Abstract
Ischemic stroke triggers a cascade of pathological events that affect multiple cell types and often lead to incomplete functional recovery. Despite advances in single-cell technologies, the molecular and cellular responses that contribute to long-term post-stroke impairment remain poorly understood. To gain better insight into the underlying mechanisms, we generated a single-cell transcriptomic atlas from distinct brain regions using a mouse model of permanent focal ischemia at one month post-injury. Our findings reveal cell- and region-specific changes within the stroke-injured and peri-infarct brain tissue. For instance, GABAergic and glutamatergic neurons exhibited upregulated genes in signaling pathways involved in axon guidance and synaptic plasticity, and downregulated pathways associated with aerobic metabolism. Using cell-cell communication analysis, we identified increased strength in predicted interactions within stroke tissue among both neural and non-neural cells via signaling pathways such as those involving collagen, protein tyrosine phosphatase receptor, neuronal growth regulator, laminin, and several cell adhesion molecules. Furthermore, we found a strong correlation between mouse transcriptome responses after stroke and those observed in human nonfatal brain stroke lesions. Common molecular features were linked to inflammatory responses, extracellular matrix organization, and angiogenesis. Our findings provide a detailed resource for advancing our molecular understanding of stroke pathology and for discovering therapeutic targets in the repair phase of stroke recovery.
Introduction
Stroke remains a leading cause of disability and death, affecting one in four adults in their lifetime1,2. Over half of stroke patients are left with permanent disabilities including partial paralysis and cognitive deficits due to the brain’s limited ability to regenerate damaged neural circuits. Post-stroke damage develops from a complex interplay of pathological processes that involve all major cellular components of the brain including neurons, glia cells, resident and infiltrating immune cells, blood vessels, and peri-vascular mural cells.3 Each cell type undergoes significant changes in response to stroke and the contributions of individual cell types to recovery remain not fully understood. Some cellular responses were associated with enhances recovery, while others may be detrimental4-7. These phenotypic alterations are often regulated at the transcriptional level.
Several single cell/nucleus RNA sequencing studies characterized the cellular heterogeneity in the healthy human and mouse brain8-14. Recent cellular atlases were generated in response to several neurodegenerative diseases15-17 and acute neurological injuries including spinal cord injury and stroke, supporting the functional plasticity of individual brain cells18-21. Some molecular stroke atlases focused characterization of single cell types e.g., immune cells, pericytes or vascular cells18,19,22, some used acute time points22-24 and none looked at a permanent focal ischemia mouse model.
Therefore, we performed snRNAseq on mouse brains one-month post-stroke using a mouse model that simulates permanent stroke conditions to (a) generate a molecular atlas of cell types from stroke-injured and peri-infarct regions, (b) infer molecular communication networks among individual cells and (c) compare these transcriptomic changes to those observed in human chronic, non-fatal brain stroke lesions. These findings enhance our mechanistic understanding of stroke repair and may improve therapeutic targeting during the transition phase of subacute to chronic stroke.
Results
Cellular and molecular profiles of adult intact, peri-infarct and stroke-injured brain
To molecularly profile the stroke-injured brain, we induced large cortical strokes in the sensorimotor cortex of C57BL/6J mice (Fig 1A). All mice showed a severe ≈70% reduction in cerebral blood flow in the stroke core, and ≈45% reduction in the ischemic border zone (ibz) compared to the intact hemisphere at 2 h after stroke induction (Fig 1B, C).
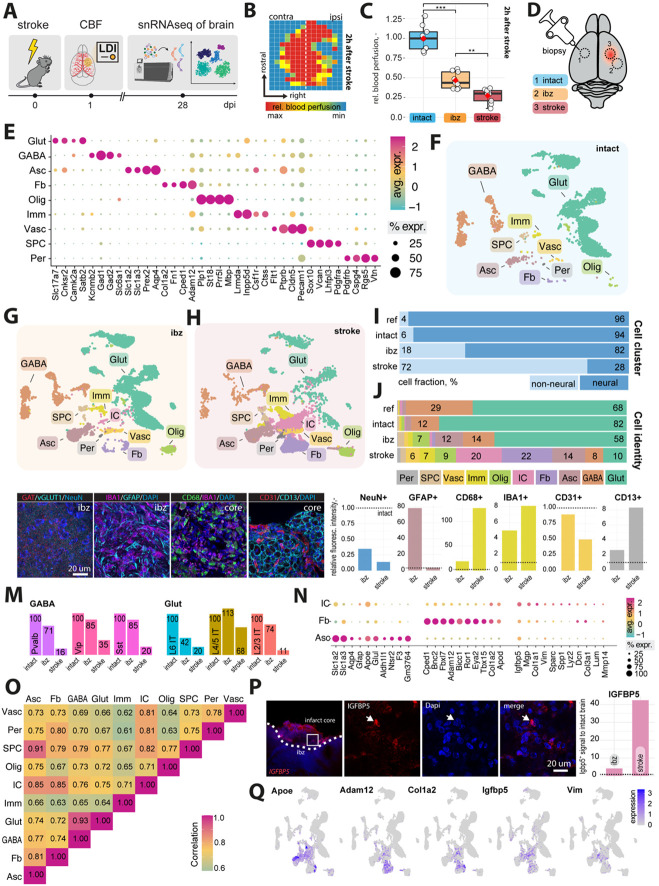
Fig 1: Cellular profiling of the stroke-injured mouse brain.
(A) Scheme of experimental workflow (B) Laser Doppler imaging (LDI) illustrating relative perfusion in the mouse brain (C) Bar plot showing quantification of relative blood perfusion in the stroke core and the ischemic border zone (ibz) compared to the left hemisphere acutely after stroke (D) Illustration of brain regions for biopsy to perform snRNAseq (E) Dot plot representation of canonical cell type markers across different cell populations from the intact contralesional hemisphere, labeled by cell type: glutamatergic neurons (Glut), GABAergic neurons (GABA), astrocytes (Asc), fibroblasts (FB), oligodendrocytes (Olig), immune cells (Imm), vascular cells (Vasc), stem/progenitor cells (SPC), and mural cells (Per) (F-H) UMAP visualization of cell clusters from intact, ibz and stroke tissue. (I) Bar plot showing distribution of cell type by neural and non-neural cells and individual cell types (J) across the reference, intact, ibz and stroke samples. (K) Representative histological overview of brain sections stained with (from left to right) Gat/vGlut, IBA1/GFAP, CD68/IBA1, CD31/CD13, co-stained with DAPI. (L) Quantification of NeuN+, GFAP+, IBA1+, CD68+, CD31+ and CD13+ expression relative to intact tissue (dotted line). (M) Bar graph showing relative amount of major GABA and glutamatergic neuronal subtypes. (N) Dot plot showing expression of canonical cell type marker of injury-associated cells (IC), FB, and Asc. (O) Heatmap showing correlation of gene expression profiles between each cell type from stroke tissue. (P) Representative histological overview of brain sections stained with IGFBP5 (left) and quantification of IGFBP5 expression relative to intact tissue (right). (Q) Feature plot showing the expression patterns of Apoe, Adam12, Cola1a2, and Vim in cells from stroke tissue. The data was generated with a cohort of n = 9 mice.
Four weeks after stroke, we performed a microbiopsy of a) intact, b) ibz, and c) stroke core tissue. All samples were processed for single nucleus RNA sequencing (snRNAseq) using the 10X Genomics Chromium platform, generating transcriptomes from approximately 35,000 nuclei (Fig 1D).
We performed clustering and annotation of nine major cell populations of the mouse brain guided by the known marker expression patterns from molecular atlases11,17,25 : Glutamatergic neurons (Glut: e.g., Slc17a7, Satb2), GABAergic neurons (GABA: e.g. Gad1, Gad2), astrocytes (Asc: Slc1a2, Slc1a3), fibroblasts (FB: Col1a, Fn1), oligodendrocytes (Olig: Mbp, Plp1), immune cells (Imm: Inpp5d, Csf1r), vascular cells (Vasc: Flt1, Cldn5), stem and progenitor cells (SPC: Sox10, Vcan), and mural cells (Per: Pdgfrb, Cspg4) (Fig. 1E). These cell types and marker expression of cell types matched previous single-cell/snRNAseq data from adult non-injured mouse cortices12,26,27.
We used these cell-type categories from the intact adult mouse brain to characterize changes specific to ibz and stroke-injured brain tissue (Fig 1F-H). All cell types were determined with a >99% confidence in all datasets. We observe an increase in the proportion of non-neural cells in the ibz (+12%) and in the stroke-injured tissue (+66%, Fig. 1I). Notably, the ratio of both glutamatergic and GABAergic cells to the total cell population was reduced in the stroke-injured tissue (Glut: intact: 82%, ibz: 58%, stroke: 10%: GABA: intact: 12%, ibz: 14%, stroke: 8%), whereas the relative number of certain non-neural cell types increased especially e.g., fibroblasts (intact: 1%, ibz: 1%, stroke: 22%), astrocytes (intact: 4%, ibz: 12%, stroke: 14%), oligodendrocytes (intact: 2%, ibz: 7%, stroke: 9%), vascular cells (intact:1%, ibz: 3%, stroke: 6%) and immune cells (intact:1%, ibz: 2%, stroke: 7%, Fig. 1J).
In addition, we aimed to confirm the abundance of major cell populations in immunohistochemical stainings of intact and stroke brain tissue. We selected markers specific to mature neurons (NeuN+), astrocytes (GFAP+), macrophages (CD68+), microglia (Iba-1+), endothelial cells (CD31+) and pericytes (CD13+) and stained stroked and intact coronal brain sections 28 days after injury (Fig. 1K). We found that relative NeuN+ expression was significantly reduced in stroke-injured and ibz tissue, whereas GFAP+-expression increased in the ibz compared to the intact side. CD68+, highly expressed in macrophages28, and IBA1+, expressed in microglia/monocytes23, was found to be elevated in the stroke core and the ibz after injury, compared to marker expression in intact tissue. Interestingly, we also found increase in CD13+ signals in the stroke core that were not associated with CD31+ vasculature, potentially indicating recently described CD13+ infiltrating monocytes which have been described to aggravate acute stroke injure but promote chronic post-stroke recovery.29 Although the relative number of vascular cells, compared to other cell types, increased in the stroke snRNAseq dataset, the overall coverage of CD31+ vasculature in stroke tissue is lower in the injured hemisphere compared to the intact hemisphere, consistent with previous stroke studies30-33.
Analysis of neural subtypes using snRNAseq revealed a decrease in most glutamatergic and GABAergic subclusters in stroke-injured tissue (Fig. 1M). For instance, the ratio of GABAergic parvalbumin (PV)-expressing, vasoactive intestinal polypeptide (Vip)-expressing, and somatostatin (Sst)-expressing neurons, as well as glutamatergic layer 2/3 and layer 6 intratelencephalic (IT) neurons was reduced by >80% relative to the intact tissue. These findings align with previous functional studies showing that the loss of specific interneurons, such as PV and SST-expressing neurons, can worsen stroke outcomes and rescuing these populations may serve as a therapeutic target34-37.
Additionally, within the stroke core tissue, we identified a distinct cluster of cells, absent in the intact tissue, which we have termed 'injury-associated cells' (IC) (Fig. 1J, N). IC cells are positive for astrocytic markers such as Apoe and Slc1a2 but also express genes related to ECM modeling such as Col1a2 and Col3a1. ICs transcriptionally segregated from other clusters by expression of genes involved in formation of scar tissue e.g., Dcn, Lum, Col3a1, and Col1a1, but also promotion of remodeling and tissue repair e.g., Mmp14, Vim, Igfbp5, and Sparc. Correlation analysis between all cell types revealed that the IC cell cluster shows most gene expression similarities (r = 0.85) to astrocytes and fibroblasts (Fig. 1O). Next, we selected one of the top IC markers and stained intact and stroked coronal brain sections (Fig. 1P, Q). We found that insulin like growth factor binding protein 5 (Igfbp5) was strongly expressed within the infarction core, but barely in the intact hemisphere (Fig. 1P, Q).
IC may represent a reactive or reparative cell type, such as reactive glia or fibroblasts, which have been shown to become proliferate and activated in response to CNS injury38-41. Importantly, ICs did also not show expression of canonical markers associated with fibrotic pericytes4.
Transcriptomic shift and pathway enrichment in brain cells following stroke
Next, we examined how long-term cerebral ischemia affects gene expression and pathway enrichment in individual brain cells (Fig 2A). We found that most cell types exhibited differentially expressed genes (DEG) after stroke. As expected, most DEG were observed between nuclei from stroke-injured brains compared to intact tissue. Most DEGs were observed in FB, Glut and GABA nuclei (Fig 2A). Interestingly, most DEGs overlapped between stroke/intact and ibz/intact tissue for neural (Glut and GABA) nuclei (Fig 2B), whereas non-neural cells exhibited a more distinct, regional-specific DEG signature (Fig 2C).
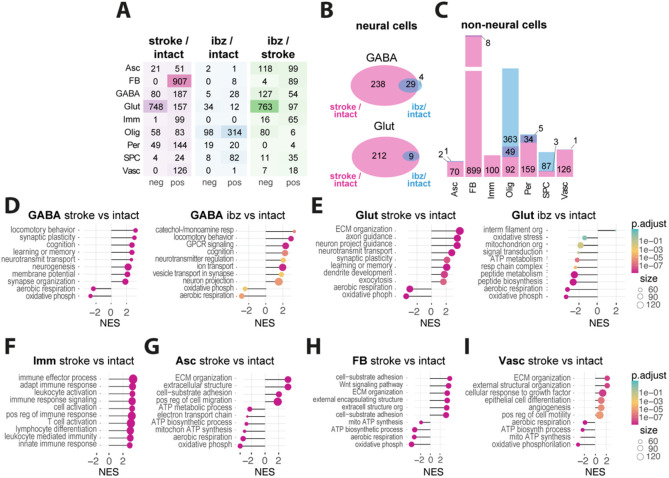
Fig 2: Comparative analysis of transcriptomic responses in mouse and human stroke.
(A) Heatmap showing number of significantly up- and downregulated genes per cell types in stroke vs intact (left), ibz vs intact (middle) and ibz vs stroke (right) tissue. (B) Venn diagram showing the overlap and unique differentially expressed genes from neural cells between stroke and ibz tissue (C) Bar plot showing the common and differential expressed genes in non-neural cells (right). (D-I) Gene set enrichment analysis (GSEA) of biological pathways that are enriched in stroke vs intact and ibz vs intact tissue for (D) GABA, (E) Glut, and GSEA from stroke vs intact tissue in (F) Imm, (G) Asc, (H) FB, and (I) Vasc. Each panel displays the normalized enrichment score (NES) for pathways that are overrepresented (positive NES) or underrepresented (negative NES) in the post-stroke environment compared to intact tissue.
Gene set-enrichment analysis (GSEA) revealed that upregulated pathways in GABA and Glut neural cells predominantly involved pro-regenerative responses including synaptic plasticity, neurotransmitter transport, synapse organization, and axon guidance (all p < 0.001), while downregulated pathways in all neural cells included aerobic respiration and oxidative phosphorylation (Fig. 2 D, E; all p < 0.001). This shift in cellular metabolism from energy-efficient aerobic respiration to alternative metabolic processes may potentially reflect an adaptive response to the altered microenvironment post-stroke42.
Immune cells mainly showed an upregulation in inflammation-associated pathways such as leukocyte activation and positive regulation of immune response (all p < 0.001). Notably, immune cells were the only cell types that did not exhibit altered aerobic metabolism (Fig 2F).
Astrocytes and fibroblasts revealed enrichment in pathways linked to remodeling such as extracellular matrix (ECM) organization and cell adhesion and migration processes (all p < 0.001) (Fig 2 G, H). Additionally, ECs showed enrichment in angiogenesis and remodeling pathways, alongside a downregulated in aerobic metabolism (Fig 2I; all p < 0.001).
Together, these data suggest major transcriptional changes of all major brain cells at 28 days after stroke involving pro-regenerative and remodeling pathways, while also indicating a persistently inflammatory and hypoxic environment.
Mapping of intercellular molecular communication after stroke
To quantitatively infer and identify relevant communication networks after stroke, we used CellChat43,44 to analyze signaling patterns involved in ligand-receptor interactions between individual cell types (Fig 3A). Our analysis suggests that the total number and strength of predicted interactions are increased in stroke tissue (Fig 3B, C; number of interactions: stroke / intact: +104%, stroke / ibz: +71%: interaction strength: stroke / intact: +95%; ibz / intact+104%).
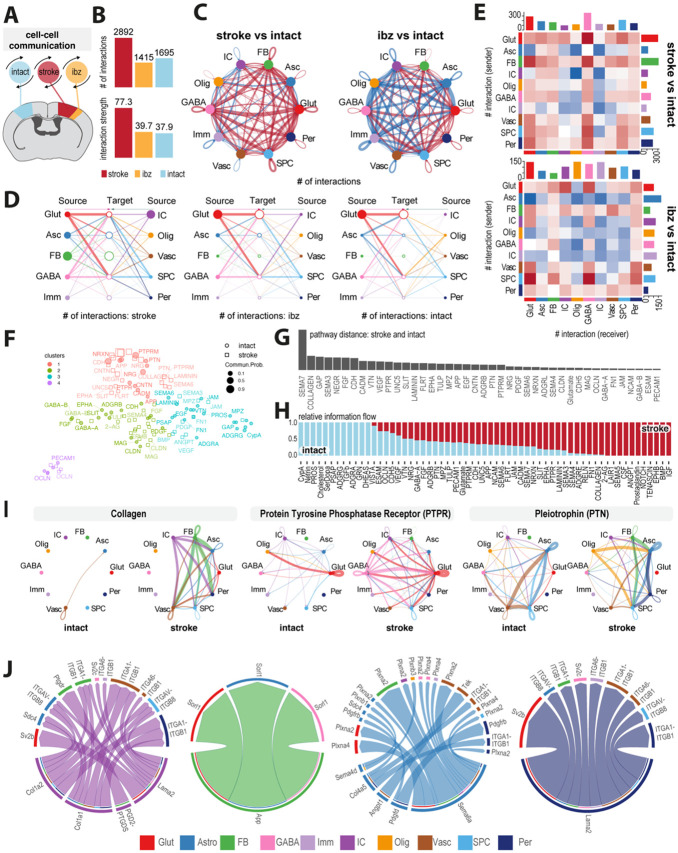
Fig 3: Mapping of intercellular molecular signaling post-stroke.
(A) Schematic of cell-cell interaction analysis (B) Bar plot showing number and strength of interactions in cells from intact, ibz and stroke tissue. (C) Network diagram contrasting total number of cell-cell interactions between individual cell types in stroke vs intact (left) and ibz vs intact (right). Red lines indicate increased interaction, blue lines indicate reduced interaction, relative to intact tissue. (D) Hierarchy plot of interaction between all individual cell types to target cells in stroke (left), ibz (middle) and intact (right) datasets. (E) Heatmap showing differential interactions between cell types from stroke vs intact (upper) and ibz vs intact tissue (lower). Red squares indicating increased signaling and blue squared indicating decreased signaling, relative to cells from intact tissue. (F) Scatter plot projecting signaling groups onto a 2D space according to their functional similarity between cells from stroke and intact tissue. (G) Bar plot showing signaling pathway distance between stroke and intact tissue (H) Stacked bar plot illustrating the proportional relative information flow in signaling pathways between intact and stroke tissue. (I) Cell-cell communication networks for selected pathways: COLLAGEN, Protein Tyrosine Phosphatase Receptor (PTPR), and Pleiotrophin (PTN) across cell types in intact (left) and stroke (right) tissue. (J) Chord diagrams showing the most upregulated signaling ligand-receptor pairings in injury-associated cells (IC), fibroblasts (FB), astrocytes (Asc), and pericytes (Per).
In stroke-injured tissue, we observed an upregulation of interactions among individual cell types (Fig 3C), with the majority of communication occurring between information sending Glut, GABA, Asc, IC and FB and information receiving Glut, GABA and Asc (Fig. 3D). By contrast, cells derived from the ibz and intact tissue exhibited a lower number of predicted interactions especially among non-neural cell populations such as Imm, Vasc, Per, IC and Olig compared to corresponding cell types from stroke-injured tissue (Fig 3D, E). In stroke-affected tissue, Glut and Asc, particularly as senders, display significantly increased interactions, with IC also showing heightened communication compared to intact tissue. Conversely, in the ibz, these interactions are less pronounced, with non-neural cells like Imm and Vasc cells engaging in fewer communications overall. The data suggests a substantial upregulation of neural cell interactions post-stroke, with a notable contribution from IC cells in stroke conditions (Fig 3E).
To better understand the involved signaling pathways in stroke compared to intact tissue, we grouped and clustered signaling pathways in four groups separated by functional similarity (Fig 3F). Most divergent pathways were linked with important biological functions such as neuronal guidance and plasticity (SEMA3, SEMA7, UNC5, SLIT, EPHA), vascular repair and ECM remodeling (COLLAGEN, LAMININ, CDH, CADM, ANGPT, FGF, MMP9).
Most of these pathways demonstrated a considerably higher information flow in stroke-injured tissue (Fig 3G, H). For instance, stroke tissue showed enhanced communication via the COLLAGEN, PTPR, PTN, NEGR, LAMININ, CNTN and CADM pathways, involving multiple cell types that either did not participate or exhibited only minimal signaling interactions in intact tissue (Fig 3I). Interactions of individual cell types in these pathways reveals that ICs preferentially signal to non-neural cells through networks related to COLLAGEN and LAMININ networks involving e.g., Col1a2, Col1a1, Lama2. These signals are predicted to be received by astrocytes (e.g., Itgav-Itgb8), fibroblasts (itga1-itgb1, Ptgdr) and vascular cells (Itga1-itgb1, Itga6-itgb1). This signaling pattern appears to be distinct from the signaling of other non-neural cells such as fibroblasts (e.g. App), astrocytes (e.g., Sema6a, Sema4d, Angpt1) and pericytes (e.g., Lama2) (Fig 3J).
Overall, these findings show the surprisingly complex and dynamic communication among individual cell types in stroke-injured brains.
Comparative analysis of transcriptome responses in mouse and human stroke
To decode and highlight transcriptomic changes that may be relevant to human stroke, we compared our mouse pseudo-bulk and ortholog-transformed RNAseq data with publicly available human stroke-lesion RNAseq datasets from cortical lesions and contralesional brain tissue of patients who experienced a nonfatal ischemic stroke up to five years before death (GSE56267)45 (Fig 4A).
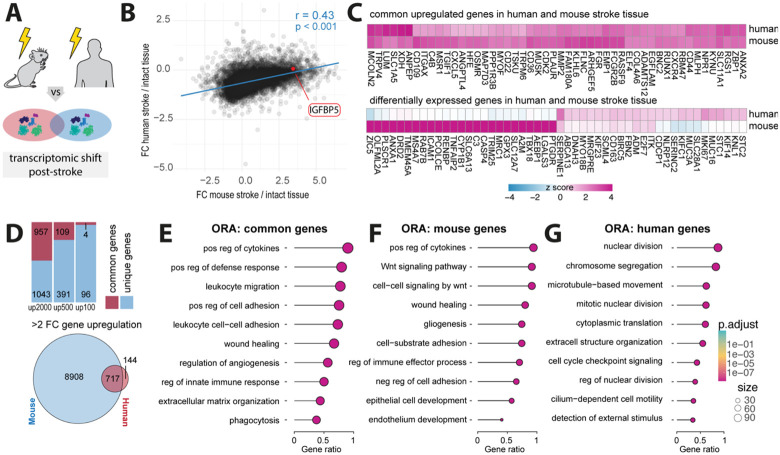
Fig 4: Comparative gene expression profiles in mouse and human post-stroke.
(A) Schematic of human and mouse stroke (B) Scatter plot displaying the Person correlation for gene expression changes between mouse and human post-stroke. (C) Heatmap of common upregulated genes in human and mouse stroke (upper) and differentially expressed genes in human and mouse stroke (lower) (D) Stacked barplot of common and unique upregulated genes in the top 2000, 500 and 100 gene sets from both mouse and human datasets (upper) and Venn diagram of shared and unique genes with a fold change >2. (E-G) Overrepresentation analysis of top 2000 genes present in (E) both mouse and human datasets, (F) genes exclusively identified in mouse stroke dataset (G) and genes only present in human stroke dataset. All p-values ***,< 0.001.
We performed a Pearson correlation analysis of the genes shared by mouse and human datasets post-stroke, which revealed similarities in gene expression changes (r = 0.43, p < 0.001) (Fig 4B). Interestingly, we found that IGFBP5, previously identified as upregulated in IC cells of stroke mice, was also upregulated in the human stroke dataset (Fig 4B). We then calculated z-scores of log2-fold changes and revealed shared and differentially expressed genes between the mouse and human datasets (Fig 4C). The overlapping upregulated gene expression featured genes associated with inflammation (e.g., CXCL5, CD44, CD36), neural plasticity (e.g., GAP43, RUNX1), ECM remodeling (e.g., ADAM12, MMP2, COL4A6) and vascular remodeling (e.g., ANGPTL4, CLDN5).
We observed that 48% of the top 2000 genes, 22% of the top 500 genes, and 4% of the top 100 genes were commonly upregulated in both mouse and human post-stroke. Moreover, of the 861 human genes exhibiting more than a 2-fold upregulation following stroke, 717 (83%) were also upregulated in the mouse stroke dataset (Fig 4D).
We then conducted an over-representation analysis (ORA) of biological processes among the top 2000 upregulated genes that were (a) common: upregulated in mouse and human, (b) mouse-specific, and (c) human-specific. The common ORA predominately featured inflammation-related pathways including regulation of cytokines and immune cells activation, along with angiogenesis, ECM regulation and wound healing responses (Fig 4E; all p < 0.001).
Mouse-specific ORAs similarly highlighted pathways of inflammation, wound healing, and gliogenesis (Fig 4F; all p < 0.001). In contrast, human-specific ORAs were primarily associated with pathways involved in cell division and structural organization (Fig 4G; all p < 0.001), potentially highlighting species-specific differences in the cellular repair mechanisms post-stroke.
In summary, our analysis underscores the substantial cross-species similarities in stroke-induced transcriptomic changes, especially in key pathways related to inflammation and tissue remodeling, highlighting conserved biological mechanisms that could inform the development of therapeutic strategies for human stroke recovery.
Discussion
In this study, we have provided a resource for exploring single-cell transcriptomic data from distinct brain areas one month after stroke injury. Understanding the transcriptomic shifts of individual brain cells and the intracellular signaling mechanisms involved may further help to identify novel therapeutic targets. Our comparative analysis to human chronic stroke dataset has shown a considerable overlap in molecular gene and pathway enrichment suggesting similarities in the pathophysiology of chronic stroke between mice and humans.
We observed a strong reduction of GABAergic and glutamatergic neurons one month following stroke within the injury core and peri-infarct areas. Interestingly, gene expression profiles of the surviving cells, irrespective of their type, indicated a downregulation of genes involved in aerobic metabolism, implying a state of persistent hypoxia within the stroke regions. This observation aligns with previous studies that have documented prolonged alterations in cerebral blood flow post-stroke in both murine models and human patients46,47. While all neurons mainly rely on aerobic metabolism48, neurons with especially high energy demands, such as glutamatergic pyramidal neurons and fast-spiking GABAergic neurons (e.g., PV-expressing interneurons), may be even more susceptible to hypoxia36.
Our findings indicate a considerable reduction in the glutamatergic and GABAergic neurons, which has been previously shown to affect stroke recovery35,49. In glutamatergic neurons, we observed upregulation of axon guidance and synaptic organization pathways, partially mediated through semaphorin (Sema) signaling. Plexin D1–Sema3e receptor-ligand interaction has been implicated in controlling synapse formation and affecting post-stroke recovery50,51. Various studies have reported an increase of guidance factors, including Sema3e after stroke30,52,53. There is evidence suggesting that blockage of Sema3e/PlexinD1 pathway might offer therapeutic benefits for restoring function after stroke41. However, other findings indicate that genetic deletion of PlexinD1 signaling can lead to impairments in the BBB52.
In our study, we observed a pronounced upregulation of genes and pathways associated with ECM remodeling in non-neural cells including astrocytes, fibroblasts, and vascular cells. This upregulation is consistent with the well-known formation of a fibrotic scar in the stroke core, closely bordered by a glial scar after stroke. Importantly, we observed the ECM remodeling molecular signature in multiple non-neural cell types within the stroke, supporting the previously described cellular composition of the scar including ECM-producing PDGFRβ+ stromal fibroblasts, pericytes, reactive astrocytes, microglia, and monocyte-derived macrophages54. While the glial scar has been known for its dual role, potentially protecting the brain from further damage55-57, the fibrotic scar is primarily regarded as detrimental, particularly due to its inhibitory effect on regeneration54,58. Through cell-cell communication networks, we predicted that most upregulated signaling pathways include major components of ECM remodeling including collagen, integrin, and laminin pathways in multiple non-neural cells. Several integrin subunits α1β1, αvβ3, and α6β1 have been shown to regulate vascular remodeling and attenuate BBB permeability following stroke59-61. Several integrin subtypes have also been described to be involved in astrocyte scar formation and the phenotypic switch of astrocytes62-64. Notably, certain integrins, such as αIIbβ3, α4β7/α4β1, and αLβ2, are druggable targets that have been explored in cardiovascular diseases and inflammatory bowel disease65. Future research therefore may explore integrin-targeted therapies in stroke.
We identified a cell cluster of cells, termed ‘injury associated cells’ (IC) in the stroke core that was barely present in peri-infarct nor intact tissue. There is evidence suggesting that ICs may be fibroblast-like cells that migrate to injury sites after stroke. However, their molecular characterization is challenging since there are only few fibroblast-specific markers and many of those markers can be substantially altered after injury66. For instance, we observed presence of Col1α1 and Vim, markers that have been used to identify fibroblasts, however, those markers can also be found on other cell types such as activated astrocytes67,68 or injury-associated pericyte-like cells (type A pericytes)69. The function of Col1α1+ associated cells in the injured brain has been described as both neuroprotective and detrimental.63,64 Studies have shown that Col1α1+ fibroblast-like cells are a crucial source of retinoic acid, promoting neural progenitor differentiation and improving recovery in rodent stroke models69.
Furthermore, research on chronic mouse ischemic stroke models reveals that Col1+ fibroblast-like cells in peri-infarct areas strongly express periostin, which has been linked to improved recovery in neonatal hypoxic-ischemic mice by promoting neural stem cell proliferation and differentiation70. On the other hand, blocking PDGFRα, which is expressed in fibroblast-like cells has been shown to preserve the BBB71. The origin of Col1α1+ fibroblast-like cells remains unknown, though some evidence suggests a potential pericyte origin72. These cells likely represent a heterogeneous group of fibroblasts, possibly including cells derived from diverse sources such as dural, arachnoid, pial, and perivascular fibroblasts, as well as meningothelial cells with distinct properties and functions in stroke. Recent single-cell transcriptomic studies help us to understand the complex diversity of fibroblasts in developmental and adult brain tissue73,74, and future research on stroke tissue could further reveal the unique characteristics of Col1α1+ fibroblast-like cells. Moreover, ICs were identified to express insulin-like growth factor-binding protein 5 (IGFBP5). Additional immunostaining of stroked and intact brain sections revealed upregulation of IGFBP5 in the stroke core, compared to peri-infarct nor the intact tissue. We also found upregulation of IGFBP5 in the analyzed human stroke dataset. IGFBP5, the most conserved member of the IGFBP family, plays various biological roles, such as influencing the inflammatory response75, fibrosis76, cell adhesion77, and cell migration and proliferation78. Recent research shows that IGFBP-5 has specific roles depending on the cell type and the physiological or pathological context. It may be involved in the development of atherosclerosis by binding to extracellular matrix (ECM) components PAI-1 and osteopontin, which are found in atherosclerotic plaques and have been shown to promote atherosclerosis in loss-of-function studies79-81. In vitro studies with primary human idiopathic pulmonary fibrosis (IPF) fibroblasts have shown that both exogenous and endogenously expressed IGFBP-5 increase the expression of ECM component-associated genes and pro-fibrotic genes82. Recent findings also suggest that IGFBP5 is essential for regulating angiogenesis. IGFBP5 is induced during reparative angiogenesis in a hind limb ischemia model, and blocking of IGFBP5 has been shown to enhance angiogenesis by boosting ATP metabolism and stabilizing HIF1α via E3 ubiquitin ligase VHL83. These results suggest that IGFBP5 could be an interesting pharmacological target for treating conditions related to impaired angiogenesis, such as stroke.84
Our comparative analysis of transcriptomic changes in human and mouse stroke tissue revealed a shared upregulation of genes and pathways, indicative of a conserved response to stroke across species. This analysis has highlighted key genes like CXCL5 and C4B, which are involved in the inflammatory response, suggesting an orchestrated immune activation in the chronic phase of stroke. CXCL5 has been reported as a potential CSF biomarker correlating with brain damage in stroke patients85, but also beyond the acute phase patients exhibit a proinflammatory signature after stroke. Elevated CXCL5 proteins have been reported in blood from stroke patients up to 7 years after injury86. Another shared pathway was ECM remodeling involving genes such as MMP2 and COL4. Alterations in COL4 expression after stroke have been described in rodent models of MCAO and non-human primates87,88. Furthermore, an association between Col4 mutations and ischemic stroke has been described in humans, suggesting that Collagen IV plays an important role in the pathogenesis and recovery of ischemic stroke in both species89.
The upregulation of ANGPTL4 suggests the role of angiogenesis in post-stroke recovery. Post-stroke angiogenesis has been reported as an important recovery in experimental and human stroke30,90-92. More recently, ANGPTL4 was associated with poor prognosis in acute ischemic stroke patients93. The cross-species comparison enhances the validity of these findings and suggests that druggable pathways that show beneficial effects in the mouse may target the same pathway in chronic human stroke.
We recognize that our molecular stroke atlas is only a first step towards deciphering the molecular and cellular interaction following a stroke. We acknowledge limitations such as unintended biases in nuclei isolation and tissue collection from stroke-affected, peri-infarct, and intact tissue, of each mouse. These biases might affect the relative cell proportions and will require future validation using spatially resolved datasets. Additional work should extend to validating our findings in longer-term studies, beyond one month, and in alternative stroke models such as the permanent and transient middle cerebral artery occlusion (pMCAo) models in rodents, non-human primates, and human stroke patients. Despite these limitations, our results offer valuable insights for upcoming research into the long-term mechanisms of stroke pathology and the development of therapeutic strategies.
Materials and Methods
Experimental design:
The study was designed to generate a single-cell atlas of stroke-injured mouse tissue one month following permanent focal cerebral stroke. we used nine stroked adult male and female mice (3-5 months old) with a C57BL/6J background. We validated a successful stroke induction using Laser Doppler imaging and collected tissue from stroke, peri-infarct and intact cortex. We dissociated the tissue and isolated nuclei for subsequent snRNAseq analysis. We compared the mouse transcriptome with a publicly available human stroke dataset (GSE56267).
Photothrombotic stroke induction:
Photothrombotic stroke was induced as previously described 32,94-96. Briefly, anesthesia was induced with 4% isoflurane delivered in oxygen. When their respiration rate reached approximately 50 breaths per minute, indicating deep anesthesia, they were placed into a stereotactic frame (Davids Kopf Instruments). A custom-made face mask provided a steady supply of 1-2% isoflurane. Their body temperature was regulated at 36-37 °C using a heating pad. The absence of the toe pinch reflex confirmed deep anesthesia, and Vitamin A eye lubricant from Bausch&Lomb was applied to prevent eye dryness during the procedure. The head was shaved from the neck to the snout, sanitized and Emla™ Creme 5% was applied to the scalp and ears. Ear bars were then inserted to stabilize the head. A 1 cm incision was made to expose the Lambda and Bregma, which were cleaned using a Q-tip. The stroke induction site was precisely marked using an Olympus SZ61 surgery microscope and a WPI UMP3T-1 stereotactic coordinate system, taking Bregma as the reference. Rose Bengal in 0.9% NaCl solution at 15mg/ml was injected intraperitoneally at a dose of 10μl/g bodyweight 5 minutes before a 150W, 3000K Olympus KL1500LCD cold light source was used for illumination at the marked site for 10 minutes. After the procedure, animals were placed in a recovery cage.
Laser Doppler imaging (LDI):
After stroke induction, anesthetized mice were secured in a stereotactic apparatus. The mice underwent a single-point Laser Doppler Imaging (LDI) procedure using the Moor Instruments MOORLDI2-IR device. LDI data was then extracted and the total flux within the region of interest (ROI) was measured using Fiji (ImageJ). Subsequent analysis was carried out using R software.
Tissue processing:
For RNA sequencing, animals were perfused transcardially on ice using Ringer's solution (0.9% NaCl). Subsequently, the specified cortical brain tissue was rapidly dissected on ice with the assistance of a microbiopsy instrument (Kai Medical) and a stereotaxic microscope (Olympus). The collected tissue was then immediately frozen in liquid nitrogen. For immunohistochemistry, perfusion was performed using Ringer's solution (0.9% NaCl), followed by a perfusion with a 4% paraformaldehyde (PFA) solution. The brain was extracted and post-fixed for 6 hours in 4% PFA. The brains were stored in 0.1 M PBS. Before immunohistochemistry, the brains were sectioned into 40 μm coronal slices using a Thermo Scientific HM 450 sliding microtome. Next, brain sections were rinsed with 0.1 M PBS. They were then treated with 500 μl of blocking buffer (5% donkey serum in 1x PBS with 0.1% Triton® X-100) and incubated for 1 hour at room temperature. Following blocking, the sections were incubated with primary antibodies (Table 1) on an Unimax 1010 shaker set to approximately 90 rpm, and this was maintained overnight at 4°C. The next day, after washing, the sections were incubated with appropriate secondary antibodies (Table 2), for 2 hours at room temperature. Additionally, the sections were treated with DAPI (Sigma, diluted 1:2000 in 0.1 M PBS) to stain the nuclei. Finally, the sections were arranged on Superfrost Plus™ microscope slides, immersed in Mowiol® mounting medium, and securely coverslipped.
Table 1:
Primary Antibody List.
| Antigen | Target | Host | Dilution | Company |
|---|---|---|---|---|
| CD13 | Pericytes | goat | 1:200 | R&D Systems |
| CD31 | Vascular endothelial cells | rat | 1:50 | BD Biosciences |
| GFAP | Astrocytes | mouse | 1:200 | R&D Systems |
| Iba1 | Microglia | goat | 1:200 | R&D Systems |
| CD68 | Macrophages | rat | 1:200 | BioLegend |
| vGlut1 | Glutamate transporter in synaptic vesicles | mouse | 1:300 | Synaptic Systems |
| GAT1 | GABA transporter in synaptic vesicles | rabbit | 1:200 | Abcam |
| IGFBP5 | Insulin-like growth factor-binding protein 5 | rabbit | 1:100 | Proteintech |
| NeuN | Neurons | rat | 1:200 | Abcam |
Table 2:
Secondary Antibody List.
| Reactivity | Host | Conjugate | Dilution | Company |
|---|---|---|---|---|
| anti-rabbit | Donkey | Cy5 | 1:100 | Jackson |
| anti-rat | Donkey | Cy3 | 1:500 | Jackson |
| anti-goat | Donkey | AlexaFluor 488 | 1:500 | Jackson |
| anti-mouse | Donkey | Cy3 | 1:500 | Jackson |
| anti-rabbit | Donkey | AlexaFluor 488 | 1:500 | Jackson |
| Anti-mouse | Donkey | AlexaFluor 488 | 1:500 | Jackson |
| Anti-goat | Donkey | Cy3 | 1:500 | Jackson |
| Anti-rabbit | Donkey | AF647 | 1:100 | Jackson |
Isolation of nuclei from frozen brains:
Nuclei were extracted from frozen cortical brain tissues as previously described97. Tissues were rapidly frozen in liquid nitrogen and subsequently pulverized using a Dounce homogenizer in a lysis solution composed of 10 mM Tris-HCl (pH 7.5), 10 mM NaCl, 3 mM MgCl2, and 0.1% Nonidet P40 dissolved in nuclease-free water. After a 15-minute incubation, the homogenate was strained through a 30 μm cell strainer. The strained suspension was then subjected to a low-speed centrifugation at 500g for 5 minutes at 4°C to sediment the nuclei. These nuclei were washed and passed through a 40 μm cell strainer twice, using sterile PBS supplemented with 2% BSA and 0.2 U/μl RNase inhibitor. The nuclei were then suspended in 500 μl of the washing buffer and combined with 900 μl of 1.8 M sucrose solution. This mixture was carefully overlaid onto 500 μl of 1.8 M sucrose and centrifuged at 13,000g for 45 minutes at 4°C, facilitating myelin removal. The final pellet was resuspended in the washing buffer and filtered once more through a 40 μm cell strainer to ensure purity.
Single-nucleus RNA sequencing:
Single-nucleus RNA sequencing (snRNAseq) was performed as previously described.97 For the droplet-based library construction, nuclei isolated from both stroked and non-stroked mouse cortices were processed on the Chromium system by 10x Genomics, following the manufacture’s guidelines. RNA capture and subsequent amplification were facilitated by the Chromium Single Cell 3' Reagent Kits v3. Sequencing of the resulting libraries was executed on an Illumina sequencing platform. The analysis pipeline, including demultiplexing of samples, processing of barcodes, and enumeration of single cells, was conducted using the Cell Ranger Single-Cell Software Suite supplied by 10x Genomics.
Clustering and annotation cell types:
For the clustering and annotation of cell types, single-nucleus RNA sequencing (snRNAseq) data alignment and gene quantification were performed using Cellranger v3.1.0, which followed default settings and referenced the mm10 2020-A dataset. Cells were screened, excluding any with greater than 5% mitochondrial gene expression or with less than 500 nFeature_RNA. Normalization and scaling of gene counts were performed using Seurat v5.0.192, to adjust for total unique molecular identifier counts per cell. Using the initial 30 principal components, cell clustering was accomplished via the FindNeighbors function, with subsequent clustering by the FindClusters function. Dimensionality was reduced through Uniform Manifold Approximation and Projection (UMAP) employing the RunUMAP function. Distinct cell types were identified based on established markers, categorizing into Glutamatergic neurons (Glut: e.g., Slc17a7, Satb2), GABAergic neurons (GABA: e.g., Gad1, Gad2), Astrocytes (Asc: Slc1a2, Slc1a3), Fibroblasts (FB: Col1a, Fn1), Oligodendrocytes (Olig: Mbp, Plp1), Immune cells (IC: Inpp5d, Csf1r), vascular cells (Vasc: Flt1, Cldn5), stem and progenitor cells (SPC: Sox10, Vcan), and mural cells (Per: Pdgfrb Cspg4). The cell types and marker expression of cell types matched sc/snRNAseq datasets12,26,27. FindMarkers function was used to pinpoint cell type–specific marker genes, considering genes with a Bonferroni correction adjusted P value <0.05 as significant markers. The downstream analysis (including Gene Set Enrichment analysis (GSEA), and over representation analysis (ORA) was performed using R package ClusterProfiler.
Cell-cell communication with CellChat:
Differential cell-cell interaction networks were generated using CellChat version 2.1.043,44. In brief, DifferentialConnectome was applied to the Seurat objects (version 5.01), which contained integrated data of mouse stroke, ibz and intact datasets. The compareInteractions function was utilized to compute the total number of interactions and their strengths, while network centrality was scored using the netAnalysis_computeCentrality function. All analyses were conducted in line with the 'Full tutorial for CellChat analysis of a single dataset with detailed explanation of each function' found on the GitHub page.
Human RNAseq dataset:
Human stroke RNA sequencing data was obtained from the NCBI Gene Expression Omnibus, accession number GSE56267. For the purpose of comparing differential gene expression profiles between mouse and human stroke, we employed a pseudo-bulk approach on the mouse snRNAseq data using the AggregateExpression() function in the Seurat package. We processed both datasets using Z-score normalization and translated mouse gene identifiers to their human orthologs. To evaluate the relationship between gene expression in mouse and human stroke cases, Pearson's correlation test was used. Subsequent analyses, which included Gene Set Enrichment Analysis (GSEA) and Over Representation Analysis (ORA), were conducted using the ClusterProfiler package in R, providing comprehensive insights into the biological significance of the expression data.
Statistical analysis:
Statistical analysis was performed using RStudio (Version 4.04). Sample sizes were designed with adequate power in line with previous studies from our group98-100 and relevant literature. One-way analyses of variance (ANOVA) followed by Tukey multiple comparison test was performed for cerebral blood flow measurements. The assumption of normality was tested by Kolmogorov–Smirnov tests and by inspecting residuals with QQ plots. Data is expressed as mean ±SD; statistical significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
Acknowledgement
This work is supported by the Swiss 3R Competence Center (OC-2020-002) and the Swiss National Science Foundation (CRSK-3_195902) and (PZ00P3_216225) to RR. The work was also supported by the National Institutes of Health (R01NS117827) and by the development funds to the Center for Neurodegeneration and Regeneration at the Zilkha Neurogenetic Institute to BVZ. In addition, RR and CT acknowledge support from the Mäxi Foundation.
Footnotes
Competing Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability:
Raw single nucleus RNA sequencing data was deposited in the NCBI Gene Expression Omnibus (GEO) and will be available following publication. Data are now also available to explore via an interactive web browser: https://rustlab.shinyapps.io/Stroke-Atlas/
References
- 1.Tsao C. W. et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 147, e93–e621 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Feigin V. L. et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Neurology 20, 795–820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rust R. et al. Brain repair mechanisms after cell therapy for stroke. Brain awae204 (2024) doi: 10.1093/brain/awae204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias D. O. et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. bioRxiv 2020.04.30.068965 (2020) doi: 10.1101/2020.04.30.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamboni M., Llorens-Bobadilla E., Magnusson J. P. & Frisén J. A Widespread Neurogenic Potential of Neocortical Astrocytes Is Induced by Injury. Cell Stem Cell 27, 605–617.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellver-Landete V. et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun 10, 518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Y. et al. Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav Immun 80, 394–405 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Almanzar N. et al. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callaway E. M. et al. A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 598, 86–102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn O. et al. Atlas of the aging mouse brain reveals white matter as vulnerable foci. Cell 186, 4117–4133.e22 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler E. A. et al. A single-cell atlas of the normal and malformed human brain vasculature. Science 375, eabi7377 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Z. et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 624, 317–332 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardwick S. A. et al. Single-nuclei isoform RNA sequencing unlocks barcoded exon connectivity in frozen brain tissue. Nat Biotechnol 40, 1082–1092 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakken T. E. et al. Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 598, 111–119 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603, 885–892 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia F. J. et al. Single-cell dissection of the human brain vasculature. Nature 603, 893–899 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathys H. et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer’s disease pathology. Cell 186, 4365–4385.e27 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Bonilla L. et al. Analysis of brain and blood single-cell transcriptomics in acute and subacute phases after experimental stroke. Nat Immunol 1–14 (2024) doi: 10.1038/s41590-023-01711-x. [DOI] [PubMed] [Google Scholar]
- 19.Callegari K. et al. Molecular profiling of the stroke-induced alterations in the cerebral microvasculature reveals promising therapeutic candidates. Proceedings of the National Academy of Sciences 120, e2205786120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matson K. J. E. et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat Commun 13, 5628 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rust R. Ischemic stroke-related gene expression profiles across species: a meta-analysis. J Inflamm 20, 21 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buizza C., Enström A., Carlsson R. & Paul G. The Transcriptional Landscape of Pericytes in Acute Ischemic Stroke. Transl. Stroke Res. (2023) doi: 10.1007/s12975-023-01169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beuker C. et al. Stroke induces disease-specific myeloid cells in the brain parenchyma and pia. Nat Commun 13, 945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahashi-Oda C. et al. CD300a blockade enhances efferocytosis by infiltrating myeloid cells and ameliorates neuronal deficit after ischemic stroke. Science Immunology 6, eabe7915 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Barisano G. et al. A “multi-omics” analysis of blood–brain barrier and synaptic dysfunction in APOE4 mice. Journal of Experimental Medicine 219, e20221137 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao Z. et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 184, 3222–3241.e26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Z. et al. A transcriptomic and epigenomic cell atlas of the mouse primary motor cortex. Nature 598, 103–110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chistiakov D. A., Killingsworth M. C., Myasoedova V. A., Orekhov A. N. & Bobryshev Y. V. CD68/macrosialin: not just a histochemical marker. Lab Invest 97, 4–13 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Nguyen J. N. et al. CD13 facilitates immune cell migration and aggravates acute injury but promotes chronic post-stroke recovery. J Neuroinflammation 20, 232 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rust R. et al. Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc Natl Acad Sci USA 201905309 (2019) doi: 10.1073/pnas.1905309116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rust R. et al. Anti-Nogo-A antibodies prevent vascular leakage and act as pro-angiogenic factors following stroke. Sci Rep 9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber R. Z. et al. Characterization of the blood brain barrier disruption in the photothrombotic stroke model. Front. Physiol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rust R. et al. A Practical Guide to the Automated Analysis of Vascular Growth, Maturation and Injury in the Brain. Front. Neurosci. 14, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motaharinia M. et al. Longitudinal functional imaging of VIP interneurons reveals sup-population specific effects of stroke that are rescued with chemogenetic therapy. Nat Commun 12, 6112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y., Chen S., Wu Y. & Murphy T. H. Prolonged Deficits in Parvalbumin Neuron Stimulation-Evoked Network Activity Despite Recovery of Dendritic Structure and Excitability in the Somatosensory Cortex following Global Ischemia in Mice. J. Neurosci. 34, 14890–14900 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Povysheva N., Nigam A., Brisbin A. K., Johnson J. W. & Barrionuevo G. Oxygen–Glucose Deprivation Differentially Affects Neocortical Pyramidal Neurons and Parvalbumin-Positive Interneurons. Neuroscience 412, 72–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balbi M. et al. Gamma frequency activation of inhibitory neurons in the acute phase after stroke attenuates vascular and behavioral dysfunction. Cell Rep 34, 108696 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Kenigsbuch M. et al. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat Neurosci 25, 876–886 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leng K. et al. CRISPRi screens in human iPSC-derived astrocytes elucidate regulators of distinct inflammatory reactive states. Nat Neurosci 25, 1528–1542 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habib N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci 23, 701–706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C. et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct Target Ther 7, 65 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Y. et al. Aerobic glycolysis is the predominant means of glucose metabolism in neuronal somata, which protects against oxidative damage. Nat Neurosci 26, 2081–2089 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Jin S. et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun 12, 1088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin S., Plikus M. V. & Nie Q. CellChat for systematic analysis of cell-cell communication from single-cell and spatially resolved transcriptomics. 2023.11.05.565674 Preprint at 10.1101/2023.11.05.565674 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Huttner H. B. et al. The age and genomic integrity of neurons after cortical stroke in humans. Nat Neurosci 17, 801–803 (2014). [DOI] [PubMed] [Google Scholar]
- 46.He F. et al. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes. Science Advances 6, eaba1933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalil A. A. et al. Non-invasive monitoring of longitudinal changes in cerebral hemodynamics in acute ischemic stroke using BOLD signal delay. J Cereb Blood Flow Metab 40, 23–34 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan L., Chow B. W. & Gu C. Neuronal regulation of the blood–brain barrier and neurovascular coupling. Nat Rev Neurosci 21, 416–432 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mascaro A. L. A. et al. Combined Rehabilitation Promotes the Recovery of Structural and Functional Features of Healthy Neuronal Networks after Stroke. Cell Reports 28, 3474–3485.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Ding J. B., Oh W.-J., Sabatini B. L. & Gu C. Semaphorin 3E–Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat Neurosci 15, 215–223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y.-F. et al. Sema3E/PlexinD1 inhibition is a therapeutic strategy for improving cerebral perfusion and restoring functional loss after stroke in aged rats. Neurobiol Aging 70, 102–116 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Yu R. et al. Vascular Sema3E-Plexin-D1 Signaling Reactivation Promotes Post-stroke Recovery through VEGF Downregulation in Mice. Transl. Stroke Res. 13, 142–159 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overman J. J. et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proceedings of the National Academy of Sciences 109, E2230–E2239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dias D. O. et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun 12, 5501 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liddelow S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi X. et al. Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat Commun 12, 6943 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dias D. O. et al. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 173, 153–165.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L., Welser J. V. & Milner R. Absence of the αvβ3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in α5β1 integrin expression. J Cereb Blood Flow Metab 30, 1031–1043 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee C. Z., Xue Z., Zhu Y., Yang G.-Y. & Young W. L. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke 38, 2563–2568 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Li L., Liu F., Welser-Alves J. V., McCullough L. D. & Milner R. Upregulation of Fibronectin and the α5β1 and αvβ3 Integrins on Blood Vessels within the Cerebral Ischemic Penumbra. Exp Neurol 233, 283–291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagos-Cabré R. et al. αVβ3 Integrin regulates astrocyte reactivity. J Neuroinflammation 14, 194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson K. M., Milner R. & Crocker S. J. Extracellular matrix composition determines astrocyte responses to mechanical and inflammatory stimuli. Neurosci Lett 600, 104–109 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chu P.-H. et al. Astrocyte-associated fibronectin promotes the proinflammatory phenotype of astrocytes through β1 integrin activation. Molecular and Cellular Neuroscience 125, 103848 (2023). [DOI] [PubMed] [Google Scholar]
- 65.Slack R. J., Macdonald S. J. F., Roper J. A., Jenkins R. G. & Hatley R. J. D. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov 21, 60–78 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu L. & Yao Y. Central Nervous System Fibroblast-Like Cells in Stroke and Other Neurological Disorders. Stroke 52, 2456–2464 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Leary L. A. et al. Characterization of Vimentin-Immunoreactive Astrocytes in the Human Brain. Frontiers in Neuroanatomy 14, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z. et al. Beneficial Effects of GFAP/Vimentin Reactive Astrocytes for Axonal Remodeling and Motor Behavioral Recovery in Mice after Stroke. Glia 62, 2022–2033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly K. K. et al. Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC Neuroscience 17, 49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimamura M. et al. Long-term expression of periostin during the chronic stage of ischemic stroke in mice. Hypertens Res 37, 494–499 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Ma Q. et al. PDGFR-α inhibition preserves blood-brain barrier after intracerebral hemorrhage. Annals of Neurology 70, 920–931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Göritz C. et al. A Pericyte Origin of Spinal Cord Scar Tissue. Science 333, 238–242 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Pietilä R. et al. Molecular anatomy of adult mouse leptomeninges. Neuron 0, (2023). [DOI] [PubMed] [Google Scholar]
- 74.DeSisto J. et al. Single-Cell Transcriptomic Analyses of the Developing Meninges Reveal Meningeal Fibroblast Diversity and Function. Dev Cell 54, 43–59.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao K. et al. microRNA-181a Promotes Mitochondrial Dysfunction and Inflammatory Reaction in a Rat Model of Intensive Care Unit-Acquired Weakness by Inhibiting IGFBP5 Expression. J Neuropathol Exp Neurol 81, 553–564 (2022). [DOI] [PubMed] [Google Scholar]
- 76.Nguyen X.-X., Muhammad L., Nietert P. J. & Feghali-Bostwick C. IGFBP-5 Promotes Fibrosis via Increasing Its Own Expression and That of Other Pro-fibrotic Mediators. Front Endocrinol (Lausanne) 9, 601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vijayan A. et al. IGFBP-5 enhances epithelial cell adhesion and protects epithelial cells from TGFβ1-induced mesenchymal invasion. Int J Biochem Cell Biol 45, 2774–2785 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Han N. et al. Local application of IGFBP5 protein enhanced periodontal tissue regeneration via increasing the migration, cell proliferation and osteo/dentinogenic differentiation of mesenchymal stem cells in an inflammatory niche. Stem Cell Res Ther 8, 210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim K. S. et al. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell 18, 4543–4552 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nam T. J., Busby W. H., Rees C. & Clemmons D. R. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology 141, 1100–1106 (2000). [DOI] [PubMed] [Google Scholar]
- 81.Matsui Y. et al. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 23, 1029–1034 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Pilewski J. M., Liu L., Henry A. C., Knauer A. V. & Feghali-Bostwick C. A. Insulin-Like Growth Factor Binding Proteins 3 and 5 Are Overexpressed in Idiopathic Pulmonary Fibrosis and Contribute to Extracellular Matrix Deposition. Am J Pathol 166, 399–407 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song F. et al. Deletion of endothelial IGFBP5 protects against ischaemic hindlimb injury by promoting angiogenesis. Clin Transl Med 14, e1725 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rust R. Insights into the dual role of angiogenesis following stroke. J. Cereb. Blood Flow Metab. 40, 1167–1171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zaremba J., Skrobański P. & Losy J. The level of chemokine CXCL5 in the cerebrospinal fluid is increased during the first 24 hours of ischaemic stroke and correlates with the size of early brain damage. Folia Morphol (Warsz) 65, 1–5 (2006). [PubMed] [Google Scholar]
- 86.Stanne T. M. et al. Longitudinal Study Reveals Long-Term Proinflammatory Proteomic Signature After Ischemic Stroke Across Subtypes. Stroke 53, 2847–2858 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukuda S. et al. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 35, 998–1004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamann G. F. et al. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab 22, 526–533 (2002). [DOI] [PubMed] [Google Scholar]
- 89.Plaisier E. et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med 357, 2687–2695 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Kufner A. et al. Magnetic resonance imaging-based changes in vascular morphology and cerebral perfusion in subacute ischemic stroke. J Cereb Blood Flow Metab 41, 2617–2627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nih L. R., Gojgini S., Carmichael S. T. & Segura T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nature Materials 17, 642 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Escudero C. et al. Vascular endothelial growth factor and poor prognosis after ischaemic stroke. European Journal of Neurology 28, 1759–1764 (2021). [DOI] [PubMed] [Google Scholar]
- 93.Zheng X. et al. Angiopoietin-like protein 4 and clinical outcomes in ischemic stroke patients. Ann Clin Transl Neurol 8, 687–695 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rust R. et al. Nogo-A is secreted in extracellular vesicles, occurs in blood and can influence vascular permeability. J Cereb Blood Flow Metab 0271678X231216270 (2023) doi: 10.1177/0271678X231216270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weber R. Z. et al. A toolkit for stroke infarct volume estimation in rodents. Neuroimage 120518 (2024) doi: 10.1016/j.neuroimage.2024.120518. [DOI] [PubMed] [Google Scholar]
- 96.Weber R. Z., Mulders G., Perron P., Tackenberg C. & Rust R. Molecular and anatomical roadmap of stroke pathology in immunodeficient mice. Frontiers in Immunology 13, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med 26, 131–142 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weber R. Z. et al. Human iPSC-derived cell grafts promote functional recovery by molecular interaction with stroke-injured brain. 2024.04.03.588020 Preprint at 10.1101/2024.04.03.588020 (2024). [DOI] [Google Scholar]
- 99.Rust R. et al. Xeno-free induced pluripotent stem cell-derived neural progenitor cells for in vivo applications. Journal of Translational Medicine 20, 421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber R. et al. Intracerebral Transplantation and In Vivo Bioluminescence Tracking of Human Neural Progenitor Cells in the Mouse Brain ∣ Protocol. JoVE (Journal of Visualized Experiments) (in press), (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw single nucleus RNA sequencing data was deposited in the NCBI Gene Expression Omnibus (GEO) and will be available following publication. Data are now also available to explore via an interactive web browser: https://rustlab.shinyapps.io/Stroke-Atlas/