Abstract
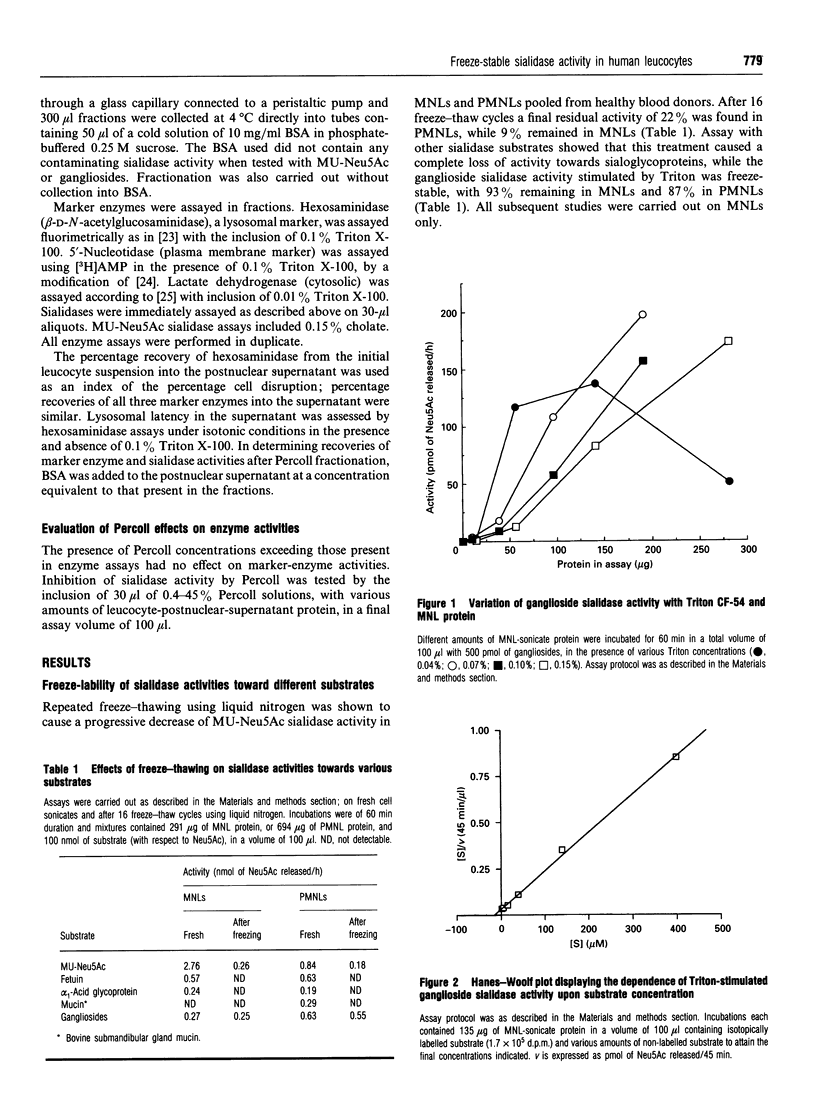
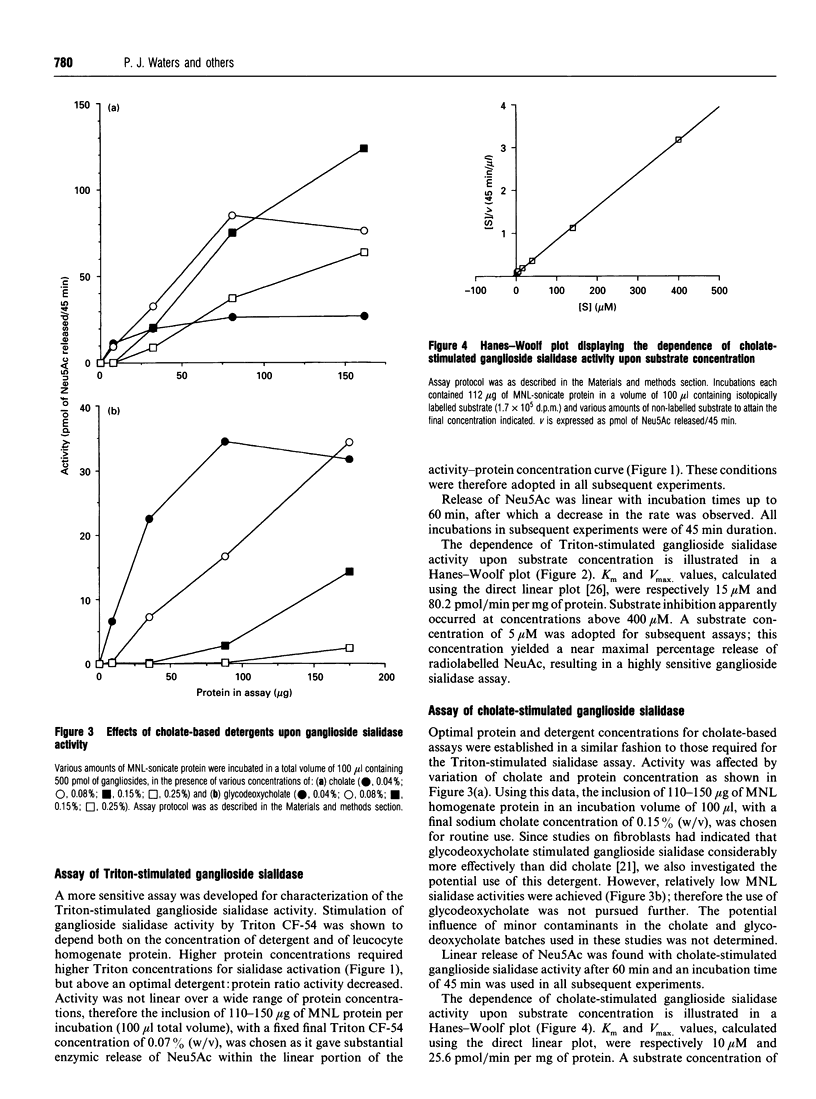
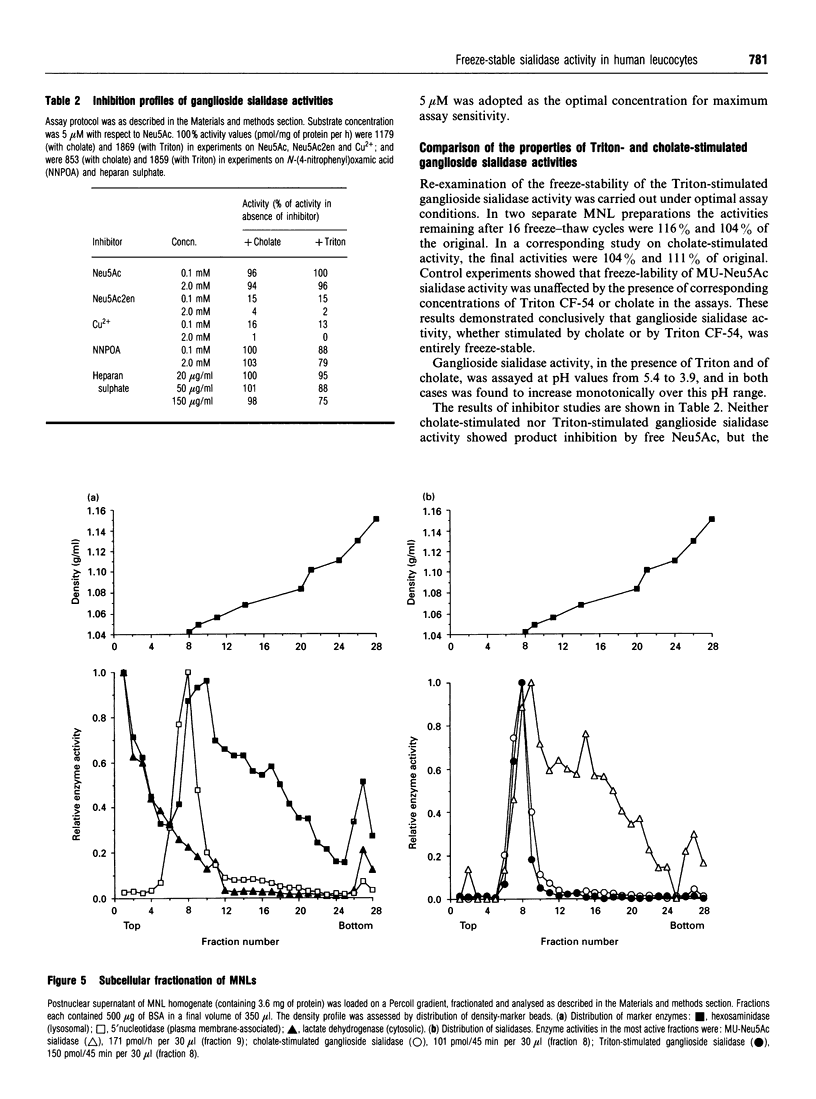
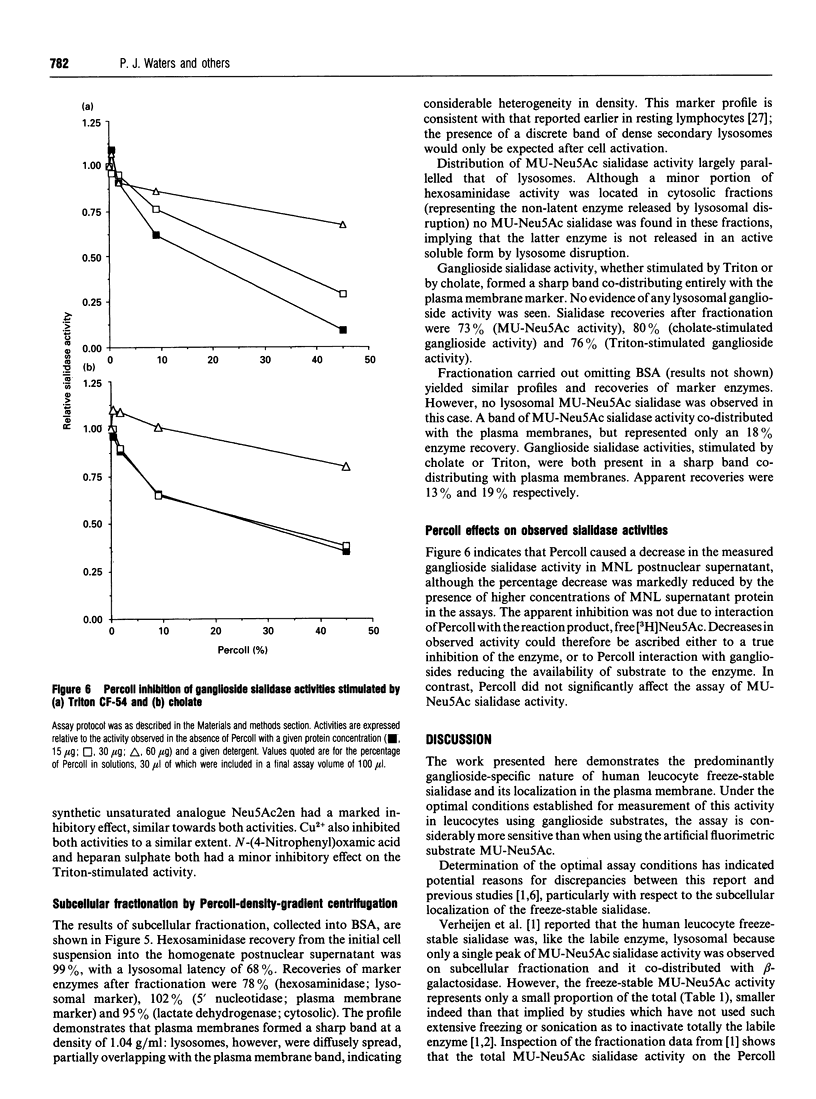
Human leucocytes contain a freeze-stable sialidase (neuraminidase; EC 3.2.1.18) activity in addition to the better-characterized lysosomal freeze-labile enzyme. In order to discriminate between the sialidase activities detected with the synthetic fluorimetric substrate 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid (MU-Neu5Ac), different tritiated sialoglycoconjugate substrates were prepared. Using this sensitive radioactive assay system, leucocyte sialidase activity towards glycoproteins was shown to be labile to repeated freeze-thawing, but a Triton-stimulated activity towards gangliosides was entirely freeze-stable. Assay conditions were optimized for this freeze-stable ganglioside sialidase activity. Subcellular fractionation of mononuclear leucocytes (MNLs) on Percoll-density gradients showed that this ganglioside sialidase activity was entirely associated with the plasma membrane. Study of the detergent requirements showed that MNLs also demonstrated ganglioside sialidase activity when sodium cholate was present in place of Triton. Cholate-stimulated ganglioside sialidase activity was found to be entirely freeze-stable and localized at the plasma membrane. Studies on whole homogenates of MNLs demonstrated that the Triton-stimulated and cholate-stimulated activities showed similar acidic pH optima at < or = 3.9 and were both strongly inhibited by 2-deoxy-2,3-didehydro-N-acetylneuraminic acid and Cu2+, but not by free N-acetylneuraminic acid, N-(4-nitrophenyl)oxamic acid or heparan sulphate. These results suggest that human MNLs contain, in addition to the lysosomal freeze-labile sialidase, a single sialidase activity which is freeze-stable, ganglioside-specific, plasma membrane-associated and stimulated both by Triton and by cholate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Separation of lymphocytes, granulocytes, and monocytes from human blood using iodinated density gradient media. Methods Enzymol. 1984;108:88–102. doi: 10.1016/s0076-6879(84)08076-9. [DOI] [PubMed] [Google Scholar]
- Corfield A. P., Clamp J. R. Mucin sialidase in the rat large intestine. Biochem Soc Trans. 1984 Aug;12(4):605–607. doi: 10.1042/bst0120605. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Wright D. G. Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J Clin Invest. 1991 Dec;88(6):2067–2076. doi: 10.1172/JCI115536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerhut R., van der Horst G. T., Verheijen F. W., Conzelmann E. Degradation of gangliosides by the lysosomal sialidase requires an activator protein. Eur J Biochem. 1992 Sep 15;208(3):623–629. doi: 10.1111/j.1432-1033.1992.tb17227.x. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M., Uda Y., Tsuji S., Miyatake T., Martin B. M., Tayama M., O'Brien J. S., Kishimoto Y. Human placental sialidase complex: characterization of the 60 kDa protein that cross-reacts with anti-saposin antibodies. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1211–1216. doi: 10.1016/0006-291x(91)90670-3. [DOI] [PubMed] [Google Scholar]
- Hong V. N., Beauregard G., Potier M., Bélisle M., Mameli L., Gatti R., Durand P. Studies on the sialidoses: properties of human leucocyte neuraminidases. Biochim Biophys Acta. 1980 Dec 4;616(2):259–270. doi: 10.1016/0005-2744(80)90143-6. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Hiraiwa M., O'Brien J. S. Saposins: structure, function, distribution, and molecular genetics. J Lipid Res. 1992 Sep;33(9):1255–1267. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landolfi N. F., Leone J., Womack J. E., Cook R. G. Activation of T lymphocytes results in an increase in H-2-encoded neuraminidase. Immunogenetics. 1985;22(2):159–167. doi: 10.1007/BF00563513. [DOI] [PubMed] [Google Scholar]
- Lieser M., Harms E., Kern H., Bach G., Cantz M. Ganglioside GM3 sialidase activity in fibroblasts of normal individuals and of patients with sialidosis and mucolipidosis IV. Subcellular distribution and and some properties. Biochem J. 1989 May 15;260(1):69–74. doi: 10.1042/bj2600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I., Bou-Gharios G., Abraham D. The activation of resting lymphocytes is accompanied by the biogenesis of lysosomal organelles. Eur J Immunol. 1990 Oct;20(10):2161–2170. doi: 10.1002/eji.1830201003. [DOI] [PubMed] [Google Scholar]
- Pilatte Y., Bignon J., Lambré C. R. Sialic acids as important molecules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology. 1993 Jun;3(3):201–218. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- Potier M., Lamontagne S., Michaud L., Tranchemontagne J. Human neuraminidase is a 60 kDa-processing product of prosaposin. Biochem Biophys Res Commun. 1990 Nov 30;173(1):449–456. doi: 10.1016/s0006-291x(05)81079-4. [DOI] [PubMed] [Google Scholar]
- Sagawa J., Miyagi T., Tsuiki S. Characterization of the major sialidases of various types of rat blood cells: their comparison with rat liver sialidases. J Biochem. 1990 Mar;107(3):452–456. doi: 10.1093/oxfordjournals.jbchem.a123066. [DOI] [PubMed] [Google Scholar]
- Saito M. [Bioactive sialoglycosphingolipids (gangliosides): differentiation-inducers as well as differentiation-markers in human hematopoietic cells]. Hum Cell. 1989 Mar;2(1):35–44. [PubMed] [Google Scholar]
- Sandhoff K., van Echten G., Schröder M., Schnabel D., Suzuki K. Metabolism of glycolipids: the role of glycolipid-binding proteins in the function and pathobiochemistry of lysosomes. Biochem Soc Trans. 1992 Aug;20(3):695–699. doi: 10.1042/bst0200695. [DOI] [PubMed] [Google Scholar]
- Schauer R., Wember M., Tschesche H. Isolation and characterization of an oligosaccharide- and glycoprotein-specific sialidase from human leucocytes. Hoppe Seylers Z Physiol Chem. 1984 Apr;365(4):419–426. doi: 10.1515/bchm2.1984.365.1.419. [DOI] [PubMed] [Google Scholar]
- Schneider-Jakob H. R., Cantz M. Lysosomal and plasma membrane ganglioside GM3 sialidases of cultured human fibroblasts. Differentiation by detergents and inhibitors. Biol Chem Hoppe Seyler. 1991 Jun;372(6):443–450. doi: 10.1515/bchm3.1991.372.1.443. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Sakuraba H., Potier M., Akagi M., Sakai M., Beppu H. beta-Galactosidase-neuraminidase deficiency in adults: deficiency of a freeze-labile neuraminidase in leukocytes and fibroblasts. Hum Genet. 1981;58(4):387–389. doi: 10.1007/BF00282820. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Yamada T., Tsutsumi A., Miyatake T. Neuraminidase deficiency and accumulation of sialic acid in lymphocytes in adult type sialidosis with partial beta-galactosidase deficiency. Ann Neurol. 1982 May;11(5):541–543. doi: 10.1002/ana.410110517. [DOI] [PubMed] [Google Scholar]
- Tsvetkova I. V., Petushkova N. A., Zolotuchina T. V., Kucharenko V. I., Rosenfeld E. L. Biochemical study of sialidosis type I in a Russian family. J Inherit Metab Dis. 1987;10(1):18–23. doi: 10.1007/BF01799483. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectins and other mammalian sialic acid-binding lectins. Curr Opin Cell Biol. 1992 Apr;4(2):257–266. doi: 10.1016/0955-0674(92)90041-a. [DOI] [PubMed] [Google Scholar]
- Veh R., Corfield A. P., Sander M., Schauer R. Neuraminic acid-specific modification and tritium labelling of gangliosides. Biochim Biophys Acta. 1976 Jan 18;486(1):145–160. doi: 10.1016/0005-2760(77)90079-0. [DOI] [PubMed] [Google Scholar]
- Verheijen F. W., Janse H. C., van Diggelen O. P., Bakker H. D., Loonen M. C., Durand P., Galjaard H. Two genetically different MU-NANA neuraminidases in human leucocytes. Biochem Biophys Res Commun. 1983 Dec 16;117(2):470–478. doi: 10.1016/0006-291x(83)91224-x. [DOI] [PubMed] [Google Scholar]
- Warner T. G., Louie A., Potier M. Photolabeling of the alpha-neuraminidase/beta-galactosidase complex from human placenta with a photoreactive neuraminidase inhibitor. Biochem Biophys Res Commun. 1990 Nov 30;173(1):13–19. doi: 10.1016/s0006-291x(05)81014-9. [DOI] [PubMed] [Google Scholar]
- Waters P. J., Flynn M. D., Corrall R. J., Pennock C. A. Increases in plasma lysosomal enzymes in type 1 (insulin-dependent) diabetes mellitus: relationship to diabetic complications and glycaemic control. Diabetologia. 1992 Oct;35(10):991–995. doi: 10.1007/BF00401431. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tsuji S., Ariga T., Miyatake T. Lysosomal sialidase deficiency in sialidosis with partial beta-galactosidase deficiency. Biochim Biophys Acta. 1983 Jan 4;755(1):106–111. doi: 10.1016/0304-4165(83)90279-9. [DOI] [PubMed] [Google Scholar]
- Zeigler M., Sury V., Bach G. The identification of lysosomal ganglioside sialidase in human cells. Eur J Biochem. 1989 Aug 1;183(2):455–458. doi: 10.1111/j.1432-1033.1989.tb14949.x. [DOI] [PubMed] [Google Scholar]
- van der Horst G. T., Galjart N. J., d'Azzo A., Galjaard H., Verheijen F. W. Identification and in vitro reconstitution of lysosomal neuraminidase from human placenta. J Biol Chem. 1989 Jan 15;264(2):1317–1322. [PubMed] [Google Scholar]


