Abstract
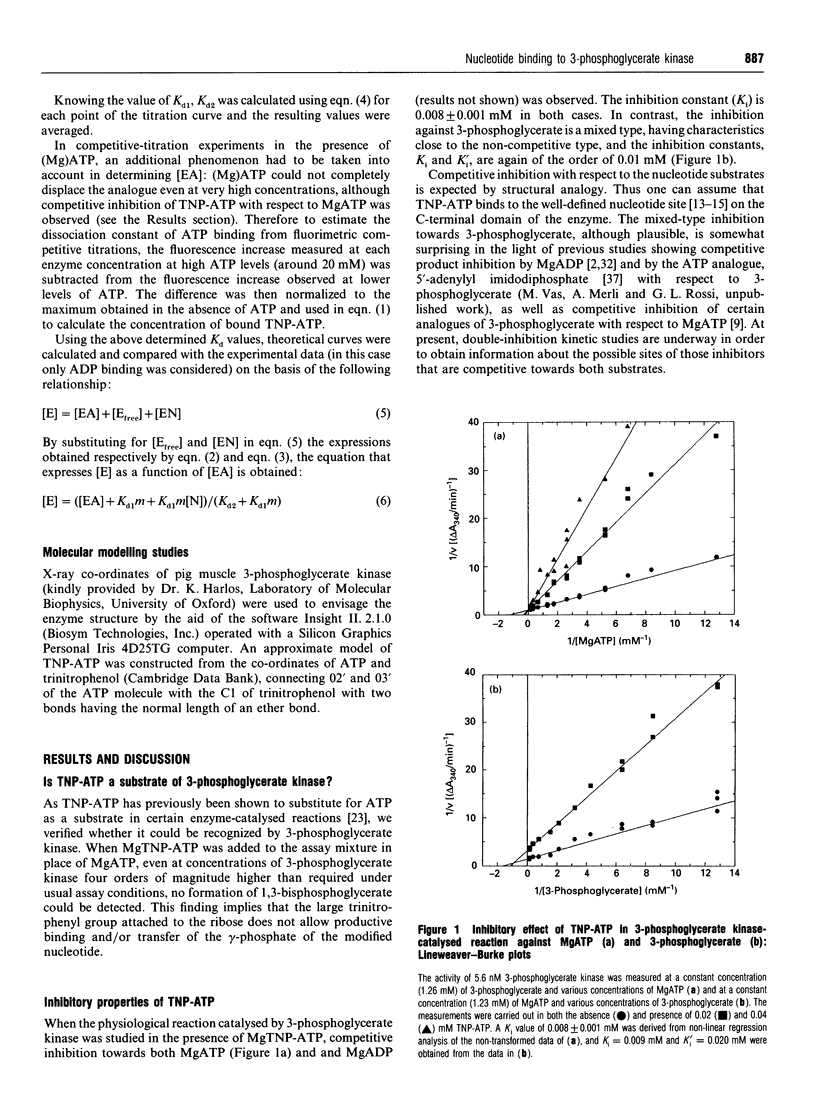
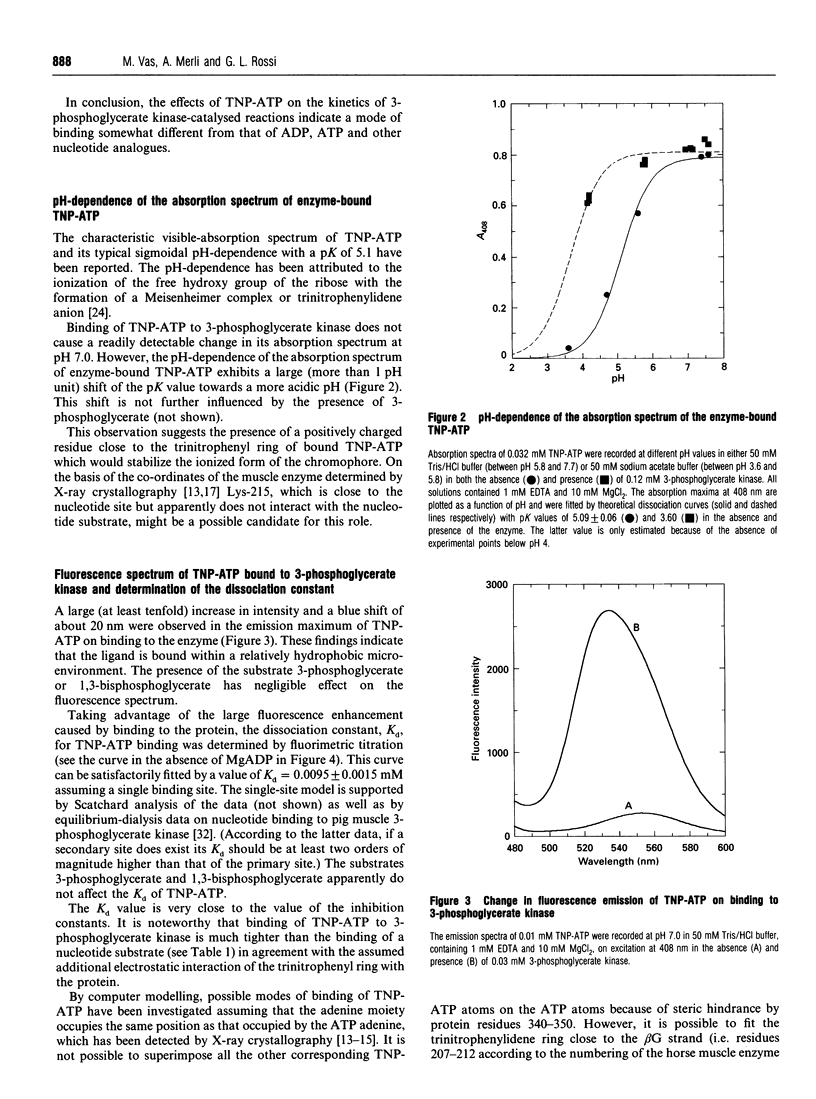
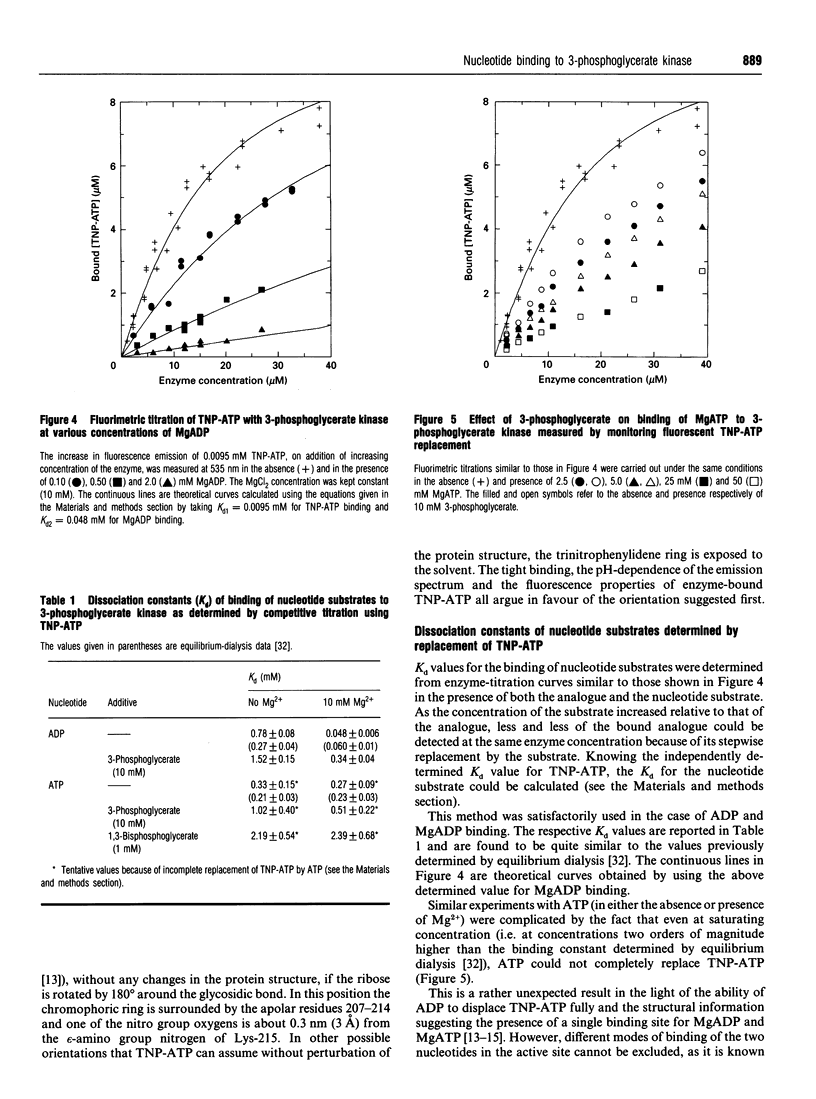
The analogue of ATP, 2'(3')-O-(2,4,6-trinitrophenyl)adenosine 5'-triphosphate (TNP-ATP), binds tightly to pig muscle 3-phosphoglycerate kinase. A dissociation constant Kd of 0.0095 +/- 0.0015 mM was determined by fluorimetric titration on the basis of 1:1 stoichiometry. TNP-ATP is a strong competitive inhibitor towards MgATP and MgADP with a Ki of 0.008 +/- 0.001 mM for both substrates. It is also a mixed-type inhibitor towards 3-phosphoglycerate with similar inhibition constants. Binding of TNP-ATP to 3-phosphoglycerate kinase is accompanied by a tenfold intensity increase and a blue shift of about 20 nm in its fluorescence emission spectrum and a shift of the pK of its trinitrophenyl group towards a more acidic pH. These findings suggest that the negatively charged trinitrophenyl group of TNP-ATP significantly contributes to the binding of the analogue. By stepwise replacement of the fluorescent TNP-ATP, the dissociation constants (Kd) for ADP and MgADP binding were determined and found to be 0.78 +/- 0.08 and 0.048 +/- 0.006 mM respectively, which are consistent with the values previously determined by equilibrium dialysis [Molnár and Vas (1993) Biochem J. 293, 595-599]. In similar competitive-titration experiments, ATP and MgATP did not completely substitute for TNP-ATP. For the fraction of the analogue that could be substituted, the dissociation constants for MgATP and ATP were estimated to be 0.27 +/- 0.09 and 0.33 +/- 0.15 mM respectively, close to the values determined by equilibrium dialysis. Using the same method, a significant weakening of binding of both (Mg)ADP and (Mg)ATP could be detected in the presence of 3-phosphoglycerate: their respective Kd values became 0.34 +/- 0.04 and 0.51 +/- 0.22 mM. The reciprocal effect, i.e. weakening of 3-phosphoglycerate binding in the presence of the nucleotide substrates, has been observed previously [Vas and Batke (1984) Eur. J. Biochem. 139, 115-123]. Similarly, a much weaker binding of (Mg)ATP could be observed in the presence of 1,3-bisphosphoglycerate (Kd = 2.30 +/- 0.68 mM). The possible reason for the mutual weakening of substrate binding is discussed in the light of the available structural data.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks R. D., Blake C. C., Evans P. R., Haser R., Rice D. W., Hardy G. W., Merrett M., Phillips A. W. Sequence, structure and activity of phosphoglycerate kinase: a possible hinge-bending enzyme. Nature. 1979 Jun 28;279(5716):773–777. doi: 10.1038/279773a0. [DOI] [PubMed] [Google Scholar]
- Cserpán I., Vas M. Effects of substrates on the heat stability and on the reactivities of thiol groups of 3-phosphoglycerate kinase. Eur J Biochem. 1983 Mar 1;131(1):157–162. doi: 10.1111/j.1432-1033.1983.tb07243.x. [DOI] [PubMed] [Google Scholar]
- Davies G. J., Gamblin S. J., Littlechild J. A., Watson H. C. The structure of a thermally stable 3-phosphoglycerate kinase and a comparison with its mesophilic equivalent. Proteins. 1993 Mar;15(3):283–289. doi: 10.1002/prot.340150306. [DOI] [PubMed] [Google Scholar]
- Deville-Bonne D., Laine R., Garel J. R. Substrate antagonism in the kinetic mechanism of E. coli phosphofructokinase-1. FEBS Lett. 1991 Sep 23;290(1-2):173–176. doi: 10.1016/0014-5793(91)81253-5. [DOI] [PubMed] [Google Scholar]
- Dékány K., Vas M. Inactivation of pig muscle 3-phosphoglycerate kinase by thiol modification depends on reagent size. Eur J Biochem. 1984 Feb 15;139(1):125–130. doi: 10.1111/j.1432-1033.1984.tb07985.x. [DOI] [PubMed] [Google Scholar]
- ELODI P., SZORENYI E. Crystallisation and comparative studies of D-3-phosphoglyceraldehyde dehydrogenase from muscle of various mammals. Acta Physiol Acad Sci Hung. 1956;9(4):339–350. [PubMed] [Google Scholar]
- Faller L. D. Binding of the fluorescent substrate analogue 2',3'-O-(2,4,6-trinitrophenylcyclohexadienylidene)adenosine 5'-triphosphate to the gastric H+,K(+)-ATPase: evidence for cofactor-induced conformational changes in the enzyme. Biochemistry. 1990 Apr 3;29(13):3179–3186. doi: 10.1021/bi00465a004. [DOI] [PubMed] [Google Scholar]
- Faller L. D. Competitive binding of ATP and the fluorescent substrate analogue 2',3'-O-(2,4,6-trinitrophenylcyclohexadienylidine) adenosine 5'-triphosphate to the gastric H+,K+-ATPase: evidence for two classes of nucleotide sites. Biochemistry. 1989 Aug 8;28(16):6771–6778. doi: 10.1021/bi00442a034. [DOI] [PubMed] [Google Scholar]
- Gregory J. D., Serpersu E. H. Arrangement of substrates at the active site of yeast phosphoglycerate kinase. Effect of sulfate ion. J Biol Chem. 1993 Feb 25;268(6):3880–3888. [PubMed] [Google Scholar]
- Gupta R. K., Gupta P., Yushok W. D., Rose Z. B. Measurement of the dissociation constant of MgATP at physiological nucleotide levels by a combination of 31P NMR and optical absorbance spectroscopy. Biochem Biophys Res Commun. 1983 Nov 30;117(1):210–216. doi: 10.1016/0006-291x(83)91562-0. [DOI] [PubMed] [Google Scholar]
- Harlos K., Vas M., Blake C. F. Crystal structure of the binary complex of pig muscle phosphoglycerate kinase and its substrate 3-phospho-D-glycerate. Proteins. 1992 Feb;12(2):133–144. doi: 10.1002/prot.340120207. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T. Biological activities and spectroscopic properties of chromophoric and fluorescent analogs of adenine nucleoside and nucleotides, 2',3'-O-(2,4,6-trinitrocyclohexadienylidene) adenosine derivatives. Biochim Biophys Acta. 1982 Dec 17;719(3):509–517. doi: 10.1016/0304-4165(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T., Uchida K. Preparation and properties of 2'(or 3')-O-(2,4,6-trinitrophenyl) adenosine 5'-triphosphate, an analog of adenosine triphosphate. Biochim Biophys Acta. 1973 Oct 5;320(3):635–647. doi: 10.1016/0304-4165(73)90143-8. [DOI] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. K., Nick J., Cohn M. Reactivity and metal-dependent stereospecificity of the phosphorothioate analogs of ADP and ATP and reactivity of Cr(III)ATP in the 3-phosphoglycerate kinase reaction. Structure of the metal nucleotide substrates. J Biol Chem. 1982 Jul 10;257(13):7650–7656. [PubMed] [Google Scholar]
- Johnson J. L., Reinhart G. D. MgATP and fructose 6-phosphate interactions with phosphofructokinase from Escherichia coli. Biochemistry. 1992 Nov 24;31(46):11510–11518. doi: 10.1021/bi00161a032. [DOI] [PubMed] [Google Scholar]
- João H. C., Williams R. J. The anatomy of a kinase and the control of phosphate transfer. Eur J Biochem. 1993 Aug 15;216(1):1–18. doi: 10.1111/j.1432-1033.1993.tb18110.x. [DOI] [PubMed] [Google Scholar]
- LARSSON RAZNIKIEWICZ M. KINETIC STUDIES ON THE REACTION CATALYZED BY PHOSPHOGLYCERATE KINASE. I. THE EFFECT OF MG2+ AND ADENOSINE 5'-TRIPHOSPHATE. Biochim Biophys Acta. 1964 Apr 6;85:60–68. doi: 10.1016/0926-6569(64)90167-1. [DOI] [PubMed] [Google Scholar]
- LaDine J. R., Cross R. L. The adenine nucleotide-binding site on yeast 3-phosphoglycerate kinase. Affinity labeling of Lys-131 by pyridoxal 5'-diphospho-5'-adenosine. J Biol Chem. 1991 Apr 15;266(11):7194–7198. [PubMed] [Google Scholar]
- Larsson-Raźnikiewicz M., Arvidsson L. Inhibition of phosphoglycerate kinase by products and product homologues. Eur J Biochem. 1971 Oct 26;22(4):506–512. doi: 10.1111/j.1432-1033.1971.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Larsson-Raźnikiewicz M. Kinetic studies on the reaction catalyzed by phosphoglycerate kinase. II. The kinetic relationships between 3-phosphoglycerate, MgATP2-and activating metal ion. Biochim Biophys Acta. 1967 Jan 11;132(1):33–40. doi: 10.1016/0005-2744(67)90189-1. [DOI] [PubMed] [Google Scholar]
- Lee C. S., O'Sullivan W. J. Properties and mechanism of human erythrocyte phosphoglycerate kinase. J Biol Chem. 1975 Feb 25;250(4):1275–1281. [PubMed] [Google Scholar]
- Molnár M., Vas M. Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium dialysis binding and enzyme kinetic data. Biochem J. 1993 Jul 15;293(Pt 2):595–599. doi: 10.1042/bj2930595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. M., Reed G. H. Coordination scheme and stereochemical configuration of manganese(II) adenosine 5'-diphosphate at the active site of 3-phosphoglycerate kinase. Biochemistry. 1985 Sep 24;24(20):5328–5333. doi: 10.1021/bi00341a009. [DOI] [PubMed] [Google Scholar]
- Pineda T., Kwon O. S., Serpersu E. H., Churchich J. E. Interaction of ATP analogs with yeast 3-phosphoglycerate kinase. Affinity labeling of the hinge region. Eur J Biochem. 1993 Mar 15;212(3):719–726. doi: 10.1111/j.1432-1033.1993.tb17710.x. [DOI] [PubMed] [Google Scholar]
- Rao B. D., Cohn M., Scopes R. K. 31P NMR study of bound reactants and products of yeast 3-phosphoglycerate kinase at equilibrium and the effect of sulfate ion. J Biol Chem. 1978 Nov 25;253(22):8056–8060. [PubMed] [Google Scholar]
- Rao R., Al-Shawi M. K., Senior A. E. Trinitrophenyl-ATP and -ADP bind to a single nucleotide site on isolated beta-subunit of Escherichia coli F1-ATPase. In vitro assembly of F1-subunits requires occupancy of the nucleotide-binding site on beta-subunit by nucleoside triphosphate. J Biol Chem. 1988 Apr 25;263(12):5569–5573. [PubMed] [Google Scholar]
- Ray B. D., Moore J. M., Rao B. D. 31P NMR studies of enzyme-bound substrate complexes of yeast 3-phosphoglycerate kinase: III. Two ADP binding sites and their Mg(II) affinity; effects of vanadate and arsenate on enzymic complexes with ADP and 3-P-glycerate. J Inorg Biochem. 1990 Sep;40(1):47–57. doi: 10.1016/0162-0134(90)80039-z. [DOI] [PubMed] [Google Scholar]
- Schierbeck B., Larsson-Raźnikiewicz M. Product inhibition studies of yeast phosphoglycerate kinase evaluating properties of multiple substrate binding sites. Biochim Biophys Acta. 1979 May 10;568(1):195–204. doi: 10.1016/0005-2744(79)90286-9. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Binding of substrates and other anions to yeast phosphoglycerate kinase. Eur J Biochem. 1978 Nov 2;91(1):119–129. doi: 10.1111/j.1432-1033.1978.tb20944.x. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. The steady-state kinetics of yeast phosphoglycerate kinase. Anomalous kinetic plots and the effects of salts on activity. Eur J Biochem. 1978 Apr 17;85(2):503–516. doi: 10.1111/j.1432-1033.1978.tb12266.x. [DOI] [PubMed] [Google Scholar]
- Sheridan M., Wilton D. C. The binding of the fluorescent ATP analogue 2'(3')-trinitrophenyladenosine-5'-triphosphate to rat liver fatty acid-binding protein. FEBS Lett. 1992 Dec 21;314(3):486–488. doi: 10.1016/0014-5793(92)81532-q. [DOI] [PubMed] [Google Scholar]
- Sheridan M., Wilton D. C. The binding of the fluorescent ATP analogue 2'(3')-trinitrophenyladenosine-5'-triphosphate to rat liver fatty acid-binding protein. FEBS Lett. 1992 Dec 21;314(3):486–488. doi: 10.1016/0014-5793(92)81532-q. [DOI] [PubMed] [Google Scholar]
- Tanswell P., Westhead E. W., Williams R. J. Nuclear-magnetic-resonance study of the active-site structure of yeast phosphoglycerate kinase. Eur J Biochem. 1976 Mar 16;63(1):249–262. doi: 10.1111/j.1432-1033.1976.tb10227.x. [DOI] [PubMed] [Google Scholar]
- Tompa P., Hong P. T., Vas M. The phosphate group of 3-phosphoglycerate accounts for conformational changes occurring on binding to 3-phosphoglycerate kinase. Enzyme inhibition and thiol reactivity studies. Eur J Biochem. 1986 Feb 3;154(3):643–649. doi: 10.1111/j.1432-1033.1986.tb09446.x. [DOI] [PubMed] [Google Scholar]
- Vas M., Batke J. Adenine nucleotides affect the binding of 3-phosphoglycerate to pig muscle 3-phosphoglycerate kinase. Eur J Biochem. 1984 Feb 15;139(1):115–123. doi: 10.1111/j.1432-1033.1984.tb07984.x. [DOI] [PubMed] [Google Scholar]
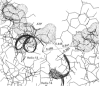
- Vas M. Modelling of substrate binding to 3-phosphoglycerate kinase with analogues of 3-phosphoglycerate. Eur J Biochem. 1990 Dec 12;194(2):639–645. doi: 10.1111/j.1432-1033.1990.tb15663.x. [DOI] [PubMed] [Google Scholar]
- Watson H. C., Walker N. P., Shaw P. J., Bryant T. N., Wendell P. L., Fothergill L. A., Perkins R. E., Conroy S. C., Dobson M. J., Tuite M. F. Sequence and structure of yeast phosphoglycerate kinase. EMBO J. 1982;1(12):1635–1640. doi: 10.1002/j.1460-2075.1982.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiksell E., Larsson-Raźnikiewicz M. Substrate binding to phosphoglycerate kinase monitored by 1-anilino-8-naphthalenesulfonate. J Biol Chem. 1982 Nov 10;257(21):12672–12677. [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]
- Zheng R. L., Kemp R. G. The mechanism of ATP inhibition of wild type and mutant phosphofructo-1-kinase from Escherichia coli. J Biol Chem. 1992 Nov 25;267(33):23640–23645. [PubMed] [Google Scholar]