Abstract
The ancient Ginkgo biloba tree grows across various regions, with distinctive leaves emitting a unique fragrance. Its extract contains flavonoids, organic acids, and terpenoids. Ginkgolide and bilobalide, which are G. biloba leaf extracts, offer diverse pharmaceutical benefits, including antioxidant, anti-inflammatory, and neuroprotective properties. The antioxidant and anti-inflammatory properties of these compounds are crucial for mitigating neurodegeneration, particularly in diseases such as Alzheimer’s disease. Additionally, their effectiveness in countering oxidative stress and inflammation highlights their potential to prevent cardiovascular ailments. This study also suggests that these compounds have a promising impact on lipid metabolism, suggesting their significance in addressing obesity-related metabolic disorders. In conclusion, ginkgolides and bilobalide exhibit promising effects in sustaining the integrity of the nervous and endocrine systems, along with the modulation of lipid metabolism. The diverse health benefits suggest that these compounds could serve as promising therapeutic interventions for various conditions, including neurological, cardiovascular, and metabolic diseases.
Keywords: Bilobalide, Ginkgolide, Cognitive impairment, Blood circulation, Metabolic syndrome
Introduction
The Ginkgo biloba tree, which has flourished for 170 million years since the Mesozoic era, is often referred to as a “living fossil” (Jacobs and Browner, 2000). Originally native to China, it spans regions such as Korea, Europe, North America, New Zealand, and Argentina (Belwal et al., 2018). Characterized by its distinctive gray hue, G. biloba is a gymnosperm tree with fan-shaped leaves that emit a unique fragrance from their seeds (Strømgaard and Nakanishi, 2004). The tree’s dioecious nature of the tree stems from the separation of male and female reproductive structures into distinct trees, with leaves being the primary chemical constituent in addition to ginkgolides and bilobalide (Huh and Staba, 1992). G. biloba, known for its leaves, fruits, and bark, has been extensively used in traditional and modern medicine. G. biloba fruits promote skin health and cognitive function and have anti-inflammatory properties (Hotta et al., 2013), whereas the bark aids digestion and exerts anti-inflammatory and antimicrobial effects (Mahadevan and Park, 2008 ). In particular, G. biloba leaf extract (GBE) aids in regulating blood pressure, enhancing circulation, and providing antioxidant benefits (Cui et al., 2022a; 2022b).
GBE, which contains the major bioactive compounds, primarily comprises flavonoids, organic acids, and terpenoids (Bilia, 2002; Smith and Luo, 2004). Flavonoids, including flavones, biflavones, and flavonol glycosides, are notable secondary metabolites in plants, and their substantial pharmaceutical impact has been well-documented (Liu et al., 2022). Organic acids, primarily ginkgolic acid, exhibit allergenicity, immunotoxicity, and other undesirable properties (Jaggy and Koch, 1997). Among the terpenoids, including diterpenes, sesquiterpenes, and triterpenes, ginkgolide has emerged as the key constituent of GBE (Li et al., 2009), showing potent pharmacological effects on various human ailments. Bilobalide, a sesquiterpene, has also drawn attention due to its therapeutic effects (Huang et al., 2003; Ma et al., 2022a; 2022b; 2022c).
In particular, ginkgolides, the primary diterpene trilactones derived from GBE, have gained extensive utilization as a phytomedicine (Belwal et al., 2018; Strømgaard and Nakanishi, 2004). Numerous investigations have demonstrated that ginkgolides are an ancient herbal remedy with diverse biological and pharmaceutical effects, including neuroprotection (Cui et al., 2022a; 2022b), antioxidant properties (Liu et al., 2019), anti-inflammatory actions (Chu, 2011), and anti-tumor potential. Notably, ginkgolides have the advantages of being efficacious, affordable, culturally accepted, and associated with minimal side effects (Singh et al., 2013).
Beyond their impact on nerve and blood functions, ginkgolides and bilobalide also can influence lipid metabolism, which is a pivotal consideration in treating metabolic syndrome. Obesity has become a pandemic, ranking as the sixth most common risk factor for disease worldwide (Swinburn et al., 2011). Over the past decade, the prevalence of obesity has continued to increase, and as of 2019, obesity has been reported in 27.3% of both men and women (KOSSO, 2021). Excessive body fat mass is a serious cause of metabolic syndromes, such as type 2 diabetes mellitus (DM), cardiovascular disease, kidney disease, hypertension, non-alcoholic fatty liver disease (NAFLD), and dyslipidemia (Zalesin et al., 2011). When free fatty acid fluxes into the liver increases due to obesity, hepatic lipid accumulation increases, which leads to low HDL cholesterol, high very LDL cholesterol with LDL cholesterol, hepatic triglyceride build-up, and eventually dyslipidemia (Klop et al., 2013). Insulin resistance, dyslipidemia, and obesity are associated with lipid metabolism. Despite the multifaceted effects of lipid dysregulation, a comprehensive review is required for further in-depth investigation. Consequently, this review focuses on elucidating the intricate effects of the predominant ginkgolides, including ginkgolides A–C and bilobalide, on the nervous and endocrine systems, aiming to further influence on lipid homeostasis.
Physical and chemical properties of ginkgolides and bilobalide
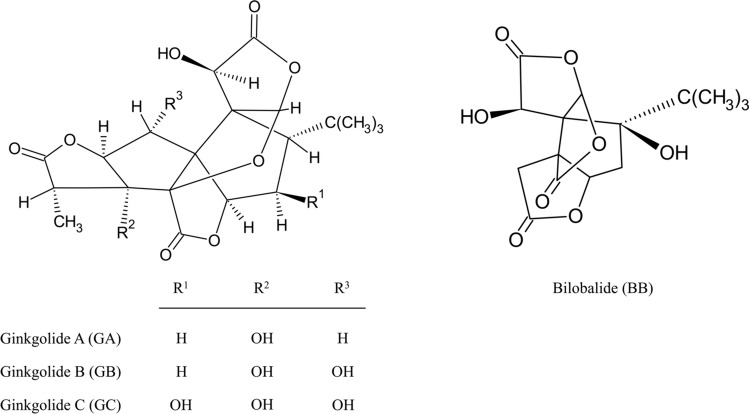
Ginkgolides consist of six rings, three lactones, and a tetrahydrofuran moiety. These constituents differ only in their hydroxyl functional group counts and placements (Biernacka et al., 2023) (Fig. 1). Ginkgolide A (C20H24O9), BN52020, is a diterpene with two secondary hydroxyl groups at C1 and C10, distinct from other ginkgolides that lack hydroxyl substitution at the 2-position (Smith et al., 1996). It is a potential platelet-activating factor (PAF) antagonist that is a pivotal mechanism governing neuroprotection and circulatory function. However, it is known to be the least potent (Sbit et al., 1987). Despite the low solubility and poor bioavailability, several studies have investigated the diverse physiological functions of ginkgolide A, such as its neuroprotective, hepatoprotective, anti-inflammation, and antioxidant effects (Strømgaard and Nakanishi, 2004; Wang et al., 2016).
Fig. 1.
The chemical structures of ginkgolides A–C and bilobalide
Ginkgolide B (C20H24O10), BN52021, is the most potent antagonist of the PAF receptor among ginkgolides (Braquet, 1997). Ginkgolide B has two hydroxyl groups on C1 and C3, similar to the sesquiterpene, bilobalide (Vogensen et al., 2003). The alkyl and alkoxy carbonyl forms of ginkgolide B has also been shown to have equivalent anti-PAF activities (Hu et al., 2000). Among them, the most active derivative of ginkgolide B was the 10-O-p-chlorobenzyl analog, which had a fourfold potency in the anti-PAF assay in comparison with other ginkgolide B derivatives (Vogensen et al., 2003). Since ginkgolide B is a natural bioactive compound used in herbal therapy, its toxicology and serious side effects have yet to be described. Owing to its fewer side effects, ginkgolide B is has attracted attention for its therapeutic and biological effects.
Ginkgolide C (C20H24O11), BN52022, is a flavone with diverse biological properties that is well established for clinical treatment in Asia (Huang et al., 2014). Ginkgolide C, similar to other diterpenoids such as ginkgolides A and B, also displays an antagonistic effect on PAF, but is 25 times less potent than ginkgolide B due to the presence of 7beta-OH (Vogensen et al., 2003). Ginkgolide C carries the highest number of hydroxyl groups of all the ginkgolides, which leads to bioactivity in rescuing several diseases caused by free radicals (Jaracz et al., 2004). In addition to these structural features, ginkgolide C functions as a potent therapeutic agent against inflammatory, neuronal, and metabolic diseases.
Bilobalide (C15H18O8) is a naturally occurring sesquiterpenoid trilactone of GBE that constitutes about 2.6–3.2% (Eisvand et al., 2020). The structure of bilobalide is similar to that of ginkgolide B; however, bilobalide has a unique tert-butyl substituent on its carbon ring (Nakanishi et al., 1971). The molecular structure of bilobalide comprises 15 carbon atoms, a configuration elucidated by Nakanishi et al. after they unraveled the ginkgolide structure (Beek, 2005). Previous studies have shown that bilobalide, similar to ginkgolides, exerts various therapeutic effects. Bilobalide possesses a PAF antagonist similar to ginkgolide B (Maerz et al., 2011). Furthermore, bilobalide protects neurons after cerebral ischemia by increasing the permeability of the blood–brain barrier (BBB) (Feng et al., 2019). In the last decade, bilobalide has long been used as an herbal medicine with neuroprotective, anti-inflammatory, antioxidant, and anti-ischemic properties. With ginkgolides, bilobalide shows high potential as a treatment for metabolic diseases.
Pharmacological effects of ginkgolides and bilobalide
Neuroprotective effects
The brain is the most important human organ that affects cognition, emotion, behavior, and judgment. Neuron, which are non-renewable basic units of the nervous system, regulates the central nervous system, memory activity, and neurotransmitter flow. Neurodegeneration is the gradual and progressive deterioration of neuronal function that results in the loss of neurons in the central nervous system (Kang et al., 2022). Neuronal loss or degeneration can lead to memory impairment, movement difficulties, cognitive defects, and emotional and behavioral problems (Solanki et al., 2016). Cognitive impairment, a worldwide health problem, is primarily attributed to a combination of factors, including genetic mutations, aging, and environmental influences (Cui et al., 2022a; 2022b). In addition, several cellular processes, including oxidative stress, mitochondrial dysfunction, acute inflammation, and neuronal apoptosis, play significant roles (Tansey et al., 2022; Parihar et al., 2008).
Oxidative stress arises from an imbalance between reactive oxygen species (ROS) and reactive nitrogen species, as well as a reduced antioxidant capacity (Persson et al., 2014). Most ROS are products of normal cell signaling in the mitochondrial respiratory chain (Jabs, 1999). Oxidative stress directly affects synaptic activity by altering metal homeostasis and antioxidant defense systems (Tönnies and Trushina, 2017). With aging, mitochondria function becomes dysregulated, thereby increasing oxidative stress. Mitochondrial dysfunction damages to mitochondrial DNA and affects nuclear DNA, particularly in the promoter region of synaptic plasticity (Lu et al., 2004). In addition, dysregulated mitochondria process the amyloid-ß protein precursor, initiating amyloid-ß accumulation (Swerdlow et al., 2014). Irregular cellular metabolism causes amyloid-ß accumulation and excess Tau protein phosphorylation, major cause of Alzheimer’s disease. These factors can exacerbate mitochondrial dysfunction and ROS production, leading to a vicious cycle (Tönnies and Trushina, 2017).
Ginkgolides and bilobalide are antioxidants used to treat neurodegeneration (Table 1). Administration of 30 mg/kg ginkgolide A for 4 days increased the activity of liver cytosolic glutathione S-transferase (GST), an important antioxidant enzyme, in vivo (Sasaki et al., 2002). Ginkgolide B is a phytotherapeutic product that has been used as an antioxidant in herbal medicines for centuries. To demonstrate the antioxidant effect of ginkgolide B, SH-SY5Y cells were subjected to oxygen–glucose deprivation (OGD) for 4 h in a glucose-free medium under hypoxic conditions. After 6 h of 0.39–50 mg/L ginkgolide B supplementation, ROS production was dose-dependently diminished in OGD-induced SH-SY5Y cells. Superoxide dismutase (SOD), a major antioxidant enzyme, significantly increased only in the 25 mg/L ginkgolide B group. In addition to SOD, ginkgolide B significantly enhanced the protein levels of hemoxyhenase-1 (HO-1) and quinone oxidoreductase1 (Nqo 1), which are important endogenous molecules involved in against oxidative stress injury (Liu et al., 2019), by up-regulating phosphorylated activated protein kinase B (PKB, AKT) and nuclear factor erythroid-2-related factor2 (Nrf2). Bilobalide 25 mg/L reduced the ROS production under oxidant stress wcia the AKT/Nrf2 pathway in OGD-exposed SH-SY5Y cells. In addition, bilobalide protects against OA by up-regulating the Nrf2/HO-1 pathway. Bilobalide 40 and 80 mg/kg also increased Nrf2/HO-1 and decreased matrix metalloproteinase-3 (MMP-3)/matrix metalloproteinase-3 (MMP-13) (Ma et al., 2022a; 2022b, 2022c). Since SOD and catalase activity were increased, bilobalide 10, 20, and 50 µM dose-dependently reduced intracellular ROS in hypoxia-induced 3T3-L1 cells (Priyanka et al., 2014). Numerous studies have demonstrated the neuroprotective and antioxidant effects of ginkgolides and bilobalide.
Table 1.
Summarized antioxidant effects of ginkgolides and bilobalide
| Components | Model | Intervention | Main results | References |
|---|---|---|---|---|
| Ginkgolide A | ddY mice | 30 mg/kg, p.o | ↑GST activity | Sasaki et al. (2002) |
| Ginkgolide B | SH-SY5Y cells | 0.39–50 mg/L |
↑ROS inhibition ↑SOD activity ↑HO-1, Nqo1 ↑p-AKT/AKT, p-Nrf2/Nrf2 |
Liu et al. (2019) |
| Bilobalide | SH-SY5Y cells | 25 mg/L |
↑SOD activity ↑HO-1, Nqo1 ↑p-AKT/AKT, p-Nrf2/Nrf2 |
Liu et al. (2019) |
| Bilobalide | New Zealand white rabbits | 40 and 80 mg/kg. i.a |
↑HO-1 ↑Nrf2 ↑MMP-3, MMP-13 |
Ma et al. (2022a; 2022b, 2022c) |
| Bilobalide | 3T3-L1 cells | 10, 20, and 50 µM |
↓ROS regeneration ↑SOD activity ↑CAT activity ↓Superoxide production |
Priyanka et al. (2014) |
GST glutathione S-transferase, ROS reactive oxygen species, SOD superoxide dismutase, HO-1 hemoxyhenase-1, Nqo1 quinone oxidoreductase1, AKT protein kinase B, Nrf2 nuclear factor erythroid-2-related factor2, MMP-3 matrix metalloproteinase-3, MMP-13 matrix metalloproteinase-13, CAT catalase
Neuroinflammation is the main cause of Alzheimer’s disease. Pro-inflammatory cytokines, including interleukin-1β (IL-1β) and the tumor necrosis factor (TNF) family, result in synaptic dysfunction, neuronal death, and prohibition of neurogenesis (Lyman et al., 2014). IL-1β increases prostaglandin E2 production, which leads to synaptic loss by releasing presynaptic glutamate (Mishra et al., 2012). In addition, TNF activates TNF receptor 1 and recruits caspase 8 if the nuclear factor kappa B (NF-κB) pathway is inhibited, leading to neuronal death (Micheau and Tschopp, 2003). Microglia, the main glial cells in the innate immune system, are macrophages found in the brain and spinal cord (Glass et al., 2010). Microglia can remodel and protect synapses from neuronal plasticity, mediated by trophic factors that contribute to memory formation (Ji et al., 2013; Parkhurst et al., 2013). Microglia tend to increase their proliferation rates in nearly all neurodegenerative conditions. Acute or chronic inflammation causes microglia to release a broad range of cytokines and chemokines, leading to neuroinflammation (Muzio et al., 2021). Anti-inflammatory effects under proper conditions are significant steps for preventing neurodegeneration via inflammatory signaling.
Numerous studies have shown that ginkgolides and bilobalide exert anti-inflammatory effects (Table 2). Ginkgolide A act as a proficient suppressor of inflammation in vitro and in vivo (Li et al., 2017). In a study, ginkgolide A attenuated serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, which can be detected in blood when the liver is destroyed by inflammation by regulating the NF-κB pathway with elevated nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBɑ) (Ye et al., 2016). Ginkgolide B modulates microglial polarization to reduce the levels of inflammatory cytokines and chemokines. Cluster of Differentiation 206 (CD206), an M2 polarization chemokine, was increased by ginkgolide B administration in mice with transient middle cerebral artery occlusion (tMCAO). The Fc gamma receptor (CD16/32), an M1 polarization chemokine, was reduced by ginkgolide B. In mRNA expression, ginkgolide B increased M2-related biomarkers, including CD206, arginase1 (Arg1), chitinase-like protein 3 (YM1), interleukin-10 (IL-10), and transforming growth factor‐beta (TGF‐β) with decreased M1-related biomarkers, including macrophage inflammatory protein-1 alpha (CCL3), inducible nitric oxide (iNOS), and TNF-ɑ (Shu et al., 2016). Ginkgolide B improves myelin generation by balancing microglia and astrocytes by reducing Toll-like receptor4 (TLR4), NF-κB, iNOS, IL-1β, and TNF-ɑ (Yin et al., 2020). Ginkgolide C also improved cerebral and myocardial-ischemia/reperfusion inflammatory damage by inhibiting TNF-ɑ, IL-1β, IL-6, and CD40/NF-κB mechanisms (Li et al., 2023; Zhang et al., 2018a; 2018b). In the case of bilobalide, bilobalide 50 µg/mL significantly reduced TNF-α, IL-1β, IL-10, and interleukin-6 (IL-6) in OGD/reperfusion-induced BV2 microglia cells. In addition, bilobalide significantly diminished the expression of TLR 2, TLR 4, and MyD88, thereby reducing the nuclear location of NF-κB (Zhou et al., 2016). Bilobalide 100 and 200 nM significantly suppressed pro-inflammatory cytokines and their mRNA expression, including TNF-α, IL-6, and IL-1β in lipopolysaccharide (LPS)-induced BV-2 microglial cells. They reduced NF-κB pathways by suppressing p-p65/p65, iNOS, and COX2 (Qin et al., 2021).
Table 2.
Summarized anti-inflammatory effects of ginkgolides and bilobalide
| Components | Model | Intervention | Main results | References |
|---|---|---|---|---|
| Ginkgolide A | C57BL/6 mice | 50, 100, and 200 mg/kg, p.o |
↓ALT, AST ↑PXR ↑IκBa |
Ye et al. (2016) |
| Ginkgolide B | C57BL/6 J mice | 1.75, 3.5, and 7 mg/kg, i.p |
↑CD206 ↓CD16/32 ↓CCL3, iNOS, TNF-ɑ ↑Arg1, YM1, IL-10, TGF-β |
Shu et al. (2016) |
| Ginkgolide B | C57BL/6 mice | 20 mg/kg, i.p |
↓TLR4, nuclear NF-κB ↓iNOS, IL-1β, TNF-ɑ |
Yin et al. (2020) |
| Ginkgolide C | Sprague–Dawley rats | 8, 16, and 32 mg/kg. i.p |
↓TNF-ɑ, IL-1β, IL-6 ↑Cytosolic NF-κB ↓p-IκBɑ/IκBɑ, IKKβ |
Li et al. (2023), Zhang et al. (2018a; 2018b) |
| Bilobalide | BV2 cells | 6.25, 12.5, 25, 50, and 100 µg/mL |
↓TNF-ɑ, IL-1β, IL-6, IL-10 ↓TLR2, TLR4, MyD88 ↓p-IkBɑ/IkBɑ, p-IKKβ/IKKβ ↓Nuclear NF-κB |
Zhou et al. (2016) |
| Bilobalide | BV2 cells | 50, 100, and 200 nM |
↓TNF-ɑ, IL-1β, IL-6 ↓Nuclear NF-κB ↓iNOS, COX2 |
Qin et al. (2021) |
ALT alanine aminotransferase, AST aspartate aminotransferase, PXR pregnane X receptor, IκBɑ nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, CD206 cluster of differentiation 206, CD16/32 Fc gamma receptor, CCL3 macrophage inflammatory protein-1 alpha, iNOS inducible nitric oxide, TNF-ɑ tumor necrosis factor-alpha, Arg1 arginase1, YM1 chitinase-like protein 3, IL-10 interleukin-10, TGF-β transforming growth factor‐beta, TLR4 toll-like receptor4, NF-κB nuclear factor-κB, IL-1β interleukin-1 beta, IL-6 interleukin-6, IKKβ IκB kinase beta, COX2 cyclooxygenase2
Cognitive impairment and cerebral ischemic stroke cause also a serious neurodegeneration in humans. Stroke is the second most common cause of dementia, which is the fourth most common cause of death (Sveinsson et al., 2014). Among the diverse stroke diseases, cerebral ischemic stroke is the third leading cause of disability and mortality (Parr et al., 2017). It is caused by a thrombus within the brain, leading to neuronal cell death, including impaired blood flow (Minnerup et al., 2012). Cerebral ischemia leads to a disruption of the blood–brain barrier (BBB), compromising its structural integrity. During this progression, the components of the BBB, such as endothelial cells featuring tight junction proteins, astrocytes, pericytes, and the basement membrane, have detrimental effects (Haley and Lawrence, 2017). Recombinant tissue plasminogen activators (rtPA), which degrades fibrin clots by catalyzing the conversion of plasminogen into plasmin, has been used to treat acute ischemic stroke (NINDS, 1995). However, rtPA can only be used in less than 5% of restricted clinical cases, with a shortened therapeutic timeframe of approximately 4.5 h (Donnan et al., 2011). Because the current treatment has these shortcomings, the necessity of herbal therapy has been in the spotlight.
One of the major neuroprotective effects of ginkgolide B is increased permeability and dysfunction of the BBB, which is a target for cerebral ischemia treatment (Table 3). In vitro, with 4 and 40 g/mL concentrations of ginkgolide B, the intracellular concentration of ginkgolide B was increased during hypoxia and reoxygenation injury by Na2S2O in endothelial cells. In addition, 15 and 30 mg/kg of ginkgolide B reduced the infarct rate and brain water content in tMCAO rats in a dose-dependent manner (Fang et al., 2010). In vitro, ginkgolide B 20 µg/mL reduced cleaved caspase 3 protein and BCL2-associated X protein (Bax)/B-cell lymphoma 2 (Bcl-2) protein in OGD/reperfusion-induced PC12 cells, proving the anti-apoptotic effects of ginkgolide B. The BBB has a protective effect. Ginkgolide B can also alleviate brain edema, which is an index of BBB integrity. Ginkgolide B at 3 and 10 mg/kg significantly diminished neurological deficit scores, cerebral edema, and water content in tMCAO rats, suggesting a protective effect against cerebral ischemia (Zhang et al., 2018a; 2018b). Ginkgolide B (5 and 10 mg/kg) significantly alleviated brain water content in rats with hypoxic-ischemic brain injury by attenuating NLR family pyrin domain containing 3 (NLRP3) activation, which can lead to increased levels of pro-inflammatory cytokines that mediates central nervous system disorders, including ischemic brain injury (Ystgaard et al., 2015; Chen et al., 2018). Ginkgolide C significantly enhanced the phosphorylation of cAMP response element-binding protein (CREB) in amyloid β-expressing neuronal cell lines. It diminished amyloid-β aggregation (Xu et al., 2007). Additionally, ginkgolide C protects against ischemia/reperfusion damage by activating HO-1, Nrf2, and CREB in vitro (Zhang et al., 2018a; 2018b). Its neuroprotective properties originate from the PI3K/AKT signaling pathway. Bilobalide also prevents ischemia-induced brain edema formation. Regarding bilobalide, 1, 3, and 10 µM of bilobalide significantly reduced water content by about 82% in rat hippocampal slices (Mdzinarishvili et al., 2007).
Table 3.
Summarized anti-ischemic effects of ginkgolides and bilobalide
| Components | Model | Intervention | Main results | References |
|---|---|---|---|---|
| Ginkgolide B |
RBME cells Wistar rats |
4 and 40 g/mL 15 and 30 mg/kg, i.v |
↓Infarct rate ↓Brain water content |
Fang et al. (2010) |
| Ginkgolide B |
PC12 cells Sprague–Dawley rats |
20 µg/mL 1, 3, and 10 mg/kg, i.v |
↓Cleaved caspase3 ↓Bax, Bcl2 ↓Neurological deficit scores ↓Cerebral edema ↓Infarct volume ↓Brain water content |
Zhang et al. (2018a; 2018b) |
| Ginkgolide B | C57BL/6 mice | 5 and 10 mg/kg, i.p |
↓Brain water content ↓NLRP3 |
Ystgaard et al. (2015) |
| Ginkgolide C | N2a cells | 10 µg/mL |
↓p-CREB/ ↓Aβ aggregation |
Xu et al. (2007) |
| Ginkgolide C | PC12 cells | 10 µmol/L |
↑Nrf2, HO-1, p-CREB/CREB ↑p-AKT/AKT |
Zhang et al. (2018a; 2018b) |
| Bilobalide | Sprague–Dawley rats | 1, 3, and 10 µM |
↓Brain water content ↓Infarct area ↓Edema area |
Mdzinarishvili et al. (2007) |
RBME rat brain microvascular endothelial, Bax BCL2 associated X protein, Bcl2 B-cell lymphoma 2, NLRP3 NLR family pyrin domain containing 3, CREB cAMP response element-binding protein, Aβ amyloid-beta
Taken together, ginkgolides and bilobalide has been shown to exert neuroprotective effects with their antioxidant and anti-inflammatory properties. Numerous in vitro and in vivo experiments have demonstrated that ginkgolides and bilobalide enhanced antioxidant enzymes and reduced pro-inflammatory cytokines. Oxidative stress and inflammatory responses play an important role not only in neurological disorders but also in the development of metabolic diseases. Thus, the physiological properties of ginkgolides and bilobalides are potentially linked to the treatment of metabolic syndrome.
Cardiovascular-protective effects with PAF antagonist
Cardiovascular disease is the primary cause of mortality, leading to approximately 17.9 million deaths annually and significant health care and economic burdens (World Health Organization, 2024; AHA, 2023). The incidence of cardiovascular disease is also increasing among young people (aged 18–50 years) in developing countries owing to the prevalence of risk factors for cardiovascular diseases, such as obesity, low physical activity, and inadequate diet (Andersson and Vasan, 2018). Cardiovascular disease refers to dysregulated heart conditions that affect the structure and function of the heart and blood vessels within the circulatory system (Balakumar et al., 2016). Therefore, improving circulatory flow is a fundamental target in cardiovascular diseases (Rijnberg et al., 2018). Risk factors for cardiovascular diseases should be modulated to ameliorate metabolic syndrome.
PAF, a pro-inflammatory phospholipid, has immunoregulatory properties. It can stimulate inflammatory signaling, including allergic responses such as elevated leukocyte adhesion, chemotaxis, and vascular permeability (Reznichenko and Korstanje, 2015). During inflammation, antigens and oxygen radicals induce endothelial cells to express PAF on their surface, including leukocyte recruitment at the inflammatory site and eventually increasing vascular permeability (Cruzado et al., 1998; Tsoupras et al., 2007). When PAF binds to its receptor (PAFR), a unique G-protein coupled with seven transmembrane receptors, multiple intracellular signaling pathways, such as inflammatory responses and blood pressure regulation, can be initiated (Honda et al., 2002; Kim et al., 2020). PAF stimulated phosphorylation of IκB via IKKɑ and IKKβ. Phosphorylated IκB activated, allowing it to enter the nucleus and induce inflammatory signals (Singh et al., 2013). Activated NF-κB produced pro-inflammatory cytokines, including IL-1, IL-6, and TNF-ɑ, stimulating macrophage M1 polarization (Liu et al., 2017). Because inflammation can destabilize and destroy atherosclerotic plaques, PAF is an important biomarker for the initiating of cardiovascular disease (Ninio et al., 2004). PAF activates atherosclerotic plaque formation by releasing inflammatory mediators and pro-inflammatory cytokines. During inflammation induced by PAF, LDL cholesterol undergoes free radical oxidation, which leads to the development of plaques and the secretion of pro-inflammatory cytokines (Ramakrishnan et al., 2017). Additionally, PAF increases platelet reactivity and stimulates plaque progression in the blood vessels (Jonge et al., 2010). Therefore, the inhibition of PAF activity in blood vessels plays a vital role in the progression of cardiovascular diseases.
Ginkgolides and bilobalide, which are widely known PAF antagonists, have drawn attention for their potential as a cardiovascular disease treatments (Harishkumar et al., 2022; Montrucchio et al., 2000) (Table 4). These compounds inhibited the binding of PAF to PAFR. Ginkgolide B is the most powerful PAF antagonist and inhibits the binding of PAF to PAFR on platelet surfaces. Ginkgolide B suppressed PAF-induced human eosinophil and neutrophil chemotaxis in a dose-dependent manner (Kurihara et al., 1989). It was found that ginkgolide B reduced TNF-ɑ, IL-1β, and iNOS in a tMCAO mice model (Gu et al., 2012). These studies demonstrated the anti-inflammatory effects of ginkgolide B, which can contribute to the inhibition of PAF activity. In vitro, ginkgolide B significantly inhibited LPS-induced NF-κB activation in rat pleural polymorphonuclear leukocytes, with TNF-ɑ reduction (Nie et al., 2004). This study suggests an underlying anti-inflammatory mechanism for ginkgolide B with NF-κB inhibition by PAF. In vivo, ginkgolide B also significantly reduced inflammatory responses, including TNF-ɑ, IL-1β, and IL-6, at both the mRNA and protein levels in the hippocampal dentate gyrus and striatum of the LPS-induced mice (Sun et al., 2021). Along with the PAF antagonist effect, ginkgolide B enhanced endothelial dysfunction in DM-induced mice through an antioxidant effect. A 20-mine ginkgolide B 10−6 M exposure significantly increased the total SOD activity in DM aortas. To clarify which SOD isoform was activated, the protein expression levels of SOD1, SOD2, and SOD3 were measured. SOD1 was significantly enhanced by ginkgolide B in DM aortas via the phosphorylation of AKT and endothelial nitric oxide synthase (eNOS). These findings suggested that ginkgolide B improves endothelial dysfunction via SOD1 activation (Taguchi et al., 2023). Ginkgolide B also attenuates cardiac dysfunction by reducing oxidative stress and PAF activity. Moreover, oral administration of 5 mg/kg ginkgolide B significantly increased SOD activity in DM-induced rats with diminished TNF-ɑ and IL-6. Additionally, it significantly reduces malondialdehyde (MDA), a product of lipid peroxidation, and increases the expression of phosphorylated AMP-activated protein kinase (AMPK), sirtuin1 (sirt1), and HO-1 (Jiang et al., 2020). Furthermore, 10 mg/kg ginkgolide B enhanced SOD activity and reduced MDA levels, with increased HO-1 mRNA expression (Lin et al., 2021).
Table 4.
Summarized anti-PAF effects of ginkgolides and bilobalide
| Components | Model | Intervention | Main results | References |
|---|---|---|---|---|
| Ginkgolide B |
Neutrophils Eosinophils |
10–9–10–4 M |
↓Neutrophil chemotaxis ↓Eosinophil chemotaxis ↓PAF binding to neutrophils and eosinophils |
Kurihara et al. (1989) |
| Ginkgolide B | ICR mice | 10, 20, and 40 mg/kg, i.v | ↓iNOS, TNF-ɑ, IL-1β | Gu et al. (2012) |
| Ginkgolide B | Peritoneal macrophages | 1 and 10 µmol/L |
↓TNF-ɑ ↓NF-kB activity |
Nie et al. (2004) |
| Ginkgolide B | C57BL/6 J | 30, 60, and 120 µmol/L, i.g | ↓TNF-ɑ, IL-1β, IL-6 | Sun et al. (2021) |
| Ginkgolide B | ICR mice | 10–9–10–6 M |
↑SOD1 activity ↑Aorta relaxation ↑p-AKT/AKT, p-eNOS/eNOS |
Taguchi et al. (2023) |
| Ginkgolide B | Sprague–Dawley rats | 5 mg/kg, p.o |
↓TNF-a, IL-6 ↑SOD activity ↓MDA ↑p-AMPK/AMPK, SIRT1, HO-1 |
Jiang et al. (2020) |
| Ginkgolide B | Sprague–Dawley rats | 10 mg/kg, i.p |
↑SOD activity ↓MDA ↑HO-1 |
Lin et al. (2021) |
| Ginkgolide C | C57BL/6 mice | 20, 30 and 40 mg/kg p.o |
↓Body weight change ↑Colon length ↓TNF-ɑ, IL-1β, IL-6, COX-2, iNOS ↓p-p65/p65, p-IκB/IκB, p-p38/p38, p-ERK1/2/ERK1/2, p-JNK1/2/JNK1/2 |
Xu et al. (2022) |
| Ginkgolide C | Chondrocytes | 0-120 µM |
↓iNOS, COX2, SOX9, MMP3, MMP13, ADAMTS4 ↑Nrf2, HO-1 ↓NF-κB activity |
Ma et al. (2022a; 2022b; 2022c) |
| Ginkgolide C | C57BL/6 J mice | 12, 24, and 48 mg/kg, i.p |
↓TNF-ɑ, IL-1β, IL-6 ↓CD40 ↓NF-κB activity |
Zhang et al. (2021) |
| Bilobalide | Wistar rats | 20 mg/kg, i.g |
↓TNF-ɑ, IL-1β, IL-6 ↓NF-κB activity |
Su et al. (2022) |
| Bilobalide | BMDM cells | 1, 3, and 10 µM |
↓TNF-ɑ, IL-1β, IL-6, IFN-γ ↓NF-κB activity, STAT |
Zhang et al. (2020) |
PAF platelet activating factor, eNOS endothelial nitric oxide synthase, MDA malondialdehyde, ERK extracellular signal-regulated kinase, JNK c-Jun N-terminal kinase, SOX9 SRY-box transcription factor 9, ADAMTS4 thrombospondin motifs 4, STAT signal transducer and activator of transcription
In addition to ginkgolide B, other ginkgolides and bilobalide have proven their PAF antagonistic properties. In the case of ginkgolide C, colitis was alleviated by the antagonistic activity of PAF. In DSS-induced ulcerative colitis animal models, the administration of ginkgolide C prevented the deterioration of body weight and colon length and reduced pro-inflammatory cytokines and enzymes through NF-κB and mitogen-activated protein kinase (MAPK) pathways (Xu et al., 2022). Furthermore, ginkgolide C treatment exerted protective effects against OA caused by chronic low-grade inflammation. This anti-inflammatory effect of the PAF antagonist was achieved via the stimulation of Nrf/HO-1, suppressing the NF-κB signaling pathway (Ma et al., 2022a; 2022b; 2022c). Ginkgolide C also improved LPS-triggered acute lung injury (ALI), significantly decreasing the expression of inflammatory cytokines such as TNF-α, IL-1β, and IL-6. The in vivo and in vitro results showed that the anti-inflammatory effect of ginkgolide C is based on downregulating CD40/NF-κB, which could be activated by PAF (Zhang et al., 2021). Bilobalide has been shown to exert protective effects on the ischemic heart through PAF antagonistic activity and PAF acetylhydrolase activation, which can degrade PAF (Maerz et al., 2011). In addition, 20 mg/kg of bilobalide reduced NF-κB p65, TNF-α, and IL-1β in STZ-induced diabetic rats (Su et al., 2022). Bilobalide 1, 3, and 10 µM significantly reduced TNF-α, IL-1β, and IL-6 in LPS and interferon-gamma (IFN-γ) induced M1-polarized BMDMs cells. Additionally, bilobalide inhibited NF-κB signaling with p-65 and signal transducer and activator protein (STAT) (Zhang et al., 2020).
Considering the increased prevalence of metabolic diseases, the regulation of PAF activity by inhibiting the inflammatory response and binding activity is crucial. In summary, ginkgolides and bilobalide are herbal medicines that exert anti-inflammatory effects by suppressing the binding of PAF to PAFR. Their anti-inflammatory and PAF modulatory effects may be associated with the treatment of metabolic diseases.
Regulation of lipid metabolism
The global prevalence of obesity has increased almost threefold since 1975, rising at an alarming rate (Blüher, 2019). Over the past decade, the prevalence of obesity has continued to increase, and as of 2019, obesity has been reported in 27.3% of both men and women (KOSSO, 2021). Obesity, commonly known as the excessive accumulation of adipose tissue, is a characterized by abnormal ectopic storage of triglycerides, contributing to a high risk of metabolic diseases, such as type 2 DM, cardiovascular diseases, and NAFLD (Neeland et al., 2019). When free fatty acid fluxes into the liver increases due to obesity, hepatic lipid accumulation increases, which leads to low HDL cholesterol, high VLDL-c with LDL cholesterol, hepatic triglyceride build-up, and eventually dyslipidemia (Klop et al., 2013). Because obesity is a critical risk factor for metabolic syndrome, the regulation of lipid metabolism is vital for disease prevention. Sarcopenic obesity, a combination disease of sarcopenia and obesity, has become a serious health problem owing to a lack of treatment. Balancing lipid metabolism is critical for attenuating sarcopenic obesity progression (Kim et al., 2023). Inflammation, which occurs in obesity and sarcopenic obesity, influences insulin resistance, energy homeostasis, and other risk factors for metabolic diseases (Saltiel and Olefsky, 2017). In obesity, macrophages become M1-polarized and produced pro-inflammatory cytokines. In addition, excessive adiposity induces the NF-κB and JNK pathways, which block insulin action (Arkan et al., 2005; Hirosumi et al., 2002). Because lipid metabolism is strongly related to inflammation, anti-inflammatory properties are important in metabolic disorders.
Notably, ginkgolides and bilobalide have powerful anti-inflammatory effects (Table 5). The anti-inflammatory properties of ginkgolide A have been observed in human coronary artery endothelial cells (HCAECs), and it effectively decreased the inflammatory response triggered by LPS. This beneficial activity appears to be related to suppressing TLR4-NF-κB signaling through the PI3K/AKT pathway. This pathway is an important regulator of lipid synthesis, including the mammalian target of rapamycin complex1 (mTORC1) and forkhead box protein1 (FOXO1) (Zhaocheng et al., 2016). In another study, ginkgolide A reduced IL-6, IL-4, and IL-13 levels and intercellular adhesion molecule-1 expression in human umbilical vein endothelial cells (HUVECs) exposed to high glucose levels. This effectiveness may be due to its ability to modulate STAT3-mediated pathways (Zhao et al., 2015). Bilobalide can also modulates inflammation. Bilobalide 15, 30, and 60 µM significantly reduced IL-β-induced pro-inflammatory cytokines, including iNOS, COX2, and MMP13 in chondrocytes. Bilobalide restored autophagic activity, decreased p-mTOR and p62, and increased LC3II dose-dependently. These results are consistent with those of in vivo studies. Bilobalide (5 and 10 mg/kg) significantly reduced iNOS and COX2 levels through the AMPK/Sirt1/mTOR pathway, which serves as a sensor of nutritional stress for regulating glucose and lipid metabolism (Liou et al., 2015) in anterior cruciate ligament transection-induced post-traumatic OA in rats (Ma et al., 2022a; 2022b; 2022c). mTOR pathway is a significant connection between inflammation and lipid metabolism. Lipotoxicity-induced inflammation by mTORC1 signaling activates NLRP3 inflammation (Ao et al., 2020). In addition, pro-inflammatory cytokines activate mTORC1 to promote lipogenic proteins, such as SREBP and PPARγ (Powell et al., 2012; Weichhart et al., 2015). Therefore, regulating inflammation by modulating the mTOR pathway is crucial for maintaining lipid metabolism homeostasis. Given that, ginkgolides and bilobalide have a chance of regulating lipid metabolism indirectly via anti-inflammatory pathways.
Table 5.
The impact of lipid homeostasis on the regulatory effects of ginkgolides and bilobalide
| Components | Model | Intervention | Main results | References |
|---|---|---|---|---|
| Ginkgolide A | HCAE cells | 10 and 20 µM |
↓TNL4 ↓NF-κB activity ↓TNF-ɑ, IL-6, IL-8, MCP-1 |
Zhaocheng et al. (2016) |
| Ginkgolide A | HUVE cells | 5, 10, 15, 20, and 30 µM |
↓IL-6, IL-4, IL-13, ICAM ↓p-STAT3 |
Zhao et al. (2015) |
| Ginkgolide A |
HepG2 cells C57BL/6 mice |
10, 50, and 100 µM 5 mg/kg, p.o |
↓Hepatocyte lipid accumulation ↓ACC ↓Body weight, epididymal fat ↓Liver TG ↓Plasma TG, TC, LDL-c ↓FAS, ACC |
Jeong et al. (2017) |
| Ginkgolide B | C57BL/6 ApoE−/− mice | 20 and 30 mg/kg, p.o |
↓Liver/body weight ↓Serum TG, TC, NEFA ↓Liver TG, TC, NEFA |
Yang et al. (2020) |
| Ginkgolide B | C57BL/6 mice | 0.1 g/100 g/diet |
↑Fecal TG ↓Abdominal WAT ↓Serum TG, total bile acid ↓SREBP1, ACC, SCD1, FAS |
Luo et al. (2017) |
| Ginkgolide B | HUVE cells | 20–100 µg/mL |
↓SREBP2, PCSK9 ↑LDLR |
Wang et al. (2019) |
| Ginkgolide C | 3T3-L1 cells | 3, 10, 30, and 100 µM |
↓PPARɑ, PPARr ↓CEBP/ɑ, CEBP/ß, SREBP1C ↑ATGL, HSL ↑Sirt1, p-AMPKa, p-ACC |
Liou et al. (2015) |
| Ginkgolide C | HepG2 cells | 3, 10, 30, and 100 µM |
↓Lipid accumulation ↓SREBP1c, PPARr, FAS ↑CPT1, PPARɑ, CD36 ↑Sirt1, p-AMPK, p-ACC |
Huang et al. (2018) |
| Bilobalide | ATDC5 cells | 7.5, 15, 30, 60, and 120 µM |
↓iNOS, COX2, MMP13 ↑ATG3, ATG4, ATG5, ATG6, ATG7, ATG9, ATG12, ATG13, ATG16, LC3II ↓p-mTOR/mTOR ↑p-AMPK/AMPK, Sirt1 |
Ma et al. (2022a; 2022b; 2022c) |
| Bilobalide | 3T3-L1 cells | 25 and 100 µM |
↓PPARγ, C/EBPɑ, SREBP1c ↓FAS, perilipin A, GLUT4 ↑ATGL, p-HSL ↑p-AMPK/AMPK ↑Adiponectin |
Bu et al. (2019) |
| Bilobalide | 3T3-L1 cells | 10, 20, and 50 µM | ↑Adiponectin | Priyanka et al. (2017) |
| Bilobalide | Wistar rats | 20 mg/kg, i.g |
↓Body weight ↓Blood glucose, HbA1c |
Su et al. (2022) |
| Bilobalide | Wistar rats | 2.5, 5, and 10 mg/kg, i.p |
↓Fasting blood glucose, HbA1C ↓Serum TG, TC, LDL-c ↑HDL-c ↓ACC ↑CPT1 |
Zhao et al. (2021) |
HCAE cells human coronary artery endothelial cells, HUVE cells human umbilical vein endothelial cells, ICAM intercellular adhesion molecule, ACC acetyl coA carboxylase, TG triglyceride, TC total cholesterol, LDL-c low-density lipoprotein-cholesterol, FAS fatty acid synthase, NEFA non-esterified fatty acid, WAT white adipose tissue, SREBP sterol regulatory element-binding protein1, SCD1 stearoyl-coA desaturase1, PCSK9 proprotein convertase subtilisin/kexin type9, LDLR LDL-c receptor, PPAR peroxisome proliferator-activating receptor, CEBP CCAAT/enhancer-binding protein, ATGL adipose triglyceride lipase, HSL hormone sensitive lipase, Sirt1 sirtuin1, AMPK AMP-activated protein kinase, CD36 cluster of differentiation36, ATG autophagy related, mTOR mammalian target of rapamycin complex, GLUT4 glucose transporter4
Despite limited research on the direct anti-obesity effects of ginkgolides and bilobalide, some studies have demonstrated their effects. Ginkgolide A attenuates NAFLD in vitro and in vivo and is directly related to fatty acid synthesis (Jeong et al., 2017). In non-esterified fatty acid (NEFA)-induced steatotic HepG2 hepatocytes, 100 µM of ginkgolide A especially diminished the area of lipid accumulation and the expression of acetyl CoA carboxylase (ACC), a de novo lipogenesis-related protein. Administration of ginkgolide A for 49 days (5 mg/kg) not only improved the phenotype of obesity and NAFLD in high-fat diet (HFD)-fed mice but also reduced the expression of lipogenesis-related proteins, such as acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS). Despite limited research on the anti-obesity effect of ginkgolide A, its physiological properties are expected to positively affect lipid metabolism based on studies on the effect of G. biloba extract on metabolic diseases (Banin et al., 2017).
For ginkgolide B, 8-week-old male C57BL/6 ApoE−/− mice were fed a 35.92% HFD for 6 weeks with ginkgolide B at 20 mg/kg (GB-L) and 30 mg/kg (GB-H) to demonstrate its anti-ferroptotic effect in HFD-induced NALFD. Liver/body weight (%) was significantly reduced in all groups. Serum triglycerides were significantly lower in the GB-H group. Total cholesterol in the serum and liver and non-esterified fatty acids in the serum were significantly reduced in all groups. Ginkgolide B regulates lipid metabolism and attenuates hepatic steatohepatitis in mice with HFD-induced NAFLD (Yang et al., 2020). In another study, C57BL/6 mice were administered a 60% high-fat diet (HFD) and 0.1/100 g of ginkgolide B for 7 weeks. The results demonstrated a significant reduction in body weight. Notably, the fecal triglyceride content significantly increased, suggesting that ginkgolide B prevented lipid absorption. Furthermore, serum triglyceride levels decreased, and total bile acid content increased. The improved lipid profiles were consistent with the downregulation of the mRNA expression levels of sterol regulatory element-binding protein1 (SREBP1), ACC, stearoyl-coA desaturase1 (SCD1), and FAS (Luo et al., 2017). These findings indicate that ginkgolide B effectively inhibited weight gain and ameliorated hypertriglyceridemia in mice with diet-induced obesity. Therefore, ginkgolide B may alleviate metabolic syndrome. Ginkgolide B is the most potent PAF antagonist among ginkgolides. In another study, ginkgolide B significantly reduced the expression of IL-1ɑ, IL-1β, and IL-6 at the mRNA and protein levels in oxidized-LDL (ox-LDL)-activated HUVECs. Moreover, ginkgolide B treatment interferes with the proprotein convertase subtilisin/kexin-9 (PCSK-9) at both the mRNA and protein levels via suppression of SREBP2 expression (Wang et al., 2019). This study established that ginkgolide B alleviates the ox-LDL-induced inflammatory cascade response and alters lipid metabolism.
In 3T3-L1 adipocytes, ginkgolide C significantly reduced adipogenesis by activating AMPK. The mRNA and protein expression of peroxisome proliferator-activating receptor alpha (PPARα), PPARγ, CCAAT/enhancer-binding protein alpha (C/EBPα), and C/EBPβ, major factors associated with adipogenesis, were significantly decreased in the ginkgolide-treated groups. Additionally, ginkgolide C activates adipose triglyceride lipase (ATGL) and hormone-sensitive lipases (HSL), resulting in lipolysis (Liou et al., 2015). Activation of the AMPK signaling pathway by ginkgolide C also inhibited lipid accumulation in HepG2 hepatocytes treated with 0.5 mM oleic acid (Huang et al., 2018). Ginkgolide C suppressed the expression of lipogenesis associated SREBP1c, PPARγ, and FAS. Furthermore, it significantly increased β-oxidation in hepatocytes by activating carnitine palmitoyltransferase1 (CPT-1), CPT-2, PPARα, and cluster of differentiation36 (CD36). Collectively, these results suggest that ginkgolide C has the potential to ameliorate excessive lipid accumulation.
Bilobalide reduces the mRNA and protein levels of some important adipogenic genes. Bilobalide 25 and 100 µM significantly suppressed intracellular lipid accumulation during 3T3-L1 differentiation. It also reduced adipogenic transcription factors, including PPARγ, C/EBPα, and SREBP1c, at both the mRNA and protein expression levels. Furthermore, bilobalide significantly suppressed the expression of adipogenic genes, such as FASN, perilipin A, and glucose transporter4 (GLUT4). Both concentrations of bilobalide increased the ATGL and p-HSL levels. Moreover, high concentrations of bilobalide promoted AMPK phosphorylation (Bu et al., 2019). These results suggested that bilobalide suppressed adipogenesis in 3T3-L1 cells. Adiponectin is a protein secreted by adipocytes and is negatively correlated with adiposity (Jung and Choi, 2014). Leptin is a product of an obesity-related gene secreted from adipocytes (Obradovic et al., 2021). Bilobalide at 10, 20, and 50 µM significantly increased adiponectin with reduced leptin in hypoxia-induced 3T3-L1 cells (Priyanka et al., 2017). These results indicate that bilobalide has the potential to improve lipid metabolism. The body weight of STZ-induced diabetic rats decreased after being fed 20 mg/kg bilobalide for 12 weeks. Additionally, bilobalide alleviated type 2 DM by lowering blood glucose and HbA1c levels (Su et al., 2022). In another study, 5 and 10 mg/kg bilobalide also reduced fasting blood glucose and HbA1c levels, respectively. Both concentrations significantly suppressed the liver triglycerides (TG), total cholesterol (TC), and LDL cholesterol levels, which are important biomarkers of lipid metabolism. Bilobalide reduced ACC and increased CPT1, a critical enzyme of β-oxidation (Zhao et al., 2021).
Taken together, all ginkgolides and bilobalide reduced de novo lipogenesis-related mRNA and protein expression in liver, such as FAS/ACC/SCD1/SREBP1c in vivo and in vitro. In white adipose tissue and adipocytes, ginkgolide C and bilobalide inhibited adipogenesis and activated lipolysis. They also enhanced ß-oxidation signaling pathways, such as CPT1/CD36/PPARa in skeletal muscle. mTORC1 acts as a key regulator of lipid homeostasis by promoting lipogenesis and adipogenesis while inhibiting a catabolic pathway including lipolysis and ß-oxidation (Ricoult and Manning, 2013). When Raptor in mTORC1 was knocked down, ATGL and HSL expression were increased in adipocytes (Chakrabarti et al., 2010). According to these lipid-modulatory pathways, ginkgolides and bilobalide appear to inhibit mTORC1, thus activating lipolysis while reducing both lipogenesis and adipogenesis. In addition, several studies proved that ginkgolides and bilobalide activated AMPK signaling pathways. AMPK is the main regulator of cellular homeostasis and regulates glucose and lipid metabolism (Carling, 2004). Activated AMPK prevents mTORC1-regulated lipid accumulation via de novo lipogenesis in hepatocytes (Li et al., 2014). FurthermAore, AMPK enhances the catabolic pathway to maintain energy status including autophagy and ß-oxidation in skeletal muscle and lipolysis by phosphorylating HSL in adipose tissue. Although there has been little research on their anti-obesity effects, the diverse pharmacological effects of ginkgolides and bilobalide seem that ginkgolides and bilobalide improved lipid homeostasis through inactivation of mTORC1 by activated AMPK.
In conclusion, G. biloba, with its rich history as an ancient "living fossil" and globally thriving presence, offers a wealth of potential in metabolic health. GBEs, containing flavonoids, organic acids, and terpenoids, such as ginkgolides and bilobalide, have shown remarkable neuroprotective and antioxidant effects. Moreover, these compounds are pivotal in regulating lipid metabolism, maintaining nerve and endocrine balance, and maintaining lipid homeostasis, all of which are critical factors in metabolic health. Ginkgolides A, B, and C, and bilobalide have unique pharmacological properties, including anti-inflammatory, antioxidant, antitumor, and neuroprotective effects. Importantly, they exhibit promise in tackling metabolic diseases, such as dyslipidemia and obesity-related complications. This holistic approach harnessing the potential of ginkgolides and bilobalide offers a promising avenue for enhancing overall metabolic well-being and presents exciting prospects for future research and therapeutic interventions in metabolic syndrome.
Funding
This study was funded by National Research Foundation of Korea, NRF-2022R1A2C1006193, Gwang-woong Go.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hayoon Kim and Sumin Kang have contributed equally to this work.
References
- American Heart Association. Heart disease and stroke statistics—2023 update. Available at: https://professional.heart.org/en/science-news/heart-disease-and-stroke-statistics-2023-update. Accessed Feb 12, 2024.
- Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nature Reviews Cardiology. 15: 230-240 (2018) 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- Ao N, Ma Z, Yang J, Jin S, Zhang K, Luo E, Du J. Liraglutide ameliorates lipotoxicity-induced inflammation through the mTORC1 signalling pathway. Peptides. 133: 170375 (2020) 10.1016/j.peptides.2020.170375 [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nature Medicine. 11: 191-198 (2005) 10.1038/nm1185 [DOI] [PubMed] [Google Scholar]
- Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacological Research. 113: 600-609 (2016) 10.1016/j.phrs.2016.09.040 [DOI] [PubMed] [Google Scholar]
- Banin RM, de Andrade IS, Cerutti SM, Oyama LM, Telles MM, Ribeiro EB. Ginkgo biloba Extract (GbE) stimulates the hypothalamic serotonergic system and attenuates obesity in ovariectomized rats. Frontiers in Pharmacology. 8: 605 (2017) 10.3389/fphar.2017.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belwal T, Giri L, Bahukhandi A, Kewlani P, Bhatt I, Rawal R. Ginkgo biloba. In: Belwal T, Giri L, Bahukhandi A, Kewlani P, Bhatt I, Rawal R. (Eds) Nonvitamin and nonmineral nutritional supplements. Elsevier Inc., India (2018) [Google Scholar]
- Biernacka P, Adamska I, Felisiak K. The potential of ginkgo biloba as a source of biologically active compounds—a review of the recent literature and patents. Molecules. 28: 3993 (2023) 10.3390/molecules28103993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilia AR. Ginkgo biloba L. Fitoterapia. 73: 276-279 (2002) 10.1016/S0367-326X(02)00071-0 [DOI] [PubMed] [Google Scholar]
- Blüher M. Obesity: global epidemiology and pathogenesis. Nature Reviews Endocrinology. 15: 288-298 (2019) 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- Braquet PG. Platelet-activating factor and its antagonists: scientific background and clinical applications of ginkgolides. In: Hori T, Ridge RW, Tulecke W, Del Tredici P, Trémouillaux-Guiller J, Tobe H (Eds) Ginkgo biloba A global treasure: from biology to Medicine. Springer, Tokyo, pp. 359-369 (1997) [Google Scholar]
- Bu S, Yuan CY, Xue Q, Chen Y, Cao F. Bilobalide suppresses adipogenesis in 3T3-L1 adipocytes via the AMPK signaling pathway. Molecules. 24: 3503 (2019) 10.3390/molecules24193503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends in Biochemical Sciences. 29: 18-24 (2004) 10.1016/j.tibs.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 59: 775-781 (2010) 10.2337/db09-1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Xu Y, Yuan J. Ginkgolide B ameliorates NLRP3 inflammasome activation after hypoxic-ischemic brain injury in the neonatal male rat. International Journal of Developmental Neuroscience. 69: 106-111 (2018) 10.1016/j.ijdevneu.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Chu X, Ci X, He J, Wei M, Yang X, Cao Q, Li H, Guan S, Deng Y, Pang D, Deng X. A novel anti-inflammatory role for ginkgolide B in asthma via inhibition of the ERK/MAPK signaling pathway. Molecules. 16: 7634-7648 (2011) 10.3390/molecules16097634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzado JM, Torras J, Riera M, Lloberas N, Herrero I, Condom E, Martorell J, Alsina J, Grinyó JM. Effect of a platelet-activating factor (PAF) receptor antagonist on hyperacute xenograft rejection; evaluation in a pig kidney-human blood xenoperfusion model. Clinical and Experimental Immunology. 113: 136-144 (1998) 10.1046/j.1365-2249.1998.00634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Wang X, Su Y, Gong C, Zhang D, Ouyang Z, Wang X. Responses of tree growth, leaf area and physiology to pavement in ginkgo biloba and platanus orientalis. Frontiers in Plant Science. 13: 1003266 (2022a) 10.3389/fpls.2022.1003266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F, Nawaz H, Kim HK, Go GW. A study on the duration of ginkgo biloba extract effective in improving cognitive function in the elderly: a systematic review and meta-analysis. Korean Society of Food Science and Technology. 54: 403-413 (2022b) [Google Scholar]
- Jonge P, Rosmalen JG, Kema IP, Doornbos B, van Melle JP, Pouwer F, Kupper N. Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: a critical review of the literature. Neuroscience and Biobehavioral Reviews. 35: 84-90 (2010) 10.1016/j.neubiorev.2009.11.025 [DOI] [PubMed] [Google Scholar]
- Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nature Reviews Neurology. 7: 400-409 (2011) 10.1038/nrneurol.2011.89 [DOI] [PubMed] [Google Scholar]
- Eisvand F, Razavi BM, Hosseinzadeh H. The effects of Ginkgo biloba on metabolic syndrome: a review. Phytotherapy Research. 34: 1798-1811 (2020) 10.1002/ptr.6646 [DOI] [PubMed] [Google Scholar]
- Fang W, Deng Y, Li Y, Shang E, Fang F, Lv P, Bai L, Qi Y, Yan F, Mao L. Blood brain barrier permeability and therapeutic time window of ginkgolide B in ischemia-reperfusion injury. European Journal of Pharmaceutical Sciences. 39: 8-14 (2010) 10.1016/j.ejps.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Feng Z, Sun Q, Chen W, Bai Y, Hu D, Xie X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: a literature review. Molecular Medicine. 25: 57 (2019) 10.1186/s10020-019-0125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 140: 918-934 (2010) 10.1016/j.cell.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JH, Ge JB, Li M, Wu F, Zhang W, Qin ZH. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. European Journal of Pharmaceutical Sciences. 47: 652-660 (2012) 10.1016/j.ejps.2012.07.016 [DOI] [PubMed] [Google Scholar]
- Haley MJ, Lawrence CB. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. Journal of Cerebral Blood Flow and Metabolism. 37: 456-470 (2017) 10.1177/0271678X16629976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harishkumar R, Hans S, Stanton JE, Grabrucker AM, Lordan R, Zabetakis I. Targeting the platelet-activating factor receptor (PAF-R): antithrombotic and anti-atherosclerotic nutrients. Nutrients. 14: 4414 (2022) 10.3390/nu14204414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 420: 333-336 (2002) 10.1038/nature01137 [DOI] [PubMed] [Google Scholar]
- Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. Journal of Biochemistry. 131: 773-779 (2002) 10.1093/oxfordjournals.jbchem.a003164 [DOI] [PubMed] [Google Scholar]
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. European Journal of Dermatology. 23: 548-549 (2013) 10.1684/ejd.2013.2102 [DOI] [PubMed] [Google Scholar]
- Hu L, Chen Z, Xie Y, Jiang Y, Zhen H. Alkyl and alkoxycarbonyl derivatives of ginkgolide B: synthesis and biological evaluation of PAF inhibitory activity. Bioorganic & Medicinal Chemistry. 8: 1515-1521 (2000) 10.1016/S0968-0896(00)00085-7 [DOI] [PubMed] [Google Scholar]
- Huang P, Zhang L, Chai C, Qian XC, Li W, Li JS, Di LQ, Cai BC. Effects of food and gender on the pharmacokinetics of ginkgolides A, B, C and bilobalide in rats after oral dosing with ginkgo terpene lactones extract. Journal of Pharmaceutical and Biomedical Analysis. 100: 138-144 (2014) 10.1016/j.jpba.2014.07.030 [DOI] [PubMed] [Google Scholar]
- Huang SH, Duke RK, Chebib M, Sasaki K, Wada K, Johnston GA. Bilobalide, a sesquiterpene trilactone from ginkgo biloba, is an antagonist at recombinant α1β2γ2L GABA(A) receptors. European Journal of Pharmacology. 464: 1-8 (2003) 10.1016/S0014-2999(03)01344-X [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen YL, Liu HC, Wu SJ, Liou CJ. Ginkgolide C reduced oleic acid-induced lipid accumulation in HepG2 cells. Saudi Pharmaceutical Journal. 26: 1178-1184 (2018) 10.1016/j.jsps.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh H, Staba EJ. The botany and chemistry of Ginkgo biloba L. Journal of Herbs, Spices & Medicinal Plants. 1: 91-124 (1992) 10.1300/J044v01n01_10 [DOI] [Google Scholar]
- Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochemical Pharmacology. 57: 231-245 (1999) 10.1016/S0006-2952(98)00227-5 [DOI] [PubMed] [Google Scholar]
- Jacobs BP, Browner WS. Ginkgo biloba: a living fossil. American Journal of Medicine. 108: 341-342 (2000) 10.1016/S0002-9343(00)00290-4 [DOI] [PubMed] [Google Scholar]
- Jaggy H, Koch E. Chemistry and biology of alkylphenols from Ginkgo biloba L. Pharmazie. 52: 735-738 (1997) [PubMed] [Google Scholar]
- Jaracz S, Nakanishi K, Jensen AA, Strømgaard K. Ginkgolides and glycine receptors: a structure-activity relationship study. Chemistry. 10: 1507-1518 (2004) 10.1002/chem.200305473 [DOI] [PubMed] [Google Scholar]
- Jeong HS, Kim KH, Lee IS, Park JY, Kim Y, Kim KS, Jang HJ. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomedicine & Pharmacotherapy. 88: 625-634 (2017) 10.1016/j.biopha.2017.01.114 [DOI] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. Public Library of Science. 8: e56293 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YX, Li W, Wang J, Wang GG. Cardiac dysfunction is attenuated by ginkgolide B via reducing oxidative stress and fibrosis in diabetic rats. Iranian Journal of Basic Medical Sciences. 23: 1078-1084 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. International Journal of Molecular Science. 15: 6184-6223 (2014) 10.3390/ijms15046184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EY, Cui F, Kim HK, Nawaz H, Kang S, Kim H, Jang J, Go G-w. Effect of phosphatidylserine on cognitive function in the elderly: a systematic review and meta-analysis. Korean Society of Food Science and Technology. 54: 52-58 (2022) [Google Scholar]
- Kim RR, Chen Z, Mann TJ, Bastard K, Scott K, Church WB. Structural and functional aspects of targeting the secreted human group IIA phospholipase A2. Molecules. 25: 4459 (2020) 10.3390/molecules25194459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Ki SW, Kim H, Kang S, Kim H, Go GW. Recent advances in nutraceuticals for the treatment of sarcopenic obesity. Nutrients. 15: 3854 (2023) 10.3390/nu15173854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 5: 1218-1240 (2013) 10.3390/nu5041218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korean Society for the Study of Obesity. 2021 Obesity fact sheet. Available at: https://www.kosso.or.kr/popup/obesity_fact_sheet.html. Accessed Feb 12, 2024.
- Kurihara K, Wardlaw AJ, Moqbel R, Kay AB. Inhibition of platelet-activating factor (PAF)-induced chemotaxis and PAF binding to human eosinophils and neutrophils by the specific ginkgolide-derived PAF antagonist, BN 52021. Journal of Allergy Clinical Immunology. 83: 83-90 (1989) 10.1016/0091-6749(89)90480-6 [DOI] [PubMed] [Google Scholar]
- Li B, Zhang B, Li Z, Li S, Li J, Wang A, Hou J, Xu J, Zhang R. Ginkgolide C attenuates cerebral ischemia/reperfusion-induced inflammatory impairments by suppressing CD40/NF-κB pathway. Journal of Ethnopharmacology. 312: 116537 (2023) 10.1016/j.jep.2023.116537 [DOI] [PubMed] [Google Scholar]
- Li H, Min Q, Ouyang C, Lee J, He C, Zou MH, Xie Z. AMPK activation prevents excess nutrient-induced hepatic lipid accumulation by inhibiting mTORC1 signaling and endoplasmic reticulum stress response. Biochimica et Biophysica Acta. 1842: 1844-1854 (2014) 10.1016/j.bbadis.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharmaceutical Research. 26: 872-882 (2009) 10.1007/s11095-008-9788-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu Y, Yao X, Hao F, Yu C, Bao Y, Wu Y, Song Z, Sun Y, Zheng L, Wang G, Huang Y, Sun L, Li Y. Ginkgolide A ameliorates LPS-induced inflammatory responses in vitro and in vivo. International Journal of Molecular Science. 18: 794 (2017) 10.3390/ijms18040794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Wu H, Zhou Z, Tao Z, Jia T, Gao W. Ginkgolide B improves multiterritory perforator flap survival by inhibiting endoplasmic reticulum stress and oxidative stress. Journal of Investigate Surgery. 34: 610-616 (2021) 10.1080/08941939.2019.1676483 [DOI] [PubMed] [Google Scholar]
- Liou CJ, Lai XY, Chen YL, Wang CL, Wei CH, Huang WC. Ginkgolide C suppresses adipogenesis in 3T3-L1 adipocytes via the AMPK signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2015: 298635 (2015) 10.1155/2015/298635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Jin Z, Xu Z, Yang H, Li L, Li G, Li F, Gu S, Zong S, Zhou J, Cao L, Wang Z, Xiao W. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 24: 441-452 (2019) 10.1007/s12192-019-00977-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2: 17023 (2017) 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XG, Lu X, Gao W, Li P, Yang H. Structure, synthesis, biosynthesis, and activity of the characteristic compounds from Ginkgo biloba L. Natural Product Reports. 39: 474-511 (2022) 10.1039/D1NP00026H [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 429: 883-891 (2004) 10.1038/nature02661 [DOI] [PubMed] [Google Scholar]
- Luo L, Li Y, Wang D, Zhao Y, Wang Y, Li F, Fang J, Chen H, Fan S, Huang C. Ginkgolide B lowers body weight and ameliorates hepatic steatosis in high-fat diet-induced obese mice correlated with pregnane X receptor activation. RSC Advances. 7: 37858-37866 (2017) 10.1039/C7RA05621D [DOI] [Google Scholar]
- Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neuroscience Reports. 79: 1-12 (2014) [DOI] [PubMed] [Google Scholar]
- Ma T, Chen H, Ruan H, Lv L, Yu Y, Jia L, Zhao J, Li X, Zang Y, Xu X, Zhang J, Gao L. Natural product, bilobalide, improves joint health in rabbits with osteoarthritis by anti-matrix degradation and antioxidant activities. Frontiers in Veterinary Science. 9: 1034623 (2022a) 10.3389/fvets.2022.1034623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Jia L, Zhao J, Lv L, Yu Y, Ruan H, Song X, Chen H, Li X, Zhang J, Gao L. Ginkgolide C slows the progression of osteoarthritis by activating Nrf2/HO-1 and blocking the NF-κB pathway. Frontiers in Pharmacology. 13: 1027553 (2022b) 10.3389/fphar.2022.1027553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Lv L, Yu Y, Jia L, Song X, Xu X, Li T, Sheng X, Wang H, Zhang J, Gao L. Bilobalide exerts anti-inflammatory effects on chondrocytes through the AMPK/SIRT1/mTOR pathway to attenuate ACLT-induced post-traumatic osteoarthritis in rats. Frontiers in Pharmacology. 13: 783506 (2022c) 10.3389/fphar.2022.783506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerz S, Liu CH, Guo W, Zhu YZ. Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes and the involvement of the platelet-activating factor receptor. Bioscience Reports. 31: 439-447 (2011) 10.1042/BSR20100128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. Journal of Food Science. 73: R14-R19 (2008) 10.1111/j.1750-3841.2007.00597.x [DOI] [PubMed] [Google Scholar]
- Mdzinarishvili A, Kiewert C, Kumar V, Hillert M, Klein J. Bilobalide prevents ischemia-induced edema formation in vitro and in vivo. Neuroscience. 144: 217-222 (2007) 10.1016/j.neuroscience.2006.08.037 [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 114: 181-190 (2003) 10.1016/S0092-8674(03)00521-X [DOI] [PubMed] [Google Scholar]
- Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: current status and future perspectives. International Journal of Molecular Sciences. 13: 11753-11772 (2012) 10.3390/ijms130911753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Kim HJ, Shin AH, Thayer SA. Synapse loss induced by interleukin-1β requires pre- and post-synaptic mechanisms. Journal of Neuroimmune Pharmacology. 7: 571-578 (2012) 10.1007/s11481-012-9342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiological Reviews. 80: 1669-1699 (2000) 10.1152/physrev.2000.80.4.1669 [DOI] [PubMed] [Google Scholar]
- Muzio L, Viotti A, Martino G. Microglia in neuroinflammation and neurodegeneration: from understanding to therapy. Frontiers in Neuroscience. 15: 742065 (2021) 10.3389/fnins.2021.742065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Habaguchi K, Nakadaira Y, Woods MC, Maruyama M, Major RT, Alauddin M, Patel AR, Weinges K, Baehr W. Structure of bilobalide, a rare tert-butyl containing sesquiterpenoid related to the C20-ginkgolides. Journal of the American Chemical Society. 93: 3544-3546 (1971) 10.1021/ja00743a051 [DOI] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. New England Journal of Medicine. 333: 1581-1587 (1995) 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- Neeland IJ, Ross R, Després J-P, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart JC, Eckel RH. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. The lancet Diabetes & endocrinology. 7: 715-725 (2019) 10.1016/S2213-8587(19)30084-1 [DOI] [PubMed] [Google Scholar]
- Nie ZG, Peng SY, Wang WJ. Effects of ginkgolide B on lipopolysaccharide-induced TNFalpha production in mouse peritoneal macrophages and NF-kappaB activation in rat pleural polymorphonuclear leukocytes. Yao Xue Xue Bao. 39: 415-418 (2004) [PubMed] [Google Scholar]
- Ninio E, Tregouet D, Carrier JL, Stengel D, Bickel C, Perret C, Rupprecht HJ, Cambien F, Blankenberg S, Tiret L. Platelet-activating factor-acetylhydrolase and PAF-receptor gene haplotypes in relation to future cardiovascular event in patients with coronary artery disease. Human molecular genetics. 13: 1341-1351 (2004) 10.1093/hmg/ddh145 [DOI] [PubMed] [Google Scholar]
- Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, Gojobori T, Isenovic ER. Leptin and obesity: role and clinical implication. Frontiers in Endocrinology. 12: 585887 (2021) 10.3389/fendo.2021.585887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palur Ramakrishnan AV, Varghese TP, Vanapalli S, Nair NK, Mingate MD. Platelet-activating factor: a potential biomarker in acute coronary syndrome? Cardiovascular Therapy. 35: 64-70 (2017) 10.1111/1755-5922.12233 [DOI] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cellular and Molecular Life Science. 65: 1272-1284 (2008) 10.1007/s00018-008-7589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 155: 1596-1609 (2013) 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr E, Ferdinand P, Roffe C. Management of acute stroke in the older person. Geriatrics (Basel). 2: 27 (2017) 10.3390/geriatrics2030027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson T, Popescu BO, Cedazo-Minguez A. Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxidative Medicine and Cellular Longevity. 2014: 427318 (2014) 10.1155/2014/427318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune response by mTOR. Annual Review of Immunology. 30: 39-68 (2012) 10.1146/annurev-immunol-020711-075024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyanka A, Nisha VM, Anusree SS, Raghu KG. Bilobalide attenuates hypoxia induced oxidative stress, inflammation, and mitochondrial dysfunctions in 3T3-L1 adipocytes via its antioxidant potential. Free Radical Research. 48: 1206-1217 (2014) 10.3109/10715762.2014.945442 [DOI] [PubMed] [Google Scholar]
- Priyanka A, Sindhu G, Shyni GL, Preetha Rani MR, Nisha VM, Raghu KG. Bilobalide abates inflammation, insulin resistance and secretion of angiogenic factors induced by hypoxia in 3T3-L1 adipocytes by controlling NF-κB and JNK activation. International Immunopharmacology. 42: 209-217 (2017) 10.1016/j.intimp.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Qin YR, Ma CQ, Wang DP, Zhang QQ, Liu MR, Zhao HR, Jiang JH, Fang Q. Bilobalide alleviates neuroinflammation and promotes autophagy in Alzheimer’s disease by upregulating lincRNA-p21. American Journal of Translational Research. 13: 2021-2040 (2021) [PMC free article] [PubMed] [Google Scholar]
- Reznichenko A, Korstanje R. The role of platelet-activating factor in mesangial pathophysiology. Americal Journal of Pathology. 185: 888-896 (2015) 10.1016/j.ajpath.2014.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricoult SJH, Manning BD. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Reports. 14: 242-251 (2013) 10.1038/embor.2013.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnberg FM, Hazekamp MG, Wentzel JJ, de Koning PJH, Westenberg JJM, Jongbloed MRM, Blom NA, Roest AAW. Energetics of blood flow in cardiovascular disease: concept and clinical implications of adverse energetics in patients with a Fontan circulation. Circulation. 137: 2393-2407 (2018) 10.1161/CIRCULATIONAHA.117.033359 [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. Journal of Clinical Investigation. 127(1): 1-4 (2017) 10.1172/JCI92035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Hatta S, Wada K, Ueda N, Yoshimura T, Endo T, Sakata M, Tanaka T, Haga M. Effects of extract of Ginkgo biloba leaves and its constituents on carcinogen-metabolizing enzyme activities and glutathione levels in mouse liver. Life Science. 70: 1657-1667 (2002) 10.1016/S0024-3205(01)01557-0 [DOI] [PubMed] [Google Scholar]
- Sbit M, Dupont L, Dideberg O, Braquet P. Structure of ginkgolide A (BN52020) monohydrate and ginkgolide C (BN52022).Ethanol.1·5 hydrate, isolated from Ginkgo biloba L. Acta Crystallographica Section C. 43: 2377-2381 (1987) [Google Scholar]
- Shu ZM, Shu XD, Li HQ, Sun Y, Shan H, Sun XY, Du RH, Lu M, Xiao M, Ding JH, Hu G. Ginkgolide B protects against ischemic stroke via modulating microglia polarization in mice. CNS Neuroscience Therapy. 22: 729-739 (2016) 10.1111/cns.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Singh IN, Mondal SC, Singh L, Garg VK. Platelet-activating factor (PAF)-antagonists of natural origin. Fitoterapia. 84: 180-201 (2013) 10.1016/j.fitote.2012.11.002 [DOI] [PubMed] [Google Scholar]
- Smith JV Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Applied Microbiology and Biotechnology. 64: 465-472 (2004) 10.1007/s00253-003-1527-9 [DOI] [PubMed] [Google Scholar]
- Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). Journal of Ethnopharmacology. 50: 131-139 (1996) 10.1016/0378-8741(96)01379-7 [DOI] [PubMed] [Google Scholar]
- Solanki I, Parihar P, Parihar MS. Neurodegenerative diseases: from available treatments to prospective herbal therapy. Neurochemistry International. 95: 100-108 (2016) 10.1016/j.neuint.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Strømgaard K, Nakanishi K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angewandte Chemie International Edition. 43:1640-1658 (2004) 10.1002/anie.200300601 [DOI] [PubMed] [Google Scholar]
- Su Q, Dong J, Zhang D, Yang L, Roy R. Protective effects of the bilobalide on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Applied Biochemistry and Biotechnology. 194: 6407-6422 (2022) 10.1007/s12010-022-04012-5 [DOI] [PubMed] [Google Scholar]
- Sun M, Sheng Y, Zhu Y. Ginkgolide B alleviates the inflammatory response and attenuates the activation of LPS-induced BV2 cells in vitro and in vivo. Experimental and Therapeutic Medicine. 21: 586 (2021) 10.3892/etm.2021.10018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveinsson OA, Kjartansson O, Valdimarsson EM. Cerebral ischemia/infarction—epidemiology, causes and symptoms. Laeknabladid. 100: 271-279 (2014) [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochimica et Biophysica Acta. 1842: 1219-1231 (2014) 10.1016/j.bbadis.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 378: 804-814 (2011) 10.1016/S0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- Taguchi K, Okudaira K, Matsumoto T, Kobayashi T. Ginkgolide B caused the activation of the Akt/eNOS pathway through the antioxidant effect of SOD1 in the diabetic aorta. Pfügers Archiv European Journal of Physiology. 475: 453-463 (2023) 10.1007/s00424-023-02790-3 [DOI] [PubMed] [Google Scholar]
- Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nature Reviews Immunology. 22: 657-673 (2022) 10.1038/s41577-022-00684-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönnies E, Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. Journal of Alzheimers Disease. 57: 1105-1121 (2017) 10.3233/JAD-161088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoupras AB, Fragopoulou E, Nomikos T, Iatrou C, Antonopoulou S, Demopoulos CA. Characterization of the de novo biosynthetic enzyme of platelet-activating factor, DDT-insensitive choline phosphotransferase, of human mesangial cells. Mediators of Inflammation. 2007: 27683 (2007) 10.1155/2007/27683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beek TA. Ginkgolides and bilobalide: their physical, chromatographic and spectroscopic properties. Bioorganic & Medical Chemistry. 13: 5001-5012 (2005) 10.1016/j.bmc.2005.05.056 [DOI] [PubMed] [Google Scholar]
- Vogensen SB, Strømgaard K, Shindou H, Jaracz S, Suehiro M, Ishii S, Shimizu T, Nakanishi K. Preparation of 7-substituted ginkgolide derivatives: potent platelet activating factor (PAF) receptor antagonists. Journal of Medical Chemistry. 46: 601-608 (2003) 10.1021/jm0203985 [DOI] [PubMed] [Google Scholar]
- Wang G, Liu Z, Li M, Li Y, Alvi SS, Ansari IA, Khan MS. Ginkgolide B mediated alleviation of inflammatory cascades and altered lipid metabolism in HUVECs via targeting PCSK-9 expression and functionality. BioMed Research International. 2019: 7284767 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ouyang B, Aa J, Geng J, Fei F, Wang P, Wang J, Peng Y, Geng T, Li Y, Huang W, Wang Z, Xiao W, Wang G. Pharmacokinetices and tissue distribution of ginkgolide A, ginkgolide B, and ginkgolide K after intravenous infusion of ginkgo diterpene lactones in a rat model. Journal of Pharmaceutical and Biomedical Analysis. 126: 109-116 (2016) 10.1016/j.jpba.2016.04.035 [DOI] [PubMed] [Google Scholar]
- Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nature Reviews Immunology. 15: 599-614 (2015) 10.1038/nri3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Cardiovascular diseases. Available at: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1. Accessed Feb 12, 2024.
- Xu D, Zhuang L, Gao S, Ma H, Cheng J, Liu J, Liu D, Fu S, Hu G. Orally administered ginkgolide C attenuates DSS-induced colitis by maintaining gut barrier integrity, inhibiting inflammatory responses, and regulating intestinal flora. Journal of Agricultural and Food Chemistry. 70: 14718-14731 (2022) 10.1021/acs.jafc.2c06177 [DOI] [PubMed] [Google Scholar]
- Xu Y, Cui C, Pang C, Christen Y, Luo Y. Restoration of impaired phosphorylation of cyclic AMP response element-binding protein (CREB) by EGb 761 and its constituents in Abeta-expressing neuroblastoma cells. European Journal of Neuroscience. 26: 2931-2939 (2007) 10.1111/j.1460-9568.2007.05905.x [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen J, Gao Q, Shan X, Wang J, Lv Z. Study on the attenuated effect of ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 445: 152599 (2020) 10.1016/j.tox.2020.152599 [DOI] [PubMed] [Google Scholar]
- Ye N, Wang H, Hong J, Zhang T, Lin C, Meng C. PXR mediated protection against liver inflammation by ginkgolide A in tetrachloromethane treated mice. Biomolecules & Therapeutics. 24: 40-48 (2016) 10.4062/biomolther.2015.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, He Y, An J, Miao Q, Sui RX, Wang Q, Yu JZ, Xiao BG, Ma CG. Dynamic balance of microglia and astrocytes involved in the remyelinating effect of ginkgolide B. Frontiers in Cellular Neuroscience. 13: 572 (2020) 10.3389/fncel.2019.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystgaard MB, Sejersted Y, Løberg EM, Lien E, Yndestad A, Saugstad OD. Early upregulation of NLRP3 in the brain of neonatal mice exposed to hypoxia-ischemia: No early neuroprotective effects of NLRP3 deficiency. Neonatology. 108: 211-219 (2015) 10.1159/000437247 [DOI] [PubMed] [Google Scholar]
- Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Medical Clinics of North America. 95: 919-937 (2011) 10.1016/j.mcna.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Zhang H, Cao N, Yang Z, Fang X, Yang X, Li H, Hong Z, Ji Z. Bilobalide alleviated dextran sulfate sodium-induced experimental colitis by inhibiting M1 macrophage polarization through the NF-κB signaling pathway. Frontiers in Pharmacology. 11: 718 (2020) 10.3389/fphar.2020.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Guo N, Yan G, Wang Q, Gao T, Zhang B, Hou N. Ginkgolide C attenuates lipopolysaccharide-induced acute lung injury by inhibiting inflammation via regulating the CD40/NF-κB signaling pathway. International Journal of Molecular Medicine. 47: 62 (2021) 10.3892/ijmm.2021.4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Han D, Li Z, Shen C, Zhang Y, Li J, Yan G, Li S, Hu B, Li J, Liu P. Ginkgolide C alleviates myocardial ischemia/reperfusion-induced inflammatory injury via inhibition of CD40-NF-κB Pathway. Frontiers in Pharmacology. 9: 109 (2018a) 10.3389/fphar.2018.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Song JK, Yan R, Li L, Xiao ZY, Zhou WX, Wang ZZ, Xiao W, Du GH. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacologica Sinica. 39: 1259-1272 (2018b) 10.1038/aps.2017.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Qin J, Shen W, Wu A. Bilobalide enhances AMPK activity to improve liver injury and metabolic disorders in STZ-induced diabetes in immature rats via regulating HMGB1/TLR4/NF-κB signaling pathway. BioMed Research International. 2021: 8835408 (2021) 10.1155/2021/8835408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Gao C, Cui Z. Ginkgolide A reduces inflammatory response in high-glucose-stimulated human umbilical vein endothelial cells through STAT3-mediated pathway. International Immunopharmacology. 25: 242-248 (2015) 10.1016/j.intimp.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Zhaocheng J, Jinfeng L, Luchang Y, Yequan S, Feng L, Kai W. Ginkgolide A inhibits lipopolysaccharide-induced inflammatory response in human coronary artery endothelial cells via downregulation of TLR4-NF-κB signaling through PI3K/Akt pathway. Pharmazie. 71: 588-591 (2016) [DOI] [PubMed] [Google Scholar]
- Zhou JM, Gu SS, Mei WH, Zhou J, Wang ZZ, Xiao W. Ginkgolides and bilobalide protect BV2 microglia cells against OGD/reoxygenation injury by inhibiting TLR2/4 signaling pathways. Cell Stress Chaperones. 21: 1037-1053 (2016) 10.1007/s12192-016-0728-y [DOI] [PMC free article] [PubMed] [Google Scholar]