., Osumi T., Rao M. S., Reddy J. K. Comparison of constitutive and inducible levels of expression of peroxisomal beta-oxidation and catalase genes in liver and extrahepatic tissues of rat. Cancer Res. 1988 Sep 15;48(18):5316–5324. [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Orentreich N., Brind J. L., Rizer R. L., Vogelman J. H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984 Sep;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Osumi T., Ishii N., Miyazawa S., Hashimoto T. Isolation and structural characterization of the rat acyl-CoA oxidase gene. J Biol Chem. 1987 Jun 15;262(17):8138–8143. [PubMed] [Google Scholar]
- Prough R. A., Webb S. J., Wu H. Q., Lapenson D. P., Waxman D. J. Induction of microsomal and peroxisomal enzymes by dehydroepiandrosterone and its reduced metabolite in rats. Cancer Res. 1994 Jun 1;54(11):2878–2886. [PubMed] [Google Scholar]
- Reddy J. K., Goel S. K., Nemali M. R., Carrino J. J., Laffler T. G., Reddy M. K., Sperbeck S. J., Osumi T., Hashimoto T., Lalwani N. D. Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Lalwai N. D. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12(1):1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Endo N., Rutledge S. J., Vogel R., Shinar D., Rodan G. A. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol Endocrinol. 1992 Oct;6(10):1634–1641. doi: 10.1210/mend.6.10.1333051. [DOI] [PubMed] [Google Scholar]
- Schwartz A. G., Whitcomb J. M., Nyce J. W., Lewbart M. L., Pashko L. L. Dehydroepiandrosterone and structural analogs: a new class of cancer chemopreventive agents. Adv Cancer Res. 1988;51:391–424. doi: 10.1016/s0065-230x(08)60227-4. [DOI] [PubMed] [Google Scholar]
- Sharma R., Lake B. G., Foster J., Gibson G. G. Microsomal cytochrome P-452 induction and peroxisome proliferation by hypolipidaemic agents in rat liver. A mechanistic inter-relationship. Biochem Pharmacol. 1988 Apr 1;37(7):1193–1201. doi: 10.1016/0006-2952(88)90770-8. [DOI] [PubMed] [Google Scholar]
- Sher T., Yi H. F., McBride O. W., Gonzalez F. J. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993 Jun 1;32(21):5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- Shimozawa N., Tsukamoto T., Suzuki Y., Orii T., Shirayoshi Y., Mori T., Fujiki Y. A human gene responsible for Zellweger syndrome that affects peroxisome assembly. Science. 1992 Feb 28;255(5048):1132–1134. doi: 10.1126/science.1546315. [DOI] [PubMed] [Google Scholar]
- Stefanini S., Farrace M. G., Argento M. P. Differentiation of liver peroxisomes in the foetal and newborn rat. Cytochemistry of catalase and D-aminoacid oxidase. J Embryol Exp Morphol. 1985 Aug;88:151–163. [PubMed] [Google Scholar]
- Sundseth S. S., Waxman D. J. Sex-dependent expression and clofibrate inducibility of cytochrome P450 4A fatty acid omega-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue-specific regulation by growth hormone and testosterone. J Biol Chem. 1992 Feb 25;267(6):3915–3921. [PubMed] [Google Scholar]
- Tugwood J. D., Issemann I., Anderson R. G., Bundell K. R., McPheat W. L., Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5' flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992 Feb;11(2):433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Martinez E. Superfamily of steroid nuclear receptors: positive and negative regulators of gene expression. FASEB J. 1991 Jun;5(9):2243–2249. doi: 10.1096/fasebj.5.9.1860615. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Morrissey J. J., Naik S., Jauregui H. O. Phenobarbital induction of cytochromes P-450. High-level long-term responsiveness of primary rat hepatocyte cultures to drug induction, and glucocorticoid dependence of the phenobarbital response. Biochem J. 1990 Oct 1;271(1):113–119. doi: 10.1042/bj2710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J. Rat hepatic P450IIA and P450IIC subfamily expression using catalytic, immunochemical, and molecular probes. Methods Enzymol. 1991;206:249–267. doi: 10.1016/0076-6879(91)06095-k. [DOI] [PubMed] [Google Scholar]
- Wu H. Q., Masset-Brown J., Tweedie D. J., Milewich L., Frenkel R. A., Martin-Wixtrom C., Estabrook R. W., Prough R. A. Induction of microsomal NADPH-cytochrome P-450 reductase and cytochrome P-450IVA1 (P-450LA omega) by dehydroepiandrosterone in rats: a possible peroxisomal proliferator. Cancer Res. 1989 May 1;49(9):2337–2343. [PubMed] [Google Scholar]
- Yamada J., Sakuma M., Ikeda T., Fukuda K., Suga T. Characteristics of dehydroepiandrosterone as a peroxisome proliferator. Biochim Biophys Acta. 1991 Apr 17;1092(2):233–243. doi: 10.1016/0167-4889(91)90162-q. [DOI] [PubMed] [Google Scholar]
- Yamada J., Sakuma M., Suga T. Induction of peroxisomal beta-oxidation enzymes by dehydroepiandrosterone and its sulfate in primary cultures of rat hepatocytes. Biochim Biophys Acta. 1992 Oct 27;1137(2):231–236. doi: 10.1016/0167-4889(92)90206-q. [DOI] [PubMed] [Google Scholar]