Abstract
Background
The quick sequential [sepsis-related] organ failure assessment (qSOFA) acts as a prompt to consider possible sepsis. The contributions of individual qSOFA elements to assessment of severity and for prediction of mortality remain unknown.
Methods
A total of 3974 patients with community-acquired pneumonia were recruited to an observational prospective cohort study. The area under the receiver operating characteristic curve (AUROC), odds ratio, relative risk and Youden’s index were employed to assess discrimination.
Results
Respiratory rate ≥22/min demonstrated the most superior diagnostic value, indicated by largest odds ratio, relative risk and AUROC, and maximum Youden’s index for mortality. However, the indices for altered mentation and systolic blood pressure (SBP) ≤100 mm Hg decreased notably in turn. The predictive validities of respiratory rate ≥22/min, altered mentation and SBP ≤100 mm Hg were good, adequate and poor for mortality, indicated by AUROC (0.837, 0.734 and 0.671, respectively). Respiratory rate ≥22/min showed the strongest associations with SOFA scores, pneumonia severity index, hospital length of stay and costs. However, SBP ≤100 mm Hg was most weakly correlated with the indices.
Conclusions
Respiratory rate ≥22/min made the greatest contribution to parsimonious qSOFA to assess severity and predict mortality. However, the contributions of altered mentation and SBP ≤100 mm Hg decreased strikingly in turn. It is the first known prospective evidence of the contributions of individual qSOFA elements to assessment of severity and for prediction of mortality, which might have implications for more accurate clinical triage decisions.
Keywords: qSOFA, community-acquired pneumonia, sepsis, contribution, triage, severity, mortality
KEY MESSAGES
Respiratory rate ≥22/min demonstrated the most superior diagnostic value.
Respiratory rate ≥22/min showed the strongest association with severity.
Respiratory rate ≥22/min, altered mentation and SBP ≤100 mm Hg predicted mortality well, adequately and poorly, respectively.
Introduction
Sepsis is a common syndrome associated with high morbidity and mortality and thus is regarded as an important global health problem [1–3]. An international task force of experts redefined this syndrome as a life-threatening organ dysfunction due to a dysregulated host response to infection in the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), and organ dysfunction can be identified as an acute change in total sequential [sepsis-related] organ failure assessment (SOFA) score ≥2 points consequent to the infection. They recommend a new clinical score termed quick SOFA (qSOFA), which incorporates respiratory rate of 22/min or greater, altered mentation, and systolic blood pressure (SBP) of 100 mm Hg or less (range: 0–3; 1 point for each of the criteria), to rapidly identify adult patients with suspected infection who are likely to have poor outcomes. The task force strongly encourages prospective validation in multiple health care settings to confirm its robustness [4,5]. The qSOFA has merits according to its proponents [6,7]. However, the contributions of individual qSOFA elements to predictive validity are unclear [5]. It might further facilitate the rationalization of clinical triage decision-making and then reduce mortality much more was the unequal weight of individual qSOFA elements elucidated.
Pneumonia is the top communicable cause of death worldwide. Community-acquired pneumonia (CAP) results in great mortality and morbidity and high costs worldwide and its usual complication is sepsis [8,9]. Therefore, it is important to assess the outcome prediction ability of qSOFA in patients with pneumonia. Ranzani et al. [10] corroborated qSOFA presented better clinical usefulness for patients with CAP in the emergency department. Hence, an observational prospective cohort study of patients with CAP was conducted to determine the contributions of individual qSOFA elements to assessment of severity and for prediction of mortality.
Material and methods
Design and setting
A total of 3974 patients with CAP were recruited to an observational prospective cohort study in the Departments of Pulmonary and Critical Care Medicine in two Chinese tertiary hospitals of two universities from 1 January 2016 to 31 December 2021. The database used for the three articles published partly overlapped the current database [11–13].
Criteria for enrolment
CAP was defined as an acute infection of the pulmonary parenchyma associated with an acute infiltrate on the chest radiograph with two or more symptoms including fever (>38 °C), hypothermia (<36 °C), rigours, sweats, new cough or change in colour of respiratory secretions, chest discomfort or dyspnoea [14]. Patients younger than 18 years, recruited during the 28 days before the study, presented severe immunosuppression, active tuberculosis, or end-stage diseases, showing a written ‘do not resuscitate’ order, having COVID-19, or being unconscious before suffering from pneumonia were excluded.
Clinical management
The study was conducted based on the principles of human experimentation guidelines of the United States Department of Health and Human Services. The report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Respiratory physicians attended patients with CAP according to the Infectious Disease Society of America/American Thoracic Society guidelines [8] and the Surviving Sepsis Campaign guidelines [15,16]. qSOFA score of 2 or higher indicated a transfer to respiratory intensive care unit (ICU). Antibiotic regimens for the empirical treatment were adherence to the guidelines and then adjusted in the light of subsequently cultured pathogens. All patients who reached clinical stability and became afebrile were discharged home.
Approval of study design
The study was approved by the Institutional Review Boards (Review Board of Sun Yat-sen University and Review Board of Peking University, No. 20152958 and No. 20153043, respectively). All procedures included in the study involving human participants were in line with the 1964 Helsinki Declaration and its later amendments.
Sample size calculation
Unit-level design prevalence, cluster-level design prevalence, test sensitivity, target cluster sensitivity, and target system sensitivity were 12%, 1%, 0.9, 0.5, and 0.95, respectively. The total number of clusters to be sampled was 598, and the maximum number of samples was 4186.
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes incorporated SOFA scores, pneumonia severity index (PSI), hospital length of stay (LOS) and costs.
Data collection
A total of 4032 patients with CAP were recruited consecutively and 58 cases were excluded due to exclusion criteria. Chest radiography and/or computer tomography scans were performed in all patients. Two senior radiologists (LHL and QZZ) classified independently the frontal and lateral chest radiographic findings and computer tomography scan images. Clinical and diagnostic data and radiological features were gathered. qSOFA and SOFA scores and PSI on admission were calculated. Laboratory variables were determined by the hospital clinical laboratories. The statistician was blinded to the study.
Statistical analysis
All statistical analyses were performed with Statistical Package for the Social Science for Windows version 16.0 (SPSS, Chicago, IL, USA) and MedCalc version 19.6.1 (Mariakerke, Belgium). Categorical variables and continuous variables with normal distribution were reported as the percentages and the mean ± standard deviation (SD), respectively. Chi-square test, Spearman rank correlation, unpaired Student’s t-test and univariate logistic regression were applied. Odds ratio (OR) and relative risk (RR) for mortality were reckoned. The receiver operating characteristic (ROC) curves were designed and the corresponding areas under the ROC curves (AUROCs) with the 95% confidence interval (CI) were computed to estimate the performances of qSOFA and its individual elements to predict mortality. AUROCs were regarded as poor at 0.6–0.7, adequate at 0.7–0.8, good at 0.8–0.9, and excellent at 0.9 or higher [17]. The sensitivities, specificities, positive predictive values (PPVs), negative predictive values (NPVs), and Youden’s index were also calculated to appraise robustness of the variables. All tests were two-sided. p Values less than 0.05 were taken as statistically significant.
Results
Patient characteristics
Baseline characteristics are summarized in Table 1. In total, 3408 (85.7%) patients had qSOFA scores of 0 or 1. In total, 1602 (40.3%) patients had concurrent sepsis. The mortality rates increased sharply as qSOFA scores raised (p < 0.001). Mortality in CAP patients with sepsis was notably higher compared with those without sepsis. The etiology of pneumonia was not detected in every patient. Table 2 describes the data.
Table 1.
Baseline characteristics of study cohort (mean ± SD, n = 3974).
| Characteristic | Value |
|---|---|
| Age (years) | 51.7 ± 22.9 |
| Sex, No. (%) | |
| Men | 1940 (48.8) |
| Women | 2034 (51.2) |
| Comorbidities, No. (%) | |
| Hypertension | 1168 (29.4) |
| Coronary heart disease | 358 (9.0) |
| Heart failure | 139 (3.5) |
| NYHA class IV | 68 (1.7) |
| COPD | 270 (6.8) |
| GOLD 3 and 4 | 159 (4.0) |
| Diabetes mellitus | 306 (7.7) |
| Chronic renal insufficiency | 171 (4.3) |
| Dialysis | 87 (2.2) |
| Liver disease | 227 (5.7) |
| Nervous system disease | 163 (4.1) |
| Tumour | 282 (7.1) |
| Alcohol abuse, No. (%) | 151 (3.8) |
| Smoking, No. (%) | 755 (19.0) |
| qSOFA score, No. (%)a, b | |
| 0/died | 2139 (53.8)/7 (0.3) |
| 1/died | 1269 (31.9)/114 (9.0) |
| 2/died | 464 (11.7)/61 (13.1) |
| 3/died | 102 (2.6)/92 (90.2) |
| Outcomes, No. (%) | |
| Ventilated patients | 274 (6.9) |
| Patients received catecholamines | 393 (9.9) |
| Sepsisc | 1602 (40.3) |
| In-hospital mortality | 274 (6.9) |
| Mortality from sepsis | 220 (13.7) |
| Mortality not from sepsis | 54 (2.3) |
COPD: chronic obstructive pulmonary disease; GOLD: global initiative for chronic obstructive lung disease; NYHA: New York Heart Association; qSOFA: quick sequential [sepsis-related] organ failure assessment; SBP: systolic blood pressure; SOFA: sequential [sepsis-related] organ failure assessment; LOS: length of stay.
a Score ranges from 0 to 3 [4,5], with higher scores indicating greater likelihood of having severe CAP.
b p < 0.001.
c Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction can be identified as an acute change in total SOFA score ≥2 points consequent to the infection [4].
Table 2.
Most common etiologies of CAP (n = 3974).
| Etiology | Patient (%) |
|---|---|
| Streptococcus pneumoniae | 1112 (28.0) |
| Mycoplasma pneumoniae | 759 (19.1) |
| Haemophilus influenzae | 428 (10.8) |
| Respiratory viruses | 261 (6.6) |
| Staphylococcus aureus | 173 (4.4) |
| Legionella species | 130 (3.3) |
| Gram-negative bacilli | 93 (2.3) |
CAP: community-acquired pneumonia.
Performances of individual qSOFA elements for the prediction of mortality
Prognostic performances of individual qSOFA elements are reported in Table 3. Respiratory rate ≥22/min demonstrated the most superior diagnostic value, indicated by largest OR, RR and AUROC, and maximum Youden’s index for mortality. However, the indices for altered mentation and SBP ≤100 mm Hg decreased notably in turn.
Table 3.
Performance of the individual criteria for the prediction of mortality among patients with CAP (n = 3974).
| Variable | Respiratory rate ≥22/min | Altered mentation | SBP ≤100 mm Hg | |
|---|---|---|---|---|
| Patients alive (%) | Yes | 1144 (81.1) | 262 (64.2) | 560 (80.9) |
| No | 267 (18.9) | 146 (35.8) | 132 (19.1) | |
| χ2 | 255.317 | 249.391 | 107.394 | |
| p Value | <0.0001 | <0.0001 | <0.0001 | |
| RR (95% CI) p Value |
63.508 (24.394–169.261) <0.0001 |
8.753 (6.462–11.957) <0.0001 |
4.758 (3.426–6.492) <0.0001 |
|
| OR (95% CI) p Value |
75.915 (28.627–207.293) <0.0001 |
12.632 (8.417–17.839) <0.0001 |
5.671 (3.916–7.915) <0.0001 |
|
| Sensitivity, % (95% CI) | 97.4 (92.8–99.5) |
53.3 (46.8–63.5) |
48.2 (39.4–56.9) |
|
| Specificity, % (95% CI) | 69.1 (67.1–71.3) |
92.9 (91.4–94.2) |
84.9 (83.2–86.4) |
|
| PPV, % (95% CI) | 18.9 (17.2–19.5) |
35.8 (30.7–39.8) |
19.1 (16.0–21.8) |
|
| NPV, % (95% CI) | 99.7 (99.3–99.9) |
96.4 (95.7–97.0) |
95.7 (95.1–96.3) |
|
| Youden’s index | 0.67 | 0.46 | 0.33 | |
| AUROC (95% CI) | 0.837 (0.820–0.849) |
0.734 (0.702–0.765) |
0.671 (0.653–0.692) |
|
CAP: community-acquired pneumonia; SBP: systolic blood pressure; RR: relative risk; CI: confidence interval; OR: odds ratio; PPV: positive predictive value; NPV: negative predictive value; AUROC: the area under the receiver operating characteristic curve.
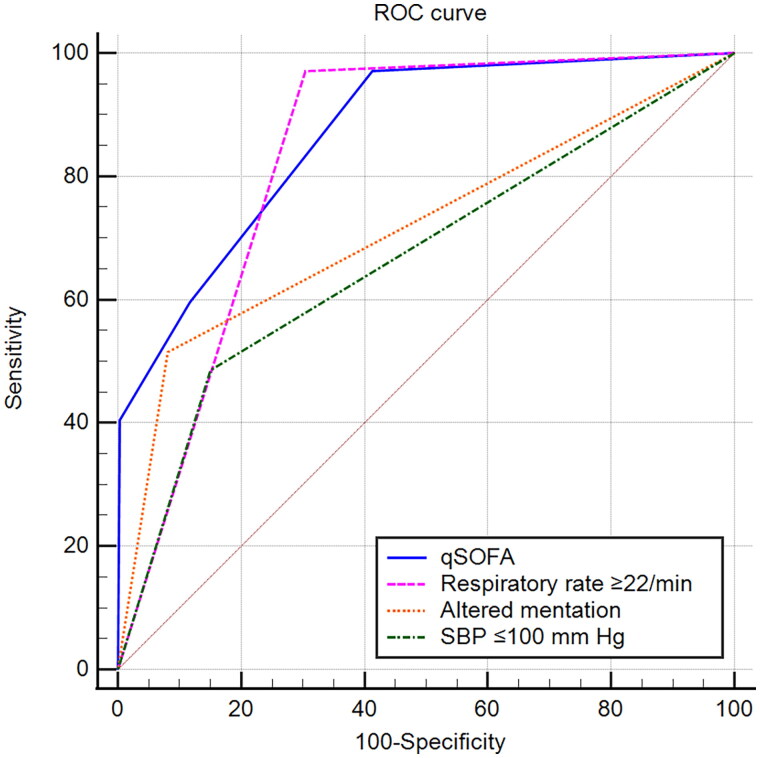
The predictive validities of respiratory rate ≥22/min, altered mentation and SBP ≤100 mm Hg were good, adequate and poor for mortality, indicated by AUROC (0.837, 0.734 and 0.671, respectively; Table 3 and Figure 1). The predictive validity of qSOFA was good for mortality (AUROC, 0.875; 95% CI, 0.857–0.886; Figure 1).
Figure 1.
ROC curves for mortality prediction by qSOFA and its individual elements.
ROC: the receiver operating characteristic; qSOFA: quick sequential [sepsis-related] organ failure assessment; SBP: systolic blood pressure.
The association of individual qSOFA elements with SOFA scores
The differences in SOFA scores between the patients meeting an individual qSOFA element and those without the criterion and their associations are shown in Table 4. Respiratory rate ≥22/min demonstrated the strongest association with SOFA scores. However, SBP ≤100 mm Hg showed the weakest correlation with the index.
Table 4.
Associations of the individual criteria with SOFA scores, PSI, hospital LOS and costs (mean ± SD, n = 3974).
| Criteria | SOFA score/PSI/hospital LOS (days)/cost ($) | t Value | p Value | rs Value | p Value |
|---|---|---|---|---|---|
| Respiratory rate ≥22/min | 3.59 ± 1.58 vs. 0.73 ± 1.49 | 12.739 | <0.001 | 0.675 | <0.001 |
| 117.46 ± 6.25 vs. 58.71 ± 3.93 | 11.361 | <0.001 | 0.604 | <0.001 | |
| 15.3 ± 7.3 vs. 10.5 ± 4.2 | 4.847 | <0.001 | 0.316 | <0.001 | |
| 2015.56 ± 937.35 vs. 766.43 ± 316.74 | 9.305 | <0.001 | 0.492 | <0.001 | |
| Altered mentation | 5.24 ± 1.37 vs. 1.34 ± 1.82 | 11.694 | <0.001 | 0.618 | <0.001 |
| 130.98 ± 9.14 vs. 73.69 ± 5.27 | 10.703 | <0.001 | 0.539 | <0.001 | |
| 16.9 ± 8.1 vs. 11.7 ± 6.3 | 4.312 | <0.001 | 0.298 | <0.001 | |
| 1512.08 ± 813.71 vs. 1175.38 ± 651.08 | 5.493 | <0.001 | 0.292 | <0.001 | |
| SBP ≤100 mm Hg | 2.64 ± 1.79 vs. 1.56 ± 1.92 | 4.613 | <0.001 | 0.309 | <0.001 |
| 96.49 ± 6.01 vs. 76.00 ± 5.04 | 3.279 | <0.001 | 0.204 | <0.001 | |
| 14.3 ± 8.35 vs. 11.8 ± 1.17 | 3.295 | <0.001 | 0.217 | <0.001 | |
| 1412.98 ± 605.11 vs. 1167.13 ± 573.19 | 3.014 | <0.001 | 0.195 | <0.001 |
SOFA: sequential organ failure assessment; PSI: pneumonia severity index; LOS: length of stay; rs: rank correlation coefficient; SBP: systolic blood pressure.
The data in longest cells indicated SOFA scores, PSI, hospital LOS and costs, respectively.
The contributions of individual qSOFA elements to PSI
The patients with an individual qSOFA element demonstrated higher PSI compared with those without the criterion (Table 4). The association of respiratory rate ≥22/min with PSI was closest, and then SBP ≤100 mm Hg presented the weakest association.
The contributions of individual qSOFA elements to hospital LOS and costs
The patients meeting an individual qSOFA element stayed in the hospital longer and cost much more compared with those without the criterion (Table 4). Respiratory rate ≥22/min was most strongly associated with hospital LOS and costs. On the other hand, SBP ≤100 mm Hg was most weakly correlated with the indices.
Discussion
This observational prospective cohort study involving 3974 patients with CAP showed that respiratory rate ≥22/min demonstrated the most superior diagnostic value, indicated by largest OR, RR, Youden’s index and AUROC for mortality from CAP, and the indices for altered mentation and SBP ≤100 mm Hg decreased notablely in turn. We first found that the predictive validities of respiratory rate ≥22/min, altered mentation and SBP ≤100 mm Hg were good, adequate and poor for mortality, respectively, and that respiratory rate ≥22/min presented the strongest associations with SOFA scores, PSI, hospital LOS and costs, and then SBP ≤100 mm Hg was most weakly correlated with the indices.
Risk prediction models are key components of treatment algorithms adopted in a wide range of medical fields. All individual qSOFA elements made contributions to mortality prediction in the current study, but respiratory rate ≥22/min predicted best, which is new evidence. Which mechanisms might be envisaged to interpret the phenomena? Enough oxygen is essential for cells, tissues and organs. Tachypnoea indicates hypoxia. Therefore, tachypnoea demonstrated the strongest association with mortality. Higher prevalence of systolic hypertension and higher systolic arterial pressure are undoubted in recent years [13]. As a result, systolic arterial pressures of many patients might not drop to <100 mm Hg, which might be the causation of worst prognostic performance of SBP ≤100 mm Hg. Although CAP is a major source of sepsis, future prospective multicentre cohort studies of patients with sepsis including suspected infection else are warranted to better understand potential generalizability.
SOFA score is an excellent operationalization of disease severity of adult patients with hospitalized CAP [18]. Since the establishment of PSI, it is a good predictor of mortality in CAP, even including SARS-CoV-2 CAP [19–23]. Respiratory rate ≥22/min demonstrated the strongest associations with SOFA scores and PSI, and then similar patterns with hospital LOS and costs. However, SBP ≤100 mm Hg showed the weakest correlations with the indices. The above-mentioned mechanisms (essential role of oxygen and hypoxia indicated by tachypnoea) might be envisaged to explicate the different associations of individual qSOFA elements with severity. Guo et al. [24] previously reported that confusion and respiratory rate ≥30/min showed similar paradigms with SOFA scores, hospital LOS and costs based on a retrospective analysis, but hypotension (SBP <90 mm Hg or diastolic blood pressure ≤60 mm Hg) did not. It is the first known prospective evidence of the associations of respiratory rate ≥22/min with these indices. However, these findings might require external validation.
Severe sepsis (The term has been left behind after 2016 Sepsis-3 guidelines), defined as new-onset acute organ dysfunction in the cohort of patients hospitalized for CAP, developed in one-half of the patients (n = 639, 48%) [8]. The percentage of concurrent sepsis discovered in the current study was very similar to the above-mentioned. Mortality in patients with CAP and fulfilling sepsis increased sharply compared with those without sepsis. These findings might be envisaged to interpret the causation of why CAP results in great mortality and morbidity and high costs worldwide.
The current findings might have implications for more accurate clinical triage decisions. It is a major challenge in the management of CAP to identify patients who might rapidly develop adverse medical outcomes among those without obvious reasons for immediate ICU admission [25]. Only 2.6% of patients with CAP met qSOFA score of 3. Among the patients with CAP and fulfilling qSOFA score of 2, the patients who breathe 22/min or more might be more severely ill, demonstrate a higher mortality rate and then have the priority for treatment and intensive care where ICU resources are limited. Most importantly, the patients with qSOFA score of <2 but fulfilling respiratory rate ≥22/min might be prioritized and even should be transferred to ICU. The current findings also have direct clinical implications regarding prompt recognition and resuscitation at the emergency department. As Guo et al. previously reported in the application of the Infectious Disease Society of America/the American Thoracic Society minor criteria for severe CAP: The individual minor criteria for severe CAP were of unequal weight in predicting hospital mortality, SOFA scores, hospital LOS, and costs [24]. The combination of arterial oxygen pressure/fraction inspired oxygen ≤250 mm Hg, confusion and uraemia predicted more severity and higher mortality compared with others, suggesting the former patients should have a higher priority for treatment in ICU and might benefit more from ICU admission [26]. The patients with non-severe CAP fulfilling the predictive findings most strongly associated with mortality (arterial oxygen pressure/fraction inspired oxygen ≤250 mm Hg, confusion and uraemia) demonstrated higher SOFA and PSI scores and mortality rates and might have the priority for treatment and intensive care [25]. Furthermore, the predictive validity of qSOFA might be better were the contributions of individual qSOFA elements accompanied by additional biomarkers. Adami et al. [27] discovered combining qSOFA 1 with the biomarker soluble urokinase plasminogen activator receptor improves its prognostic performance for unfavourable outcome and can help decisions for earlier treatment. Bolanaki et al. [28] reported biomarkers of infection and organ dysfunction, most notably procalcitonin, substantially improve early prediction of sepsis with added value to qSOFA alone as a simple screening tool on emergency department admission.
Limitations
Several limitations of this study deserve comment. First, the prospective cohort was derived from two centres in a city, but not multicentre settings located in different cities in different countries. Popularization of the findings should be cautious. Second, this study tested the questions in patients with CAP. However, its applicability to patients with suspected infection of other parts of the body might not be determined by this study. Third, the samples were relatively small. Had the scale been larger, the results might have been more powerful. Finally, many medications can influence the patient’s respiratory rate (e.g. opioids, sedative drugs, etc.).
Conclusions
Respiratory rate ≥22/min made the greatest contribution to parsimonious qSOFA to assess severity and predict mortality. However, the contributions of altered mentation and SBP ≤100 mm Hg decreased strikingly in turn. It provides new prospective evidence of the contributions of individual qSOFA elements to assessment of severity and for prediction of mortality, which might have implications for more accurate clinical triage decisions.
Acknowledgements
We are indebted to the nurses, further education physicians, and postgraduates of the Departments of Pulmonary and Critical Care Medicine for making contributions to this study.
Funding Statement
This work was supported by the Medical Science and Technology Foundation of Guangdong Province [Grant No. A2010553], the Planned Science and Technology Project of Shenzhen Municipality [Grant No. 201102078], and the Non-Profit Scientific Research Project of Futian District [Grant No. FTWS201120]. The funding sources had no role in the design and conduct of these studies; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authors contributions
QG was in charge of funding acquisition and project administration. QG and HYL made substantial contributions to conception and design and were in charge of data collection and curation, and the writing of the manuscript. LHL and QZZ read the chest radiographs and computer tomography scans. WDS, ML, XKC, HL, HLP, HQY, NL, YHL, ZDL, LHL and QZZ made substantial contributions to acquisition, analysis, and interpretation of data. MJ was in charge of statistical analysis. Each author has participated in the writing of the manuscript or revising it critically for important intellectual content, been involved in the analysis of the data, and seen and approved the submitted version.
Ethical statement
Ethical approval from the regulation committee (Ethical Committee of Shenzhen, No. 201510673) was granted for the study protocol.
Consent form
Written informed consent was obtained from the patient prior to enrolment. The patients with confusion were asked afterwards and before enrolment, a first-degree relative gave assumed consent. They were notified the content of the study on admission and then signed the documents if they approved.
Disclosure statement
All authors report no conflict of interest.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317(3):301–308. doi: 10.1001/jama.2016.20329. [DOI] [PubMed] [Google Scholar]
- 7.Rudd KE, Seymour CW, Aluisio AR, et al. Association of the quick sequential (sepsis-related) organ failure assessment (qSOFA) score with excess hospital mortality in adults with suspected infection in low- and middle-income countries. JAMA. 2018;319(21):2202–2211. doi: 10.1001/jama.2018.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dremsizov T, Clermont G, Kellum JA, et al. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129(4):968–978. doi: 10.1378/chest.129.4.968. [DOI] [PubMed] [Google Scholar]
- 9.Prina E, Ranzani OT, Torres A.. Community-acquired pneumonia. Lancet. 2015;386(9998):1097–1108. doi: 10.1016/S0140-6736(15)60733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranzani OT, Prina E, Menéndez R, et al. New sepsis definition (sepsis-3) and community-acquired pneumonia mortality. A validation and clinical decision-making study. Am J Respir Crit Care Med. 2017;196(10):1287–1297. doi: 10.1164/rccm.201611-2262OC. [DOI] [PubMed] [Google Scholar]
- 11.Guo Q, Song WD, Li HY, et al. Cold-inducible RNA-binding protein might determine the severity and the presences of major/minor criteria for severe community-acquired pneumonia and best predicted mortality. Respir Res. 2020;21(1):192. doi: 10.1186/s12931-020-01457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Q, Li HY, Song WD, et al. qSOFA predicted pneumonia mortality better than minor criteria and worse than CURB-65 with robust elements and higher convergence. Am J Emerg Med. 2022;52:1–7. doi: 10.1016/j.ajem.2021.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Li HY, Song WD, et al. Updating cut-off values of severity scoring systems for community-acquired pneumonia to orchestrate more predictive accuracy. Ann Med. 2023;55(1):2202414. doi: 10.1080/07853890.2023.2202414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phua J, See KC, Chan YH, et al. Validation and clinical implications of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Thorax. 2009;64(7):598–603. doi: 10.1136/thx.2009.113795. [DOI] [PubMed] [Google Scholar]
- 15.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ.. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 18.Ahnert P, Creutz P, Horn K, et al. Sequential organ failure assessment score is an excellent operationalization of disease severity of adult patients with hospitalized community acquired pneumonia – results from the prospective observational PROGRESS study. Crit Care. 2019;23(1):110. doi: 10.1186/s13054-019-2316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 20.Cilloniz C, Ward L, Mogensen ML, et al. Machine-learning model for mortality prediction in patients with community-acquired pneumonia: development and validation study. Chest. 2023;163(1):77–88. doi: 10.1016/j.chest.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Willis DR, Yih Y.. The pneumonia severity index: assessment and comparison to popular machine learning classifiers. Int J Med Inform. 2022;163:104778. doi: 10.1016/j.ijmedinf.2022.104778. [DOI] [PubMed] [Google Scholar]
- 22.Kaal AG, Op de Hoek L, Hochheimer DT, et al. Outcomes of community-acquired pneumonia using the Pneumonia Severity Index versus the CURB-65 in routine practice of emergency departments. ERJ Open Res. 2023;9(3):00051–2023. doi: 10.1183/23120541.00051-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley J, Sbaih N, Chandler TR, et al. Pneumonia severity index and CURB-65 score are good predictors of mortality in hospitalized patients with SARS-CoV-2 community-acquired pneumonia. Chest. 2022;161(4):927–936. doi: 10.1016/j.chest.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Q, Li HY, Zhou YP, et al. Weight of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Respir Med. 2011;105(10):1543–1549. doi: 10.1016/j.rmed.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Li HY, Guo Q, Song WD, et al. Priority for treatment and intensive care of patients with non-severe community-acquired pneumonia. Am J Med Sci. 2018;356(4):329–334. doi: 10.1016/j.amjms.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Li HY, Guo Q, Song WD, et al. Mortality among severe community-acquired pneumonia patients depends on combinations of 2007 IDSA/ATS minor criteria. Int J Infect Dis. 2015;38(9):141–145. doi: 10.1016/j.ijid.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Adami ME, Kotsaki A, Antonakos N, et al. qSOFA combined with suPAR for early risk detection and guidance of antibiotic treatment in the emergency department: a randomized controlled trial. Crit Care. 2024;28(1):42. doi: 10.1186/s13054-024-04825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolanaki M, Winning J, Slagman A, et al. Biomarkers improve diagnostics of sepsis in adult patients with suspected organ dysfunction based on the quick sepsis-related organ failure assessment (qSOFA) score in the emergency department. Crit Care Med. 2024;52(6):887–899. doi: 10.1097/CCM.0000000000006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.