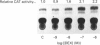Abstract
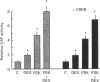
The somatostatin (SS) gene is transcriptionally regulated via the cyclic AMP (cAMP) response element (CRE), located in the proximal promoter (-41 to -48 bp). We have previously reported that glucocorticoids induce dose-dependent cell-specific alterations in the steady-state SS mRNA level. Here we have investigated direct transcriptional control of the SS gene by glucocorticoids. We have examined transcriptional interaction between glucocorticoids and the cAMP signalling pathway and mapped the 5' upstream regulatory region of the SS gene involved in glucocorticoid transactivation. Transcriptional regulation was determined by analysis of chloramphenicol acetyltransferase (CAT) activity in PC12 rat pheochromocytoma cells and A126-1B2 (protein kinase A-deficient mutant PC12) cells, by acute transfection of 5' flanking SS DNA (- 750, -250 and -71 bp) ligated to the reporter (CAT) gene. Dexamethasone (DEX) induced a dose-dependent 2.2-fold stimulation of SS gene transcription in PC12 cells, but not in A126-1B2 cells. Other steroid and thyroid hormones tested, and retinoic acid, were ineffective, while cAMP and forskolin stimulated gene transcription 4-5-fold in PC12 cells but not in A126-1B2 cells. DEX exerted an additive effect on cAMP-induced gene transcription. Deletion of the promoter from -750 to -71 bp (but not from -750 to -250 bp) abolished all stimulatory effects of DEX without affecting cAMP responsiveness. Mutation of the CRE abrogated both DEX- and cAMP-dependent gene enhancement. Gel electrophoretic mobility shift assays confirmed that the -250 to -71 bp region of the SS promoter (but not the -71 to +55 bp domain) binds specifically to a glucocorticoid response element-sensitive nuclear protein(s) from PC12 cells, suggesting a putative glucocorticoid receptor interaction with SS promoter DNA. We conclude that glucocorticoids regulate SS gene transcription positively. Glucocorticoid-induced transactivation shows dependence on protein kinase. A activity, and may be mediated via protein-protein interaction between the glucocorticoid receptor and the CRE binding protein. DNA sequences upstream from the CRE between -250 and -71 bp in the SS promoter appear to be the target of glucocorticoid action.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrisani O. M., Dixon J. E. Somatostatin gene regulation. Annu Rev Physiol. 1990;52:793–806. doi: 10.1146/annurev.ph.52.030190.004045. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Chang F. H., Bourne H. R. Dexamethasone increases adenylyl cyclase activity and expression of the alpha-subunit of Gs in GH3 cells. Endocrinology. 1987 Nov;121(5):1711–1715. doi: 10.1210/endo-121-5-1711. [DOI] [PubMed] [Google Scholar]
- Chatterjee V. K., Madison L. D., Mayo S., Jameson J. L. Repression of the human glycoprotein hormone alpha-subunit gene by glucocorticoids: evidence for receptor interactions with limiting transcriptional activators. Mol Endocrinol. 1991 Jan;5(1):100–110. doi: 10.1210/mend-5-1-100. [DOI] [PubMed] [Google Scholar]
- Drouin J., Charron J., Gagner J. P., Jeannotte L., Nemer M., Plante R. K., Wrange O. Pro-opiomelanocortin gene: a model for negative regulation of transcription by glucocorticoids. J Cell Biochem. 1987 Dec;35(4):293–304. doi: 10.1002/jcb.240350404. [DOI] [PubMed] [Google Scholar]
- Drouin J., Sun Y. L., Chamberland M., Gauthier Y., De Léan A., Nemer M., Schmidt T. J. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J. 1993 Jan;12(1):145–156. doi: 10.1002/j.1460-2075.1993.tb05640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J., Campos R., Reynolds R., Stobie K., Brubaker P. L. The rat glucagon gene is regulated by a protein kinase A-dependent pathway in pancreatic islet cells. Endocrinology. 1991 Jan;128(1):394–400. doi: 10.1210/endo-128-1-394. [DOI] [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Goodman R. H. Regulation of neuropeptide gene expression. Annu Rev Neurosci. 1990;13:111–127. doi: 10.1146/annurev.ne.13.030190.000551. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granner D., O'Brien R., Imai E., Forest C., Mitchell J., Lucas P. Complex hormone response unit regulating transcription of the phosphoenolpyruvate carboxykinase gene: from metabolic pathways to molecular biology. Recent Prog Horm Res. 1991;47:319–348. doi: 10.1016/b978-0-12-571147-0.50014-7. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992 Jul 10;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Wang X. S., Milkowski D. M., Short M. L. Glucocorticoid induction of CRE-binding protein isoform mRNAs in rat C6 glioma cells. Nucleic Acids Res. 1992 Feb 25;20(4):825–829. doi: 10.1093/nar/20.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J., Serup P., Gonzalez G., Edlund T., Montminy M. The LIM family transcription factor Isl-1 requires cAMP response element binding protein to promote somatostatin expression in pancreatic islet cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6247–6251. doi: 10.1073/pnas.89.14.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P. C., Granner D. K. Hormone response domains in gene transcription. Annu Rev Biochem. 1992;61:1131–1173. doi: 10.1146/annurev.bi.61.070192.005411. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Habener J. F. Cyclic adenosine 3',5'-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993 Jun;14(3):269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou D. N., Liu J. L., Patel Y. C. Glucocorticoids regulate somatostatin peptide and steady state messenger ribonucleic acid levels in normal rat tissues and in a somatostatin-producing islet tumor cell line (1027B2). Endocrinology. 1994 May;134(5):2259–2266. doi: 10.1210/endo.134.5.7908873. [DOI] [PubMed] [Google Scholar]
- Patel S. C., Papachristou D. N., Patel Y. C. Quinolinic acid stimulates somatostatin gene expression in cultured rat cortical neurons. J Neurochem. 1991 Apr;56(4):1286–1291. doi: 10.1111/j.1471-4159.1991.tb11423.x. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Papachristou D. N., Zingg H. H., Farkas E. M. Regulation of islet somatostatin secretion and gene expression: selective effects of adenosine 3',5'-monophosphate and phorbol esters in normal islets of Langerhans and in a somatostatin-producing rat islet clonal cell line 1027 B2. Endocrinology. 1991 Apr;128(4):1754–1762. doi: 10.1210/endo-128-4-1754. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N Engl J Med. 1983 Dec 15;309(24):1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- Rogers K. V., Vician L., Steiner R. A., Clifton D. K. The effect of hypophysectomy and growth hormone administration on pre-prosomatostatin messenger ribonucleic acid in the periventricular nucleus of the rat hypothalamus. Endocrinology. 1988 Feb;122(2):586–591. doi: 10.1210/endo-122-2-586. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough D. E., Lee S. L., Dinarello C. A., Reichlin S. Interleukin-1 beta stimulates somatostatin biosynthesis in primary cultures of fetal rat brain. Endocrinology. 1989 Jan;124(1):549–551. doi: 10.1210/endo-124-1-549. [DOI] [PubMed] [Google Scholar]
- Schneider W., Shyamala G. Glucocorticoid receptors in primary cultures of mouse mammary epithelial cells: characterization and modulation by prolactin and cortisol. Endocrinology. 1985 Jun;116(6):2656–2662. doi: 10.1210/endo-116-6-2656. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo M., Miller C. P., Habener J. F. Somatostatin gene transcription regulated by a bipartite pancreatic islet D-cell-specific enhancer coupled synergetically to a cAMP response element. J Biol Chem. 1992 Jun 25;267(18):12868–12875. [PubMed] [Google Scholar]
- Vallejo M., Penchuk L., Habener J. F. Somatostatin gene upstream enhancer element activated by a protein complex consisting of CREB, Isl-1-like, and alpha-CBF-like transcription factors. J Biol Chem. 1992 Jun 25;267(18):12876–12884. [PubMed] [Google Scholar]
- Van Buskirk R., Corcoran T., Wagner J. A. Clonal variants of PC12 pheochromocytoma cells with defects in cAMP-dependent protein kinases induce ornithine decarboxylase in response to nerve growth factor but not to adenosine agonists. Mol Cell Biol. 1985 Aug;5(8):1984–1992. doi: 10.1128/mcb.5.8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Koch Y., Baldino F., Jr, Gozes I. Steroid regulation of somatostatin mRNA in the rat hypothalamus. J Biol Chem. 1988 Jun 5;263(16):7666–7671. [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]