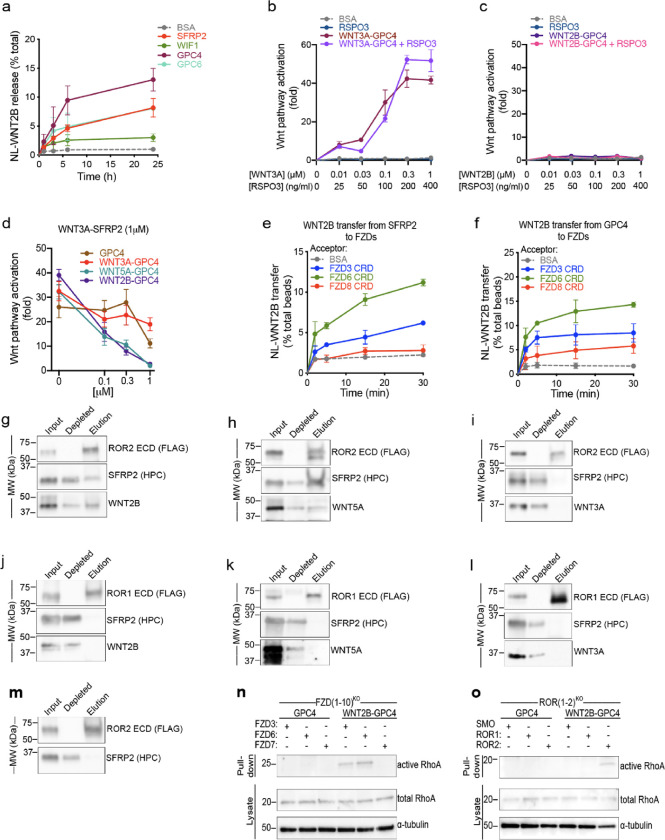
Figure 4. WNT2B released by SFRP2 and GPC4 activates the non-canonical Wnt/PCP pathway by binding to FZD3-CRD or FZD6-CRD, and ROR2-ECD.
a. HEK293 cells stably expressing NL-WNT2B were incubated with 1μM of purified SFRP2, WIF1, GPC4 or GPC6 ectodomains in serum-free media. NL-WNT2B release was measured at various time points by NanoLuc luciferase (NL) luminescence. Bovine serum albumin (BSA) served as negative control. WNT2B is released mainly by SFRP2, GPC4 and GPC6. Data represent the mean of two biological replicates, normalized to total NL-WNT in lysates, and error bars show SD.
b. R-Spondin 3 (RSPO3; 0, 25, 100, 200 and 400ng/ml) or purified WNT3A-GPC4 complex (0, 0.01, 0.03, 0.1, 0.3 and 1μM with respect to WNT3A) with or without RSPO3 (400ng/ml) was added to Wnt reporter cells. After 24h, Wnt pathway activity was measured by luciferase assay. Incubation with BSA served as negative control. RSPO3 does not potentiate WNT3A-GPC4 activity. Points represent average activation for two biological replicates, normalized to untreated cells, and error bars represent SD. See also Supplemental Figure 4a–e for protein purification and activity of WNT5A-GPC4 complex and WNT3A-carrier or WNT2B-carrier conditioned media.
c. As in (b), but with purified WNT2B-GPC4 complex. WNT2B-GPC4 complex is unable to activate canonical Wnt signaling, even with RSPO3.
d. As in (b), but purified WNT3A-SFRP2 complex (1μM) was mixed with varying amounts of GPC4 alone or in complex with WNT3A, WNT5A or WNT2B (0.1, 0.3 and 1μM). Both WNT5A-GPC4 and WNT2B-GPC4 complexes abolish WNT3A-SFRP2 activity, in contrast to GPC4 alone or in complex with WNT3A. See Supplemental Figure 4f for a similar experiment using WNT3A-GPC4 complex.
e. NL-WNT2B-SFRP2 complex was covalently captured on HaloLink beads from conditioned media, via HT7 fused to the C-terminus of SFRP2. The beads were then incubated with purified FZD-CRDs (5μM) and NL-WNT2B release was measured at different time points by NL luminescence. Incubation with BSA (5μM) served as negative control. WNT2B is preferentially transferred to FZD3-CRD and FZD6-CRD more than FZD8-CRD. Points represent average for two biological replicates, normalized by total NL-WNT on beads, and error bars represent SD.
f. As in (e), but with NL-WNT2B-GPC4 on beads.
g. Purified WNT2B-SFRP2 (5μM) was incubated with the extracellular domain (ECD) of ROR2 (2.5μM), followed by immunoprecipitation with antibodies against the FLAG tag attached to ROR. Samples were analyzed by SDS-PAGE and immunoblotting. WNT2B-SFRP2 complex interacts with ROR2-ECD. See also Supplemental Figure 4g–j for protein purification and a similar experiment using purified SFRP2.
h. As in (g), but with purified WNT5A-SFRP2 complex. WNT5A-SFRP2 complex binds to ROR2-ECD.
i. As in (g), but with purified WNT3A-SFRP2 complex. WNT3A-SFRP2 complex does not bind to ROR2-ECD.
j. As in (g), but WNT2B-SFRP2 complex (5μM) was incubated with ROR1-ECD (2.5μM). WNT2B-SFRP2 does not bind to ROR1-ECD.
k. As in (j), but with WNT5A-SFRP2 complex. WNT5A-SFRP2 does not bind to ROR1-ECD.
l. As in (j), but with WNT3A-SFRP2 complex. WNT3A-SFRP2 does not bind to ROR1-ECD.
m. As in (g) but using SFRP2 alone. SFRP2 is unable to interact with ROR2-ECD.
n. Activity of RhoA in FZD(1–10)KO cells expressing FZD3, FZD6 or FZD7 was assessed by Rhotekin-RBD pull-down assay after 6h of treatment with GPC4 alone or in complex with WNT2B (2μM). RhoA endogenous levels are shown in the lysates. RhoA activity by WNT2B-GPC4, in contrast to GPC4 alone, is rescued in cells expressing FZD3 or FZD6, but not the canonical FZD7. Blotting for α-tubulin served as loading control.
o. As in (n), but measuring activity of RhoA in ROR(1–2)KO cells expressing ROR1 or ROR2. WNT2B-GPC4, in contrast to GPC4 alone, activates RhoA only when ROR2 expression is rescued, not ROR1. Smoothened (SMO) transfection served as negative control.