Abstract
Background
Long-term clinical practice has suggested a possible association between ossification of cervical ligament (OCL) and primary osteoporosis (POP). However, there is a lack of relevant research data. This study aimed to clarify the potential relationship between OCL and POP, and propose new strategies for preventing the onset of POP.
Material/Methods
The study involved 107 patients. The patients’ diagnosis included OCL (ossification of the posterior longitudinal ligament, ossification of the ligamentum flavum, and ossification of the nuchal ligament) and POP. Bone mineral density (BMD), types of OCL, types of ossification of posterior longitudinal ligament, age, sex, serum calcium, serum phosphorus, alkaline phosphatase, type I collagen amino-terminal extension peptide, type I collagen degradation products, osteocalcin N-terminal molecular fragments, 25-hydroxyvitamin D, and history of taking steroid drugs were collected. SPSS24.0 and GraphPad Prism 8 were used to obtain the risk factors for POP.
Results
One-way analysis of variance found that OCL, ossification of posterior longitudinal ligament, alkaline phosphatase, and osteocalcin N-terminal molecular fragments had statistical significance on BMD of the femoral neck (P<0.05). The independent sample t test showed that patient sex had statistical significant effect on BMD (femoral neck) (P=0.036). Incorporating the above factors into multiple linear regression analysis, it was found that OCL, alkaline phosphatase, and osteocalcin N-terminal molecular fragments were risk factors affecting BMD of femoral neck (P<0.05).
Conclusions
OCL, osteocalcin N-terminal molecular fragments, and alkaline phosphatase are risk factors for POP.
Keywords: Osteoporosis; Ossification, Heterotopic; Ossification of Posterior Longitudinal Ligament; Ossification of the Posterior Longitudinal Ligament of the Spine
Introduction
Osteoporosis (OP) is an age-related disease that mostly affects postmenopausal women and middle-aged and elderly people [1,2]. It is a systemic metabolic disorder characterized by bone loss, bone microstructural destruction, and bone strength loss, making bones prone to fractures [3]. Primary osteoporosis (POP) is a high-conversion type of OP, and patients usually present with symptoms such as hunchback, height loss, and chronic pain. Fracture is one of the most serious complications of POP and one of the important risk factors for patient death [4,5]. Because bone fragility increases when patients develop POP, fractures can occur under the action of daily minor external forces, placing a heavy burden on patients and medical systems [6,7]. The World Health Organization attaches great importance to the prevention of POP and lists POP as one of the common diseases that endangers the health of the elderly, especially postmenopausal women [8,9].
Ossification of cervical ligament (OCL) refers to the hyperplasia and ossification of the posterior longitudinal ligament, ligamentum flavum, and nuchal ligament [10,11]. OCL is difficult to detect in the budding stage, without the assistance of radiological equipment, and hard to treat in the advanced stage. OCL was initially identified by Tsukimoto through autopsy findings and was subsequently formalized by Terayama. In 1975, a research society focused on OCL was founded in Japan, leading to extensive basic research. Epidemiological studies indicate that OCL predominantly affects the East Asian yellow race, particularly Japanese individuals, whereas its prevalence is lower in Europe and the United States. The highest incidence rate among those over 30 years old is observed in Japan, ranging from 1.9% to 4.3%, followed by China at 1.6% to 1.8%, and South Korea at 3.6% [12–14]. In the early stage of the disease, most patients have no obvious clinical symptoms. As the disease progresses, neurological symptoms gradually worsen and limb sensory and motor dysfunction occur [15–17]. OCL is the result of the combined action of multiple pathological factors. Local vascular tissue and spindle cells infiltrate within the ligament, which leads to fibroblast proliferation. The cervical ligament becomes hypertrophic or calcified, transforming into bone tissue, to form plate-like bone [18,19]. Some studies have suggested that OCL is related to genetic and environmental factors. The pathological and physiological mechanisms, however, are still unclear but may be similar to that of diffuse idiopathic osteohypertrophy [10,20]. From a biomechanical perspective, during the degeneration process of the cervical spine, segmental instability and mechanical stress distribution imbalance activate ossification pathways and upregulate the expression levels of osteogenic markers such as alkaline phosphatase and osteocalcin [15,19]. From a genomic perspective, there is a familial genetic tendency of ossification of the posterior longitudinal ligament. A study reported that the rs199772854A site of the IL17RC gene may be one of the potential pathogenic genes for posterior longitudinal ligament ossification [21]. In addition, long-term poor posture, strenuous exercise, fatigue, and abnormal hormone secretion levels are risk factors for OCL [22,23].
Both POP and OCL are age-related diseases and are the result of multiple factors. However, the correlation between the two remains controversial. In this article, we study and discuss the correlation between the POP and OCL.
Material and Methods
Study Design and Participants
This was a clinical retrospective study based on the medical record system of the First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine. The discharge time was set from January 2014 to January 2024. The first diagnostic setting was “ossification of cervical ligament (ossification of posterior longitudinal ligament, ossification of ligamentum flavum, and ossification of nuchal ligament)”. The bone mineral density (BMD), OCL, ossification of posterior longitudinal ligament, age, sex, serum calcium, serum phosphorus, alkaline phosphatase, type I collagen amino-terminal extension peptide, type I collagen degradation products, osteocalcin N-terminal molecular fragments, 25-hydroxyvitamin D, and history of taking steroid drugs of all included patients were recorded. This study was conducted in accordance with the Declaration of Helsinki. Medical record information obtained from previous diagnoses and treatments were used to conduct an accidental application for informed consent. The study was approved by the ethics committee of the regional hospital (ID: JY2024-032).
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) patients whose radiological examination clearly showed OCL (ossification of posterior longitudinal ligament, ossification of ligamentum flavum, and ossification of nuchal ligament); (2) patients who accepted dual-energy X-ray method to detect BMD [1,4]; (3) patients who had complete basic information; and (4) patients who had no history of cervical surgery.
The exclusion criteria were as follows: (1) unnatural menopause; (2) secondary hypertension; (3) diabetes and abnormal glucose tolerance; (4) severe damage to heart, lung, liver, and kidney functions; (5) gonadal disease, adrenal gland disease, parathyroid disease, thyroid disease, and pituitary disease; (6) a history of immune deficiency diseases, cancer, malignant tumors, autoimmune diseases, serious cardiovascular diseases, or other diseases that might significantly reduce life expectancy; (7) participation in any drug clinical trials within 6 months before the screening examination; and (8) patients who were judged by the researcher to be unfit to participate in this study.
Research Approaches
The dual-energy X-ray absorptiometry (Lunar-Prodigy, General Electric, USA) was used to measure the BMD of the patient’s lumbar spine, femoral neck, and hip joint for classification of POP. Instrument quality control was performed daily, and the coefficient of variation was ≤1.2%.
Based on X-ray and computed tomography examination, according to the location of the patient’s OCL, it was divided into ossification of the posterior longitudinal ligament, ossification of the ligamentum flavum, and ossification of the nuchal ligament. Ossification of posterior longitudinal ligament was divided into type focal, type segmental, type continuous, and type hybrid according to the shape and extent of ossification lesions. Type focal meant that it straddled the upper and lower posterior edges of 2 adjacent vertebral bodies, that is, it occurred at the intervertebral disc plane; type segmental meant that ossification blocks existed in the form of clouds on the posterior edge of each vertebral body, and several ossification lesions could be split separately; type continuous meant that the ossification was in the shape of a cord and spanned several vertebral bodies continuously; and type hybrid meant that there were continuous ossification and segmental ossification blocks.
Age, sex, serum calcium, serum phosphorus, alkaline phosphatase, type I collagen amino-terminal extension peptide, type I collagen degradation products, osteocalcin N-terminal molecular fragments, 25-hydroxyvitamin D, and history of taking steroid drugs were carefully and accurately documented based on the medical record system (Jiahe electronic medical record editing system, China) and hematological testing system (Huiqiao testing system, China).
Statistical Analysis
SPSS24.0 software (IBM Corp, Armonk, NY, USA) was used in the study, and the data are presented in the form of mean±standard deviation. Graphing was performed using GraphPad Prism 8 (GraphPad Software Inc, USA). BMD (lumbar spine, femoral neck, and hip joint), OCL, ossification of posterior longitudinal ligament, age, sex, serum calcium, serum phosphorus, alkaline phosphatase, type I collagen amino-terminal extension peptide, type I collagen degradation products, osteocalcin N-terminal molecular fragments, 25-hydroxyvitamin D, and history of taking steroid drugs were analyzed using the independent sample t test, one-way analysis of variance, or Pearson correlation analysis. For differences between groups, the Dunnett T3 test was used when the variances were different; the least significant difference method was used when the variances were uniform. After data analysis, if it was suggested that the variables were potential influencing factors (P<0.10), multiple linear regression analysis of each influencing factor and BMD was performed. P<0.05 was considered to indicate a statistical difference. Finally, the correlation between the influencing factors and BMD was judged by the size of the analysis coefficient value.
Results
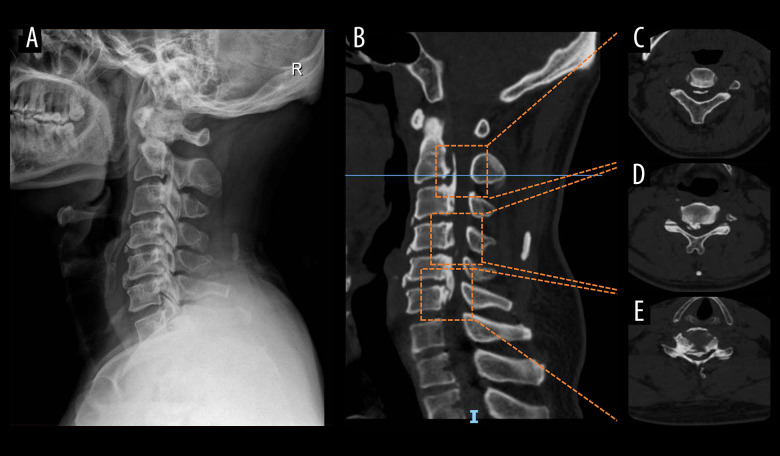
A total of 107 cases were included in the study. The detailed process of patient collection is shown in Figure 1. Demographic characteristics are detailed in Table 1. Representative radiological images of patients with OCL are shown in Figure 2.
Figure 1.
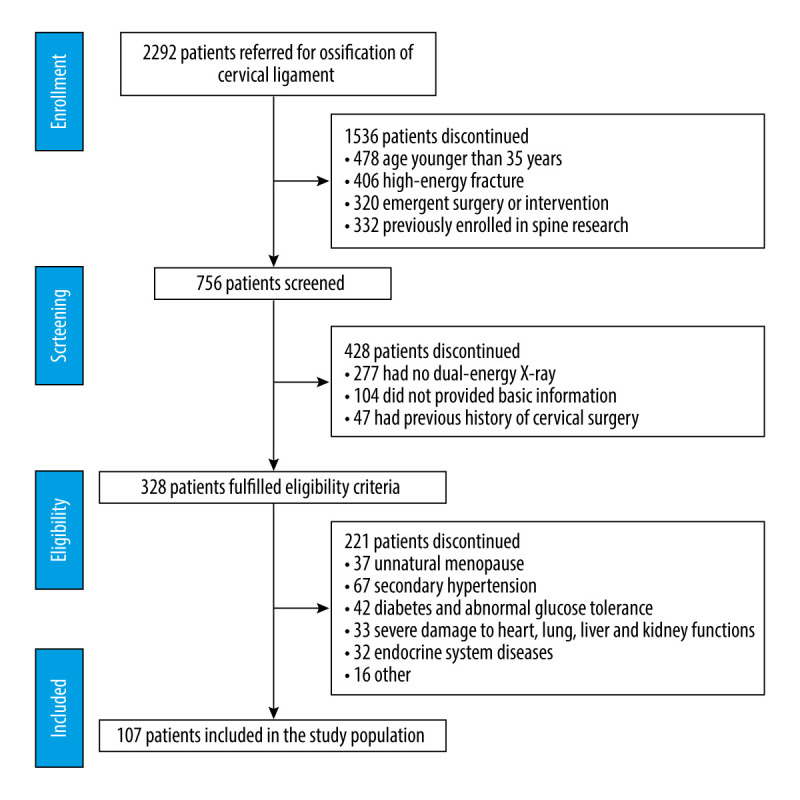
Patient flow diagram.
Table 1.
Demographic characteristics.
| Characteristic | Value |
|---|---|
| Sex (Male/Female) | 48 (44.86%)/ 59 (55.14%) |
| Age (years) | 61.66±10.42 |
| Height (m) | 1.61±0.07 |
| Weight (kg) | 61.51±12.50 |
| Body mass index (kg/m2) | 23.61±4.29 |
| Systolic blood pressure (mm Hg) | 133.50±16.75 |
| Diastolic blood pressure (mm Hg) | 81.27±11.43 |
| Blood sugar (mmol/L) | 5.39±1.37 |
| Bone mineral density (femoral neck) | −1.90±1.10 |
| Serum calcium (mmol/L) | 2.25±0.10 |
| Serum phosphorus (mmol/L) | 1.24±0.18 |
| Alkaline phosphatase (U/L) | 70.91±21.42 |
| Type I collagen amino-terminal extension peptide (pg/L) | 55.49±27.76 |
| Type I collagen degradation products (pg/L) | 0.73±0.39 |
| Osteocalcin N-terminal fragment (ug/L) | 16.53±7.35 |
| 25-Hydroxyvitamin D (pg/L) | 29.71±9.01 |
| History of taking steroid drugs | 72 (67.29%) |
Figure 2.
Representative radiologic images of patients with ossification of cervical ligament. (A) Lateral X-ray of the cervical spine; (B) sagittal view of the cervical spine computed tomography: (C) C2/3; (D) back of the C4 vertebral body; (E) C5/6.
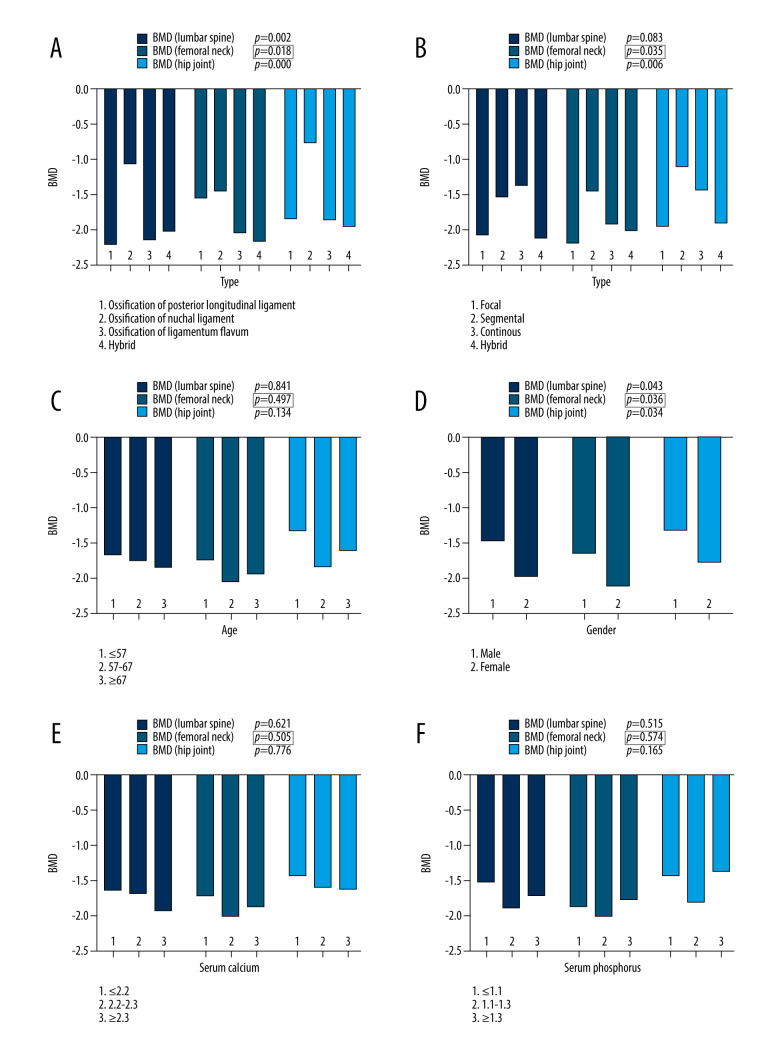
The dependent variable was POP. One-way analysis of variance was used to evaluate the effect of OCL, ossification of posterior longitudinal ligament, age, serum calcium, serum phosphorus, alkaline phosphatase, type I collagen amino-terminal extension peptide, type I collagen degradation products, osteocalcin N-terminal molecular fragments, and 25-hydroxyvitamin D on BMD (lumbar spine, femoral neck, and hip joint). The results suggested that OCL, ossification of posterior longitudinal ligament, alkaline phosphatase, and osteocalcin N-terminal molecular fragments had statistical significance on BMD (femoral neck) (P=0.018, P=0.035, P=0.045, P=0.010, respectively). The above 4 factors might be potential influencing factors leading to BMD (femoral neck) abnormalities, which were planned to be included in multiple linear regression analysis. More details are shown in Figures 3, 4, and Table 2.
Figure 3.
Statistical analysis results. (A) Types of ossification of cervical ligament and bone mineral density (BMD); (B) types of ossification of posterior longitudinal ligament and BMD; (C) age and BMD; (D) sex and BMD; (E) serum calcium and BMD; (F) serum phosphorus and BMD.
Figure 4.
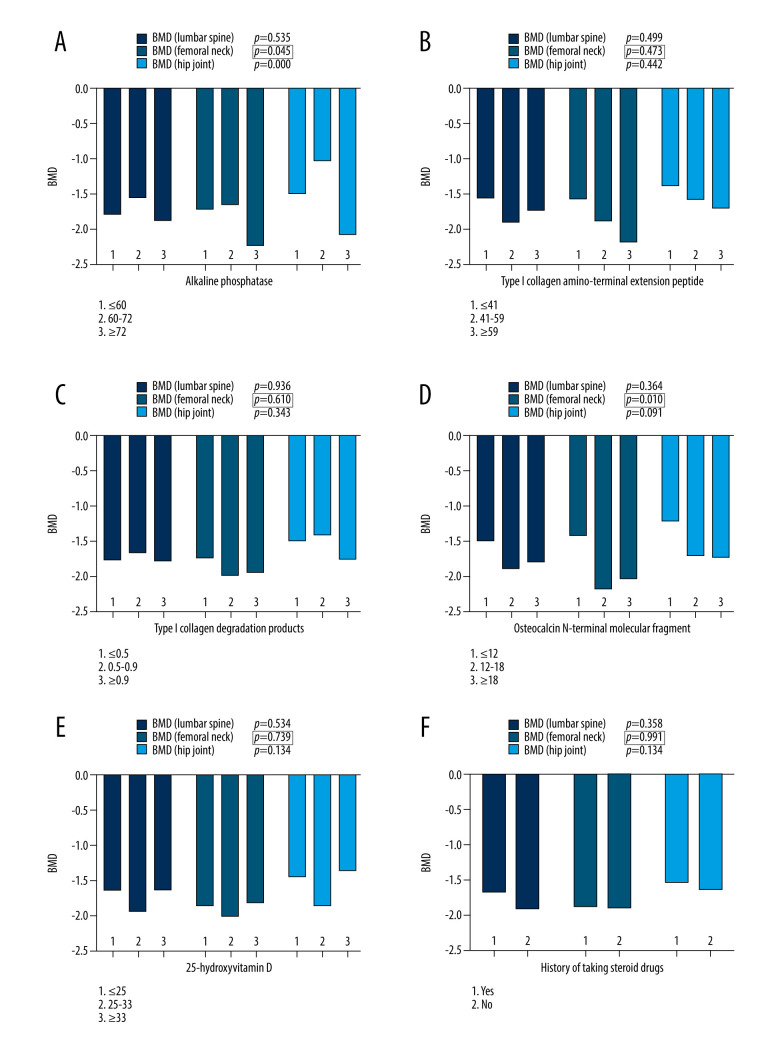
Statistical analysis results. (A) Alkaline phosphatase and bone mineral density (BMD); (B) type I collagen amino-terminal extension peptide and BMD; (C) type I collagen degradation products and BMD; (D) osteocalcin N-terminal molecular fragments and BMD; (E) 25-hydroxyvitamin D and BMD; (F) history of taking steroid drugs and BMD.
Table 2.
The significant variables before the regression analysis.
| Variable | P |
|---|---|
| Sex | 0.036 |
| Ossification of cervical ligament | 0.018 |
| Ossification of posterior Longitudinal ligament | 0.035 |
| Alkaline phosphatase | 0.045 |
| Osteocalcin N-terminal molecular fragments | 0.010 |
The independent sample t test was used to evaluate the effect of patient sex and history of taking steroid drugs on BMD (lumbar spine, femoral neck, and hip joint). The results showed that sex had statistically significant effect on BMD (femoral neck) (P=0.036). The correlation between history of taking steroid drugs and BMD (femoral neck) was not significant (P=0.991). Therefore, sex might be a potential influencing factor leading to BMD (femoral neck) abnormalities. It was included in multiple linear regression analysis. More details are shown in Figures 3D and 4F.
Multiple linear regression analysis was conducted with OCL, ossification of posterior longitudinal ligament, alkaline phosphatase, sex, and osteocalcin N-terminal molecular fragments as independent variables and BMD (femoral neck) as the dependent variable. In the original goodness-of-fit test table, R2 was 0.198; after adjustment, R2 became 0.158. In the significance F test, F=4.984 and P=0.000, there was statistical significance. In the t test, OCL, alkaline phosphatase, and osteocalcin N-terminal molecular fragments were risk factors affecting BMD (femoral neck) (P=0.005, P=0.015, P=0.025, respectively). However, ossification of posterior longitudinal ligament and sex were not risk factors (P=0.830, P=0.092, respectively). According to the standardized coefficient, the variables that affected BMD were sorted from large to small, as follows: osteocalcin N-terminal molecular fragments, alkaline phosphatase, and ossification of cervical ligament. More details are shown in Table 3.
Table 3.
The results of multiple linear regression analysis.
| Model | Unstandardized coefficient | Standardized coefficient | t | P | ||
|---|---|---|---|---|---|---|
| B | Std. error | Beta | ||||
| 1 | (Constant) | 0.717 | 0.629 | 1.141 | 0.257 | |
| Sex | −0.348 | 0.204 | −0.158 | −1.704 | 0.092 | |
| Ossification of cervical ligament | −0.280 | 0.097 | −0.272 | −2.900 | 0.005 | |
| Ossification of posterior longitudinal ligament | −0.023 | 0.106 | −0.020 | −0.216 | 0.830 | |
| Alkaline phosphatase | −0.291 | 0.118 | −0.221 | −2.465 | 0.015 | |
| Osteocalcin N-terminal molecular fragments | −0.271 | 0.119 | −0.205 | −2.283 | 0.025 | |
Discussion
POP has become an important health problem affecting people over 50 years old [1,3]. According to the results of the epidemiological survey of OP among Chinese residents in 2018, the prevalence of OP among people aged 40 to 49 years old is 3.2%, but the prevalence rate among people over 50 years old reaches 19.2%. The prevalence rate among the above 65-year-old group is as high as 32%, of which women account for the majority. The prevalence rate for women over 50 years old is 32.1%, and for women over 65 years old is 51.6%. Its incidence rate cannot be ignored [24].
A study found that OCL was an influencing factor leading to abnormal BMD [25]. One-way analysis of variance was used to evaluate the effects of different types of OCL on the BMD of the lumbar spine, femoral neck, and hip joints. It was found that OCL had a statistically significant effect on the BMD of the lumbar spine, femoral neck, and hip joints (P=0.002, P=0.018, P=0.000, respectively). In multiple linear regression analysis, OCL was found to be a risk factor affecting BMD (femoral neck) (P=0.005). Hirai et al found that the BMD of type continuous and hybrid of cervical posterior longitudinal ligament ossification was higher than that of type segmental, and the BMD of patients with OCL was higher than that of those without spinal ligament ossification [26]. It can be seen that BMD can reflect the tendency of ligament ossification. However, some studies had found that patients with OCL had reduced BMD, especially of the trabecular bone. The reason might be that the ossified site required mineral supply from other sites. During the progression of OCL, the cortical bone remained unaltered while the quantity of cancellous bone diminished. This might be attributed to the fact that cancellous bone possesses a greater blood supply than does cortical bone. Consequently, when the ossification growth factor is secreted and enters the bloodstream, it initially encounters the cancellous bone, instigating mineral transformation. Due to this mineral transfer, BMD decreases, which induces OP [27,28]. Osteocalcin N-terminal molecular fragments are the degradation products of osteocalcin. Their content in the blood is more stable than osteocalcin and can better reflect bone formation [29,30]. When OP occurs, human bone metabolism and bone turnover rate are significantly enhanced, and the level of osteocalcin N-terminal molecular fragments is elevated [31,32]. Studies have shown that when physiological or pathological bone resorption is enhanced, osteocalcin N-terminal molecular fragments are at a lower level in the blood of patients. Clinical studies have shown that BMD values are correlated with the level of osteocalcin N-terminal molecular fragments, which increased sequentially in the normal bone mass group, osteopenia group, and OP group [33,34]. This is consistent with the conclusions of the present study. Alkaline phosphatase is released by osteoblasts and has the effect of promoting bone formation and bone matrix mineralization. The number of bone trabeculae decreases, and the body’s compensatory bone formation increases, causing the level of alkaline phosphatase to increase. The increase in the level of alkaline phosphatase reflects the rising in bone formation, which also means higher bone turnover, accelerated osteoblast apoptosis, and continuous reduction in bone volume [6,35]. Upon identifying pertinent risk factors, clinicians should educate potential high-risk groups on these risks and disseminate relevant knowledge. Once patients understand the dangers associated with these risk factors, they should be advised to implement suitable preventive strategies. Lifestyle significantly influences the development of POP. The emergence of risk factors can be mitigated through interventions such as a calcium-rich diet and exercise of appropriate intensity. Regular health assessments facilitate early detection of potential risk factors. Individuals at high risk can then adopt suitable therapeutic measures to inhibit further escalation of these risk factors.
OCL is a pathological heterotopic ossification disease of spinal ligaments, whose pathogenic mechanism remains unclear [16,27,28,36]. From the perspective of genetic susceptibility, epidemiological studies have shown that OCL mainly occurs in Asian populations and has a familial genetic tendency [37–40]. Runx2 is a key factor required for osteoblast differentiation. Runx2 regulates the osteogenic differentiation of osteoblasts and promotes bone tissue formation and reconstruction. Two loci in Runx2, RS1321075 and RS12333172, are different between OCL patients and controls: one of the haplotype loci is associated with an increased incidence of OCL [41,42]. Bone morphogenetic protein (BMP) is a multifunctional growth factor that plays a vital regulatory role in the osteogenesis process [43,44]. The BMP family has more than 10 subtypes, among which the genes related to OCL include BMP-2, BMP-4, and other subtypes [18]. The expression level of BMP-2 in ligamentum flavum cells is found to be significantly increased under cyclic mechanical stress, indicating that BMP may be involved in promoting endochondral osteogenesis at the ectopic ossification site of OCL, and the ossification activity of OCL continues to exist [17,45]. Fibroblast growth factor (FGF) and its receptor FGFR play a fundamental role in bone development, regulating osteoblast proliferation, differentiation and apoptosis, among which FGF-2 is an important regulator of bone and cartilage differentiation. The direct sequencing method was used to conduct a comparative study on the relationship between FGF-2, FGFR-1, FGFR-2 and single nucleotide polymorphisms in patients and controls. The results showed that the rs1476217 polymorphism site of FGF-2 was associated with OCL [46,47].
This study had several limitations. First, the patients in the study came from the same medical institution, resulting in underrepresentation and selection bias. This limited the generalizability of the study. Second, the number of included samples was not large enough, which might affect the statistical analysis and reduce the credibility of extending the results to the general population. Third, we reported a rare ligament ossification disease, and the number of references worldwide is currently low. Our study was biased toward exploratory research and unable to refer to past clinical studies. Lack of data or previous studies can result in insufficient study hypotheses or inability to compare and verify.
Conclusions
OCL, osteocalcin N-terminal molecular fragments, and alkaline phosphatase are risk factors for POP. Considering the huge medical burden caused by POP, identifying the risk factors of POP, providing health education, and conducting early diagnosis can help prevent the occurrence of POP earlier and bring important health benefits.
Acknowledgements
We are highly grateful to the contribution from all the doctors and nurses.
Footnotes
Conflict of interest: None declared
Ethics Statement: The study was approved by the First Affiliated Hospital of Guangzhou University of Chinese Medicine (ID: JY2024-032).
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported by the Natural Science Foundation of Guangdong Province, China (number: 2021A1515012168), Administration of Traditional Chinese Medicine of Guangdong Province, China (number: 20221146; 20241091; 20203004), Basic and Applied Basic Research Fund Project in Guangdong Province, China (number: 2020A1515110948), Basic and Applied Basic Research in Jointly Funded Projects of City Schools (Institutes) Projects, China (number: 202201020295), Project of Guangzhou Science and Technology Department, China (number: 202102021040; 202201020533), Application and Foundation Research Project of Guangzhou, China (number: 202201020500; 202201020295), Guangzhou Science and Technology Plan Project, China (number: 2023B03J0379), Chinese Society of Traditional Chinese Medicine Youth Talent Lifting Project (number: 2022-QNRC2-B11), and The Hospital Young and Middle Aged Key Talent Cultivation Project of The First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine (2023.10)
References
- 1.Walker M, Shane E. Postmenopausal osteoporosis. N Engl J Med. 2023;389(21):1979–91. doi: 10.1056/NEJMcp2307353. [DOI] [PubMed] [Google Scholar]
- 2.LeBoff M, Greenspan S, Insogna K, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis Int. 2022;33(10):2049–102. doi: 10.1007/s00198-021-05900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gielen E, Dupont J, Dejaeger M, Laurent M. Sarcopenia, osteoporosis and frailty. Metab. 2023;145:155638. doi: 10.1016/j.metabol.2023.155638. [DOI] [PubMed] [Google Scholar]
- 4.Aibar-Almazán A, Voltes-Martínez A, Castellote-Caballero Y, et al. Current status of the diagnosis and management of osteoporosis. Int J Mol Sci. 2022;23(16):9465. doi: 10.3390/ijms23169465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers C, Kansagara D, Lazur B, et al. Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: A living systematic review and network meta-analysis for the American college of physicians. Ann Intern Med. 2023;176(2):182–95. doi: 10.7326/M22-0684. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg C, Ix J. Diagnosis and management of osteoporosis in advanced kidney disease: A review. Am J Kidney Dis. 2022;79(3):427–36. doi: 10.1053/j.ajkd.2021.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Gosset A, Pouillès J, Trémollieres F. Menopausal hormone therapy for the management of osteoporosis. Best Pract Res Clin Endoc Metab. 2021;35(6):101551. doi: 10.1016/j.beem.2021.101551. [DOI] [PubMed] [Google Scholar]
- 8.Reid I, Billington E. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080–92. doi: 10.1016/S0140-6736(21)02646-5. [DOI] [PubMed] [Google Scholar]
- 9.Foessl I, Dimai H, Obermayer-Pietsch B. Long-term and sequential treatment for osteoporosis. Nat Rev Endocrinol. 2023;19(9):520–33. doi: 10.1038/s41574-023-00866-9. [DOI] [PubMed] [Google Scholar]
- 10.Abiola R, Rubery P, Mesfin A. Ossification of the posterior longitudinal ligament: Etiology, diagnosis, and outcomes of nonoperative and operative management. Glob Spine J. 2016;6(2):195–204. doi: 10.1055/s-0035-1556580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshii T, Sakai K, Machino M, Furuya T. Choice of surgical procedure for cervical ossification of the posterior longitudinal ligament. J Clin Med. 2022;11(18):5396. doi: 10.3390/jcm11185396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koike Y, Takahata M, Nakajima M, et al. Genetic insights into ossification of the posterior longitudinal ligament of the spine. eLife. 2023;12:e86514. doi: 10.7554/eLife.86514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le H, Wick J, Van B, Klineberg E. Ossification of the posterior longitudinal ligament: Pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 2022;30(17):820–30. doi: 10.5435/JAAOS-D-22-00049. [DOI] [PubMed] [Google Scholar]
- 14.Tamai K, Terai H, Hoshino M, et al. A deep learning algorithm to identify cervical ossification of posterior longitudinal ligaments on radiography. Sci Rep. 2022;12(1):2113. doi: 10.1038/s41598-022-06140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasawa T, Iwasaki K, Sawada T, et al. Pathophysiological role of endothelin in ectopic ossification of human spinal ligaments induced by mechanical stress. Calcif Tissue Int. 2006;79(6):422–30. doi: 10.1007/s00223-006-0147-7. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Luk K, Karppinen J, et al. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: A population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine. 2010;35(1):51–56. doi: 10.1097/BRS.0b013e3181b3f779. [DOI] [PubMed] [Google Scholar]
- 17.Ning S, Chen Z, Fan D, et al. Genetic differences in osteogenic differentiation potency in the thoracic ossification of the ligamentum flavum under cyclic mechanical stress. Int J Mol Med. 2017;39(1):135–43. doi: 10.3892/ijmm.2016.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto M, Toyama Y, Chikuda H, et al. Outcomes of fusion surgery for ossification of the posterior longitudinal ligament of the thoracic spine: A multicenter retrospective survey. J Neurosurg-Spine. 2011;15(4):380–85. doi: 10.3171/2011.6.SPINE10816. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Lu X, Chen D, et al. Mechanical strain induces Cx43 expression in spinal ligament fibroblasts derived from patients presenting ossification of the posterior longitudinal ligament. Eur Spine J. 2011;20(9):1459–65. doi: 10.1007/s00586-011-1767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu C, Lin S, Yuan L, et al. Correlation of blood bone turnover biomarkers and Wnt signaling antagonists with AS, DISH, OPLL, and OYL. BMC Musculoskelet Disord. 2017;18(1):61. doi: 10.1186/s12891-017-1425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Liu X, Liu X, et al. IL17RC affects the predisposition to thoracic ossification of the posterior longitudinal ligament. J Orthop Surg Res. 2019;14(1):210. doi: 10.1186/s13018-019-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endo T, Takahata M, Koike Y, et al. Association between obesity and ossification of spinal ligaments in 622 asymptomatic subjects: A cross-sectional study. J Bone Miner Metab. 2022;40(2):337–47. doi: 10.1007/s00774-021-01292-5. [DOI] [PubMed] [Google Scholar]
- 23.He Z, Tung N, Makino H, et al. Assessment of cervical myelopathy risk in ossification of the posterior longitudinal ligament patients with spinal cord compression based on segmental dynamic versus static factors. Neurospine. 2023;20(2):651–61. doi: 10.14245/ns.2346124.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epidemiological survey of osteoporosis in China and results of the “Healthy Bones” special action. Chinese J Osteoporosis and Bone Miner Diseases. 2019;12(4):317–18. [Google Scholar]
- 25.Gurban C, Balas M, Vlad M, et al. Bone turnover markers in postmenopausal osteoporosis and their correlation with bone mineral density and menopause duration. Rom J Morphol Embryol. 2019;60(4):1127–35. [PubMed] [Google Scholar]
- 26.Hirai N, Ikata T, Murase M, et al. Bone mineral density of the lumbar spine in patients with ossification of the posterior longitudinal ligament of the cervical spine. J Spinal Disord. 1995;8(5):337–41. [PubMed] [Google Scholar]
- 27.Liu J, Chen Y, Shan X, Wang H. Investigation of the biomarkers involved in ectopic ossification: The shared mechanism in ossification of the spinal ligament. Front Genet. 2022;13:991834. doi: 10.3389/fgene.2022.991834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishimoto H. [Clinical study on bone mineral mass in metabolic bone disorders – I-125 photon absorptiometry]. Nihon Seikeigeka Gakkai Zasshi. 1983;57(11):1699–715. [in Japanese] [PubMed] [Google Scholar]
- 29.Liu K, Tan G, Sun W, et al. Percutaneous kyphoplasty combined with zoledronic acid for the treatment of primary osteoporotic vertebral compression fracture: A prospective, multicenter study. Arch Orthop Trauma Surg. 2023;143(7):3699–706. doi: 10.1007/s00402-022-04557-4. [DOI] [PubMed] [Google Scholar]
- 30.Deng L, Yao F, Tian F, et al. Influence of iguratimod on bone metabolism in patients with rheumatoid arthritis: A meta-analysis. Int J Clin Pract. 2022;2022:5684293. doi: 10.1155/2022/5684293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong L, Chen Q, Cheng Y, et al. The relationship between coronary artery calcification and bone metabolic markers in maintenance hemodialysis patients. BMC Nephrol. 2023;24(1):238. doi: 10.1186/s12882-023-03286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong M, Xu C, Wen S, et al. The effects of obesity on bone turnover markers in diabetic patients with diabetic ketosis or ketoacidosis. Endocr. 2023;23(13):1660–67. doi: 10.2174/1871530323666230509101203. [DOI] [PubMed] [Google Scholar]
- 33.Ning W. Changes of bone metabolism markers in the healing stage of hip fracture in the elderly of different genders. J Lab Clin Med. 2018;15:1329–31. [Google Scholar]
- 34.An N, Ji L, Meng H, et al. Study on the correlation of N-MID and β-CTX with BMD with bone mineral density in patients with type 2 diabetes mellitus. Chinese J Osteoporosis Bone Miner Diseases. 2018;24(12):1591–95. 1605. [Google Scholar]
- 35.Schini M, Vilaca T, Gossiel F, et al. Bone turnover markers: Basic biology to clinical applications. Endocr Rev. 2023;44(3):417–73. doi: 10.1210/endrev/bnac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuya T, Sakai K, Yoshii T, Machino M. Conservative treatment and surgical indication of cervical ossification of the posterior longitudinal ligament. J Clin Med. 2023;12(17):5719. doi: 10.3390/jcm12175719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maigne J, Ayral X, Guérin-Surville H. Frequency and size of ossifications in the caudal attachments of the ligamentum flavum of the thoracic spine. Role of rotatory strains in their development. An anatomic study of 121 spines. Surg Radiol Anat. 1992;14(2):119–24. doi: 10.1007/BF01794886. [DOI] [PubMed] [Google Scholar]
- 38.Qu X, Chen Z, Fan D, et al. Two novel BMP-2 variants identified in patients with thoracic ossification of the ligamentum flavum. Eur J Hum Genet. 2017;25(5):565–71. doi: 10.1038/ejhg.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong Q, Ma X, Li F, et al. COL6A1 polymorphisms associated with ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament. Spine. 2007;32(25):2834–38. doi: 10.1097/BRS.0b013e31815b761c. [DOI] [PubMed] [Google Scholar]
- 40.Elkon R, Agami R. Characterization of noncoding regulatory DNA in the human genome. Nat Biotechnol. 2017;35(25):732–46. doi: 10.1038/nbt.3863. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Zhao Y, Chen Y, et al. Runx2 polymorphisms associated with OPLL and OLF in the Han population. Clin Orthop Rel Res. 2010;468(12):3333–41. doi: 10.1007/s11999-010-1511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishiya M, Sawada T, Kanemaru K, et al. A functional RNAi screen for Runx2-regulated genes associated with ectopic bone formation in human spinal ligaments. J Pharmacol Sci. 2008;106(3):404–14. doi: 10.1254/jphs.fp0072043. [DOI] [PubMed] [Google Scholar]
- 43.Chen G, Deng C, Li Y. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siamwala J, Rajendran S, Chatterjee S. Strategies of manipulating BMP signaling in microgravity to prevent bone loss. Vitam Horm. 2015;99:249–72. doi: 10.1016/bs.vh.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi K, Ishidou Y, Yonemori K, et al. Expression and localization of bone morphogenetic proteins (BMPs) and BMP receptors in ossification of the ligamentum flavum. Bone. 1997;21(1):23–30. doi: 10.1016/s8756-3282(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 46.Ornitz D, Marie P. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015;29(14):1463–86. doi: 10.1101/gad.266551.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jun J, Kim S. Association study of fibroblast growth factor 2 and fibroblast growth factor receptors gene polymorphism in korean ossification of the posterior longitudinal ligament patients. J Korean Neurosurg Soc. 2012;52(1):7–13. doi: 10.3340/jkns.2012.52.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]