Abstract
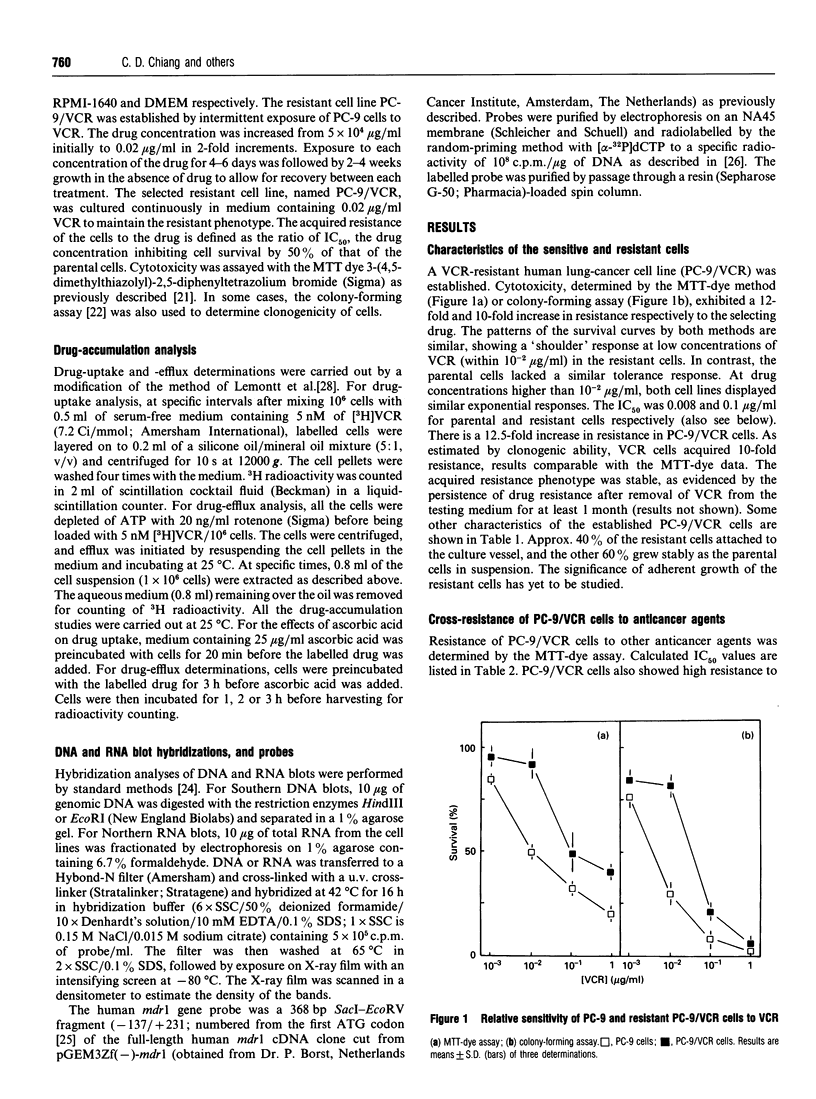
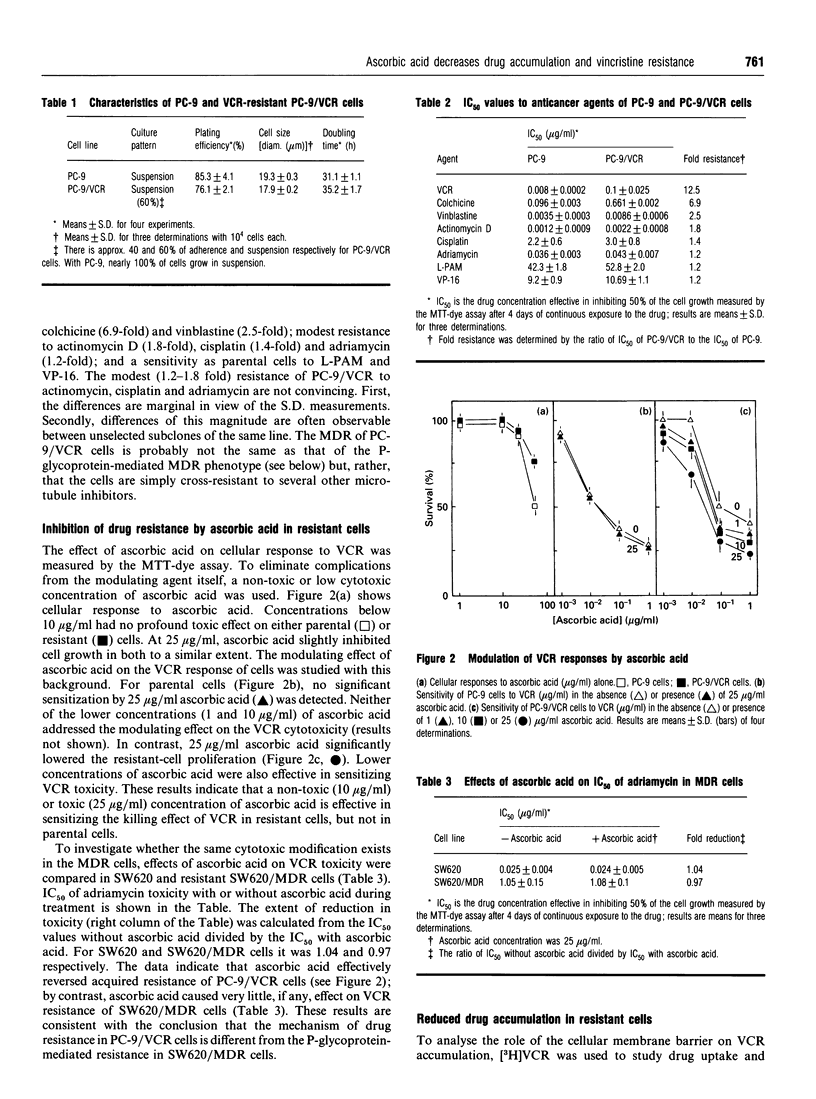
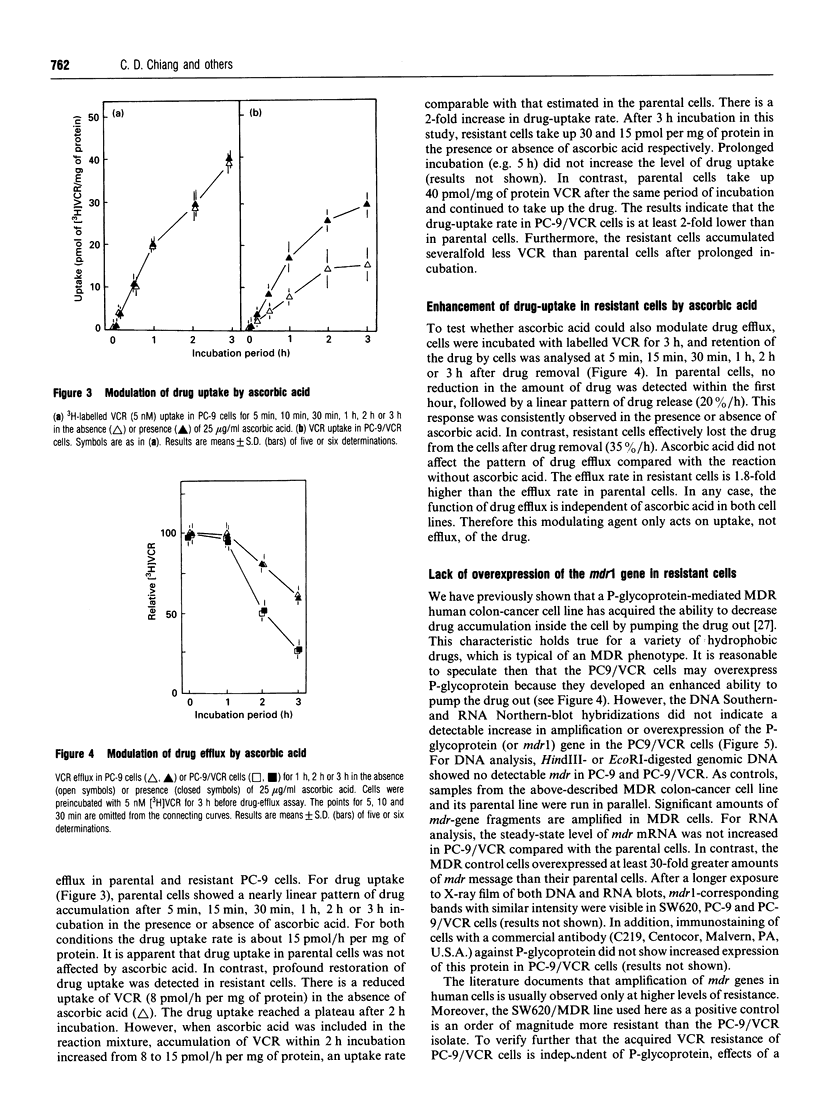
A human lung-cancer PC-9 subline with acquired resistance to vincristine (VCR), a chemotherapeutic agent, was established with incremental increases of the drug. The resistant PC-9 subline (PC-9/VCR) shows a 12-fold increase in resistance to VCR and a unique cross-resistance pattern: high cross-resistance to the potent VCR analogue colchicine (6.9-fold) and vinblastine (2.5-fold); lower cross-resistance to actinomycin D (1.8-fold), cisplatin (1.2-fold) and adriamycin (1.3-fold) and a sensitivity to melphalan and VP-16 which is similar to that of the parental cell line. A reduced accumulation of VCR in the resistant cells was demonstrated. Interestingly, the VCR resistance of the PC-9/VCR cell line was partially reversed by ascorbic acid, and the drug uptake was enhanced. In contrast, ascorbic acid had no effect on drug tolerance and drug accumulation was not observed in either PC-9 parental cells or known multidrug-resistant (MDR) cells, suggesting that VCR resistance in PC-9/VCR cells results essentially from reduced drug accumulation. It is worth noting that, whereas reduced drug accumulation in the PC-9/VCR cells was susceptible to modulation by ascorbic acid, the increased efflux rate characteristic of the resistant cells was not. Further, there was a higher efflux rate in resistant cells than in parental cells. DNA Southern- and RNA Northern-blot hybridization analyses indicate that PC-9/VCR cells do not contain amplified mdr genes or overexpress P-glycoprotein. In addition, the calcium-channel blocker verapamil, which acts as a competitive inhibitor of drug binding and efflux, did not affect the resistant phenotype of PC-9/VCR cells. These findings suggest an ascorbic acid-sensitive drug uptake mechanism which is important in mediating VCR resistance per se in human lung-cancer cells; this differs from the P-glycoprotein-mediated MDR mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Bleyer W. A., Frisby S. A., Oliverio V. T. Uptake and binding of vincristine by murine leukemia cells. Biochem Pharmacol. 1975 Mar 1;24(5):633–639. doi: 10.1016/0006-2952(75)90185-9. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Ma C. M., Cheng P. W., Lin-Chao S. Increased mdr gene expression and decreased drug accumulation in a human colonic cancer cell line resistant to hydrophobic drug. Biochem Biophys Res Commun. 1990 Oct 30;172(2):842–849. doi: 10.1016/0006-291x(90)90752-9. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Ma C. M., Lin-Chao S. Co-amplification and over-expression of two mdr genes in a multidrug-resistant human colon carcinoma cell line. FEBS Lett. 1991 Oct 21;291(2):214–218. doi: 10.1016/0014-5793(91)81287-i. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cook E. S., Nutini L. G., Fardon J. C., Proudfoot M. B., Poydock M. E. Offsetting toxicity of antineoplastic agents. J Surg Oncol. 1975;7(5):337–345. doi: 10.1002/jso.2930070503. [DOI] [PubMed] [Google Scholar]
- Danks M. K., Yalowich J. C., Beck W. T. Atypical multiple drug resistance in a human leukemic cell line selected for resistance to teniposide (VM-26). Cancer Res. 1987 Mar 1;47(5):1297–1301. [PubMed] [Google Scholar]
- Eijdems E. W., Borst P., Jongsma A. P., de Jong S., de Vries E. G., van Groenigen M., Versantvoort C. H., Nieuwint A. W., Baas F. Genetic transfer of non-P-glycoprotein-mediated multidrug resistance (MDR) in somatic cell fusion: dissection of a compound MDR phenotype. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3498–3502. doi: 10.1073/pnas.89.8.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferguson P. J., Fisher M. H., Stephenson J., Li D. H., Zhou B. S., Cheng Y. C. Combined modalities of resistance in etoposide-resistant human KB cell lines. Cancer Res. 1988 Nov 1;48(21):5956–5964. [PubMed] [Google Scholar]
- Ferguson P. J., Phillips J. R., Selner M., Cass C. E. Differential activity of vincristine and vinblastine against cultured cells. Cancer Res. 1984 Aug;44(8):3307–3312. [PubMed] [Google Scholar]
- Lai S. L., Goldstein L. J., Gottesman M. M., Pastan I., Tsai C. M., Johnson B. E., Mulshine J. L., Ihde D. C., Kayser K., Gazdar A. F. MDR1 gene expression in lung cancer. J Natl Cancer Inst. 1989 Aug 2;81(15):1144–1150. doi: 10.1093/jnci/81.15.1144. [DOI] [PubMed] [Google Scholar]
- Larsson R., Bergh J., Nygren P. Combination of cyclosporin A and buthionine sulfoximine (BSO) as a pharmacological strategy for circumvention of multidrug resistance in small cell lung cancer cell lines selected for resistance to doxorubicin. Anticancer Res. 1991 Jan-Feb;11(1):455–459. [PubMed] [Google Scholar]
- Lemontt J. F., Azzaria M., Gros P. Increased mdr gene expression and decreased drug accumulation in multidrug-resistant human melanoma cells. Cancer Res. 1988 Nov 15;48(22):6348–6353. [PubMed] [Google Scholar]
- McGrath T., Center M. S. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988 Jul 15;48(14):3959–3963. [PubMed] [Google Scholar]
- Mirski S. E., Gerlach J. H., Cole S. P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987 May 15;47(10):2594–2598. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mãrtensson J., Meister A., Mrtensson J. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4656–4660. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owellen R. J., Donigian D. W., Hartke C. A., Dickerson R. M., Kuhar M. J. The binding of vinblastine to tubulin and to particulate fractions of mammalian brain. Cancer Res. 1974 Dec;34(12):3180–3186. [PubMed] [Google Scholar]
- Owellen R. J., Hartke C. A., Dickerson R. M., Hains F. O. Inhibition of tubulin-microtubule polymerization by drugs of the Vinca alkaloid class. Cancer Res. 1976 Apr;36(4):1499–1502. [PubMed] [Google Scholar]
- Owellen R. J., Owens A. H., Jr, Donigian D. W. The binding of vincristine, vinblastine and colchicine to tubulin. Biochem Biophys Res Commun. 1972 May 26;47(4):685–691. doi: 10.1016/0006-291x(72)90546-3. [DOI] [PubMed] [Google Scholar]
- Park C. H., Amare M., Hoogstraten B. Analysis of the growth enhancing effect of L-ascorbic acid on human leukemic cells in culture. Exp Hematol. 1980 Aug;8(7):853–859. [PubMed] [Google Scholar]
- Park C. H., Amare M., Savin M. A., Hoogstraten B. Growth suppression of human leukemic cells in vitro by L-ascorbic acid. Cancer Res. 1980 Apr;40(4):1062–1065. [PubMed] [Google Scholar]
- Park C. H. Biological nature of the effect of ascorbic acids on the growth of human leukemic cells. Cancer Res. 1985 Aug;45(8):3969–3973. [PubMed] [Google Scholar]
- Pavelić K. L-ascorbic acid-induced DNA strand breaks and cross links in human neuroblastoma cells. Brain Res. 1985 Sep 9;342(2):369–373. doi: 10.1016/0006-8993(85)91139-4. [DOI] [PubMed] [Google Scholar]
- Poydock M. E., Fardon J. C., Gallina D., Ferro V., Heher C. Inhibiting effect of vitamins C and B12 on the mitotic activity of ascites tumors. Exp Cell Biol. 1979;47(3):210–217. doi: 10.1159/000162938. [DOI] [PubMed] [Google Scholar]
- Poydock M. E., Reikert D., Rice J., Aleandri L. Inhibiting effect of dehydroascorbic acid on cell division in ascites tumors in mice. Exp Cell Biol. 1982;50(1):34–38. doi: 10.1159/000163124. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K., Ramanujam M., Sakamoto A. Sodium ascorbate potentiates the growth inhibitory effect of certain agents on neuroblastoma cells in culture. Proc Natl Acad Sci U S A. 1979 Feb;76(2):829–832. doi: 10.1073/pnas.76.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S. E. Human tumor colony assay and chemosensitivity testing. Cancer Treat Rep. 1984 Jan;68(1):117–125. [PubMed] [Google Scholar]
- Slovak M. L., Hoeltge G. A., Dalton W. S., Trent J. M. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res. 1988 May 15;48(10):2793–2797. [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]



