Abstract
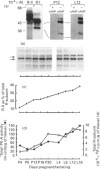
'Expressed' and 'total' activities of cyclic AMP-dependent protein kinase (PK-A) were measured in extracts of rat mammary tissue sampled throughout pregnancy and lactation. Expression of the genes encoding the catalytic subunit (C-subunit) isoforms C alpha and C beta was examined by Northern blotting, as a function of mammary development, to determine relative levels of their respective mRNAs. The content of C-subunit protein (all isoforms) was estimated immunochemically and related to levels of C-subunit catalytic activity and of mRNAs. It was found that C-subunit isoform mRNAs are expressed co-ordinately during mammary development and that a marked decline in expression, per cell, at around parturition is paralleled by a fall in 'total' PK-A activity. The 'expressed' activity of PK-A activity underwent characteristic changes throughout pregnancy and lactation, reaching a peak late in pregnancy. The PK-A activity ratio reached a peak in early lactation. C-subunit protein mass closely parallel 'total' PK-A activity throughout pregnancy and lactation, thereby demonstrating the constancy of C-subunit specific catalytic activity during these developmental events. Regulatory subunits (R-subunits) were probed with the photoaffinity label 8-azido-[32P]cAMP. The abundance of R-II as a proportion of total R-subunit increased throughout pregnancy and lactation, and quantitative analysis of the photoaffinity labelling suggested inconstancy in the ratio of R:C subunits, with highest values occurring in late pregnancy/early lactation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebe S. J., Oyen O., Sandberg M., Frøysa A., Hansson V., Jahnsen T. Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis--representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990 Mar;4(3):465–475. doi: 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- Bussmann L. E., Ward S., Kuhn N. J. Lactose and fatty acid synthesis in lactating-rat mammary gland. Effects of starvation, re-feeding, and administration of insulin, adrenaline, streptozotocin and 2-bromo-alpha-ergocryptine. Biochem J. 1984 Apr 1;219(1):173–180. doi: 10.1042/bj2190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S. Suppression of malignancy targeting cyclic AMP signal transducing proteins. Biochem Soc Trans. 1992 May;20(2):425–430. doi: 10.1042/bst0200425. [DOI] [PubMed] [Google Scholar]
- Ciardiello F., Pepe S., Bianco C., Baldassarre G., Ruggiero A., Bianco C., Selvam M. P., Bianco A. R., Tortora G. Down-regulation of RI alpha subunit of cAMP-dependent protein kinase induces growth inhibition of human mammary epithelial cells transformed by c-Ha-ras and c-erbB-2 proto-oncogenes. Int J Cancer. 1993 Feb 1;53(3):438–443. doi: 10.1002/ijc.2910530315. [DOI] [PubMed] [Google Scholar]
- Clegg R. A., Connor K. Cyclic AMP-dependent protein kinase in mammary epithelial cells; activity and subcellular distribution are acutely modulated by isoprenaline. Cell Signal. 1991;3(3):201–208. doi: 10.1016/0898-6568(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Clegg R. A., Ottey K. A. Cyclic AMP-dependent protein kinase in mammary tissue of the lactating rat. Activity ratio and responsiveness of the target enzymes acetyl-CoA carboxylase and glycogen phosphorylase to beta-adrenergic stimulation. Biochem J. 1990 Feb 1;265(3):769–775. doi: 10.1042/bj2650769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A. Regulation of fatty acid uptake and synthesis in mammary and adipose tissues: contrasting roles for cyclic AMP. Curr Top Cell Regul. 1988;29:77–128. doi: 10.1016/b978-0-12-152829-4.50005-7. [DOI] [PubMed] [Google Scholar]
- Connor K., Clegg R. A. Isoenzymes of protein kinase C in rat mammary tissue: changes in properties and relative amounts during pregnancy and lactation. Biochem J. 1993 May 1;291(Pt 3):817–824. doi: 10.1042/bj2910817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D. Determination of the cAMP-dependent protein kinase activity ratio in intact tissues. Methods Enzymol. 1983;99:227–232. doi: 10.1016/0076-6879(83)99057-2. [DOI] [PubMed] [Google Scholar]
- Ekanger R., Vintermyr O. K., Houge G., Sand T. E., Scott J. D., Krebs E. G., Eikhom T. S., Christoffersen T., Ogreid D., Døskeland S. O. The expression of cAMP-dependent protein kinase subunits is differentially regulated during liver regeneration. J Biol Chem. 1989 Mar 15;264(8):4374–4382. [PubMed] [Google Scholar]
- Gardner R. A., Travers M. T., Barber M. C., Miller W. R., Clegg R. A. Expression of PK-A catalytic subunit in mammary tissue during pregnancy and lactation. Biochem Soc Trans. 1993 Nov;21(4):398S–398S. doi: 10.1042/bst021398s. [DOI] [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Hardie D. G. Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta. 1992 Feb 12;1123(3):231–238. doi: 10.1016/0005-2760(92)90001-c. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Knight C. H., Peaker M. Development of the mammary gland. J Reprod Fertil. 1982 Jul;65(2):521–536. doi: 10.1530/jrf.0.0650521. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- MUNFORD R. E. CHANGES IN THE MAMMARY GLANDS OF RATS AND MICE DURING PREGNANCY, LACTATION AND INVOLUTION. 2. LEVELS OF DEOXYRIBONUCLEIC ACID, AND ALKALINE AND ACID PHOSPHATASES. J Endocrinol. 1963 Dec;28:17–34. doi: 10.1677/joe.0.0280017. [DOI] [PubMed] [Google Scholar]
- Massa J. S., Walker P. S., Moser D. R., Fellows R. E., Maurer R. A. Fetal development and neuronal/glial cell specificity of cAMP-dependent protein kinase subunit mRNAs in rat brain. Dev Neurosci. 1991;13(1):47–53. doi: 10.1159/000112140. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Clegg C. H., Uhler M. D., Chrivia J. C., Cadd G. G., Correll L. A., Otten A. D. Analysis of the cAMP-dependent protein kinase system using molecular genetic approaches. Recent Prog Horm Res. 1988;44:307–335. doi: 10.1016/b978-0-12-571144-9.50014-4. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Sapag-Hagar M., Greenbaum A. L. The role of cyclic nucleotides in the development and function of rat mammary tissue. FEBS Lett. 1974 Sep 15;46(1):180–183. doi: 10.1016/0014-5793(74)80363-7. [DOI] [PubMed] [Google Scholar]
- Showers M. O., Maurer R. A. A cloned bovine cDNA encodes an alternate form of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Dec 15;261(35):16288–16291. [PubMed] [Google Scholar]
- Steinberg R. A. A kinase-negative mutant of S49 mouse lymphoma cells is defective in posttranslational maturation of catalytic subunit of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1991 Feb;11(2):705–712. doi: 10.1128/mcb.11.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUCKER H. A., REECE R. P. Nucleic acid content of mammary glands of lactating rats. Proc Soc Exp Biol Med. 1963 Feb;112:409–412. doi: 10.3181/00379727-112-28060. [DOI] [PubMed] [Google Scholar]
- TUCKER H. A., REECE R. P. Nucleic acid content of mammary glands of pregnant rats. Proc Soc Exp Biol Med. 1963 Feb;112:370–372. doi: 10.3181/00379727-112-28048. [DOI] [PubMed] [Google Scholar]
- Takhar S., Munday M. R. Evidence for different isozymic forms of catalytic subunit of cyclic AMP-dependent protein kinase in heart and lactating mammary gland of the rat. Biochem Soc Trans. 1992 Aug;20(3):307S–307S. doi: 10.1042/bst020307s. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers M. T., Barber M. C. Isolation of a goat acetyl-CoA carboxylase complementary DNA and effect of milking frequency on the expression of the acetyl-CoA carboxylase and fatty acid synthase genes in goat mammary gland. Comp Biochem Physiol B. 1993 May;105(1):123–128. doi: 10.1016/0305-0491(93)90178-8. [DOI] [PubMed] [Google Scholar]
- Walter U., Greengard P. Photoaffinity labeling of the regulatory subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:154–162. doi: 10.1016/0076-6879(83)99048-1. [DOI] [PubMed] [Google Scholar]
- Weldon S. L., Taylor S. S. Monoclonal antibodies as probes for functional domains in cAMP-dependent protein kinase II. J Biol Chem. 1985 Apr 10;260(7):4203–4209. [PubMed] [Google Scholar]
- Wu J. C., Wang J. H. Sequence-selective DNA binding to the regulatory subunit of cAMP-dependent protein kinase. J Biol Chem. 1989 Jun 15;264(17):9989–9993. [PubMed] [Google Scholar]