Abstract
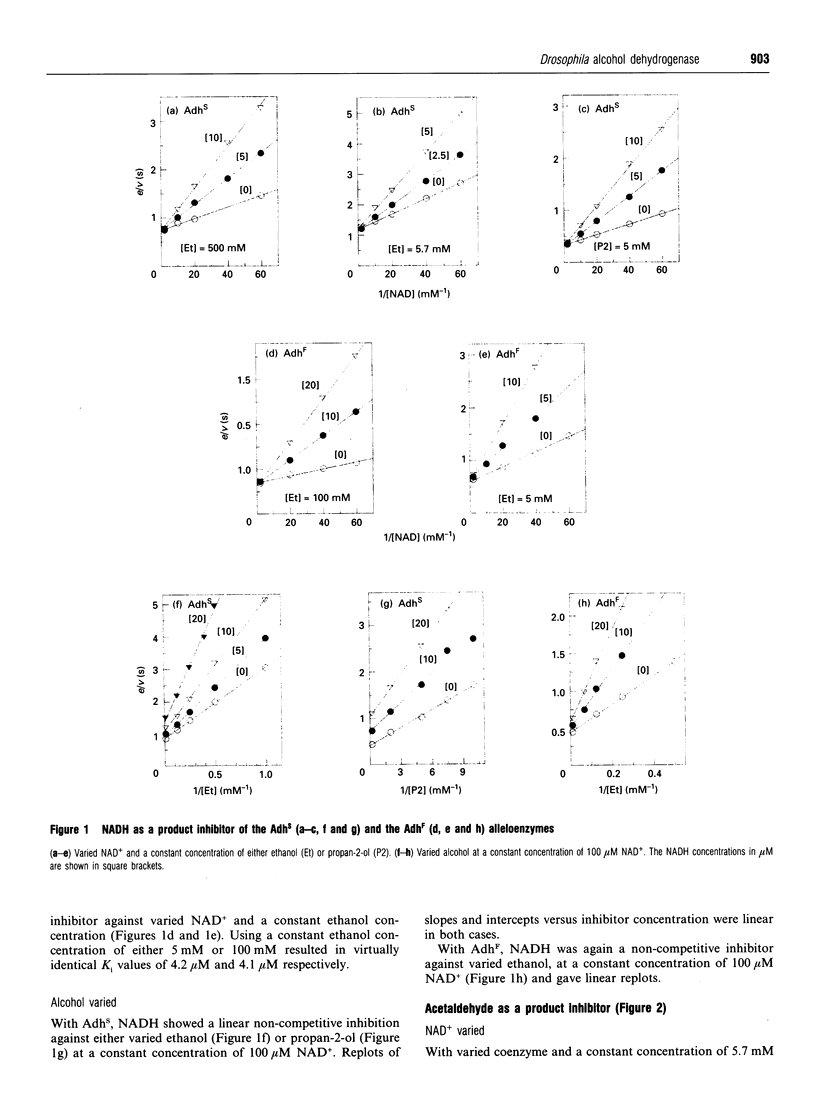
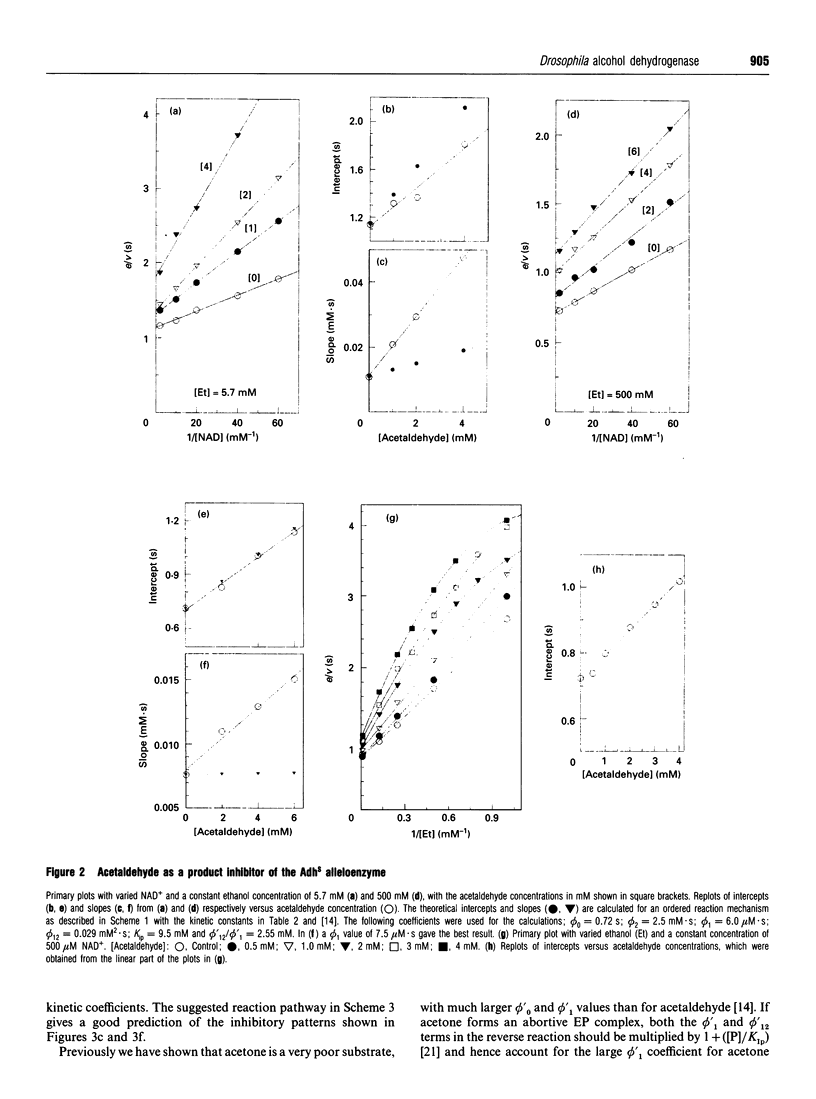
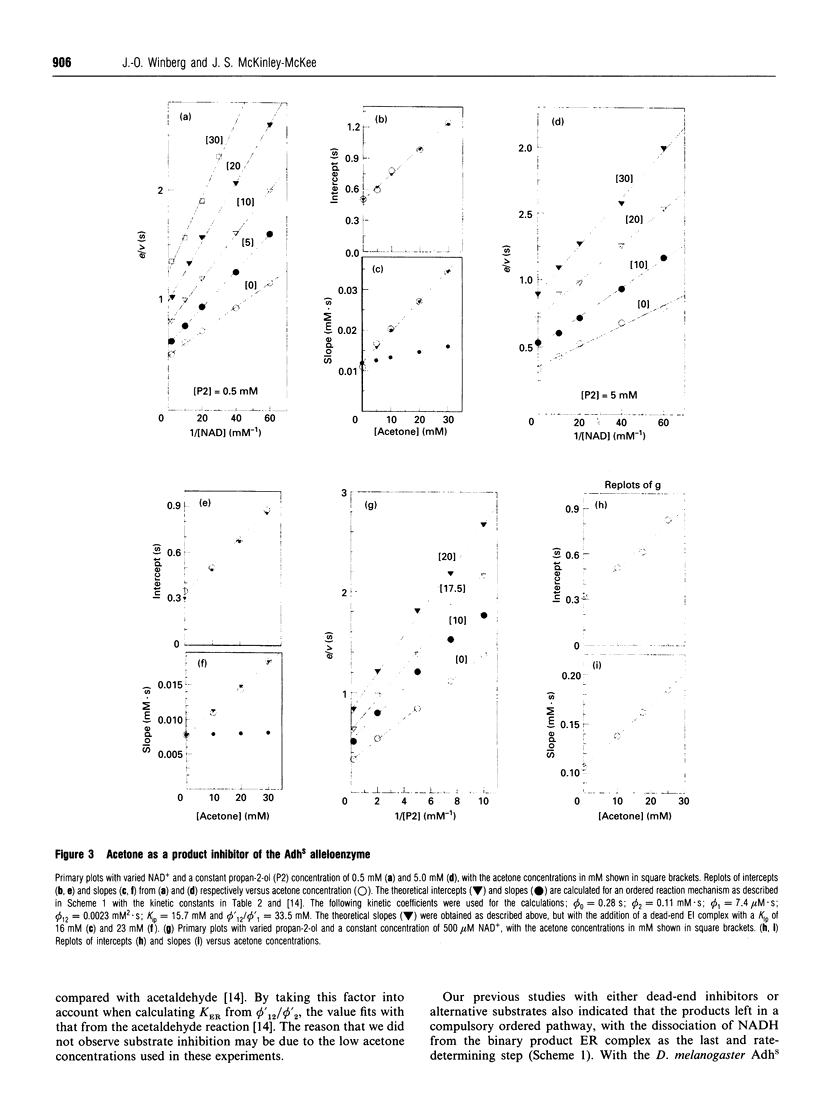
The Drosophila melanogaster alleloenzymes AdhS and AdhF have been studied with respect to product inhibition by using the two substrate couples propan-2-ol/acetone and ethanol/acetaldehyde together with the coenzyme couple NAD+/NADH. With both substrate couples the reaction was consistent with an ordered Bi Bi mechanism. The substrates added to the enzyme in a compulsory order, with coenzyme as the leading substrate, to give two interconverting ternary complexes. The second ternary complex broke down with release of products in an obligatory order, with the aldehyde/ketone leaving first. Both the acetaldehyde and acetone products formed binary complexes with the enzyme that affected NAD+ binding. However, only an enzyme-acetone complex seemed to affect NADH binding and hence the reverse reaction. The inhibitory pattern with acetaldehyde as product was also affected by the formation of a ternary enzyme-NAD(+)-acetaldehyde complex, which broke down to acetic acid and NADH. The product-inhibition pattern shown in the present work is different from that published for Drosophila Adh previously and this discrepancy can not be explained by the use of different variants of Drosophila Adh.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Chambers G. K. Gene expression, adaptation and evolution in higher organisms. Evidence from studies of Drosophila alcohol dehydrogenases. Comp Biochem Physiol B. 1991;99(4):723–730. doi: 10.1016/0305-0491(91)90135-z. [DOI] [PubMed] [Google Scholar]
- Chen Z., Jiang J. C., Lin Z. G., Lee W. R., Baker M. E., Chang S. H. Site-specific mutagenesis of Drosophila alcohol dehydrogenase: evidence for involvement of tyrosine-152 and lysine-156 in catalysis. Biochemistry. 1993 Apr 6;32(13):3342–3346. doi: 10.1021/bi00064a017. [DOI] [PubMed] [Google Scholar]
- Chen Z., Lu L., Shirley M., Lee W. R., Chang S. H. Site-directed mutagenesis of glycine-14 and two "critical" cysteinyl residues in Drosophila alcohol dehydrogenase. Biochemistry. 1990 Feb 6;29(5):1112–1118. doi: 10.1021/bi00457a003. [DOI] [PubMed] [Google Scholar]
- Cols N., Marfany G., Atrian S., Gonzàlez-Duarte R. Effect of site-directed mutagenesis on conserved positions of Drosophila alcohol dehydrogenase. FEBS Lett. 1993 Mar 15;319(1-2):90–94. doi: 10.1016/0014-5793(93)80043-t. [DOI] [PubMed] [Google Scholar]
- Heinstra P. W., Scharloo W., Thorig G. E. Alcohol dehydrogenase polymorphism in Drosophila: enzyme kinetics of product inhibition. J Mol Evol. 1988 Dec;28(1-2):145–150. doi: 10.1007/BF02143506. [DOI] [PubMed] [Google Scholar]
- Heinstra P. W., Thörig G. E., Scharloo W., Drenth W., Nolte R. J. Kinetics and thermodynamics of ethanol oxidation catalyzed by genetic variants of the alcohol dehydrogenase from Drosophila melanogaster and D. simulans. Biochim Biophys Acta. 1988 Nov 17;967(2):224–233. doi: 10.1016/0304-4165(88)90013-x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Persson B., Krook M., Kaiser R. Alcohol dehydrogenases. Biochem Soc Trans. 1990 Apr;18(2):169–171. doi: 10.1042/bst0180169. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Persson M., Jeffery J. Alcohol and polyol dehydrogenases are both divided into two protein types, and structural properties cross-relate the different enzyme activities within each type. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4226–4230. doi: 10.1073/pnas.78.7.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstad R. I., Hermansen L. F., McKinley-McKee J. S. The kinetic mechanism of sheep liver sorbitol dehydrogenase. Eur J Biochem. 1992 Dec 1;210(2):641–647. doi: 10.1111/j.1432-1033.1992.tb17465.x. [DOI] [PubMed] [Google Scholar]
- McKinley-McKee J. S., Winberg J. O., Pettersson G. Mechanism of action of Drosophila melanogaster alcohol dehydrogenase. Biochem Int. 1991 Dec;25(5):879–885. [PubMed] [Google Scholar]
- Thatcher D. R. The complete amino acid sequence of three alcohol dehydrogenase alleloenzymes (AdhN-11, AdhS and AdhUF) from the fruitfly Drosophila melanogaster. Biochem J. 1980 Jun 1;187(3):875–883. doi: 10.1042/bj1870875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. Kinetic formulations for enzymic reactions involving two substrates. Can J Biochem Physiol. 1962 Jun;40:763–804. [PubMed] [Google Scholar]
- Winberg J. O., Hovik R., McKinley-McKee J. S., Juan E., Gonzalez-Duarte R. Biochemical properties of alcohol dehydrogenase from Drosophila lebanonensis. Biochem J. 1986 Apr 15;235(2):481–490. doi: 10.1042/bj2350481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg J. O., Hovik R., McKinley-McKee J. S. The alcohol dehydrogenase alleloenzymes AdhS and AdhF from the fruitfly Drosophila melanogaster: an enzymatic rate assay to determine the active-site concentration. Biochem Genet. 1985 Apr;23(3-4):205–216. doi: 10.1007/BF00504319. [DOI] [PubMed] [Google Scholar]
- Winberg J. O., Martinoni B., Roten C., McKinley-McKee J. S. Drosophila alcohol dehydrogenase: stereoselective hydrogen transfer from ethanol. Biochem Mol Biol Int. 1993 Nov;31(4):651–658. [PubMed] [Google Scholar]
- Winberg J. O., McKinley-McKee J. S. Drosophila melanogaster alcohol dehydrogenase. Biochemical properties of the NAD+-plus-acetone-induced isoenzyme conversion. Biochem J. 1988 Apr 1;251(1):223–227. doi: 10.1042/bj2510223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg J. O., McKinley-McKee J. S. Kinetic interpretations of active site topologies and residue exchanges in Drosophila alcohol dehydrogenases. Int J Biochem. 1992 Feb;24(2):169–181. doi: 10.1016/0020-711x(92)90245-v. [DOI] [PubMed] [Google Scholar]
- Winberg J. O., McKinley-McKee J. S. The AdhS alleloenzyme of alcohol dehydrogenase from Drosophila melanogaster. Variation of kinetic parameters with pH. Biochem J. 1988 Oct 15;255(2):589–599. [PMC free article] [PubMed] [Google Scholar]
- Winberg J. O., Thatcher D. R., McKinley-McKee J. S. Alcohol dehydrogenase from the fruitfly Drosophila melanogaster. Inhibition studies of the alleloenzymes AdhS and AdhUF. Biochim Biophys Acta. 1982 May 21;704(1):17–25. doi: 10.1016/0167-4838(82)90126-1. [DOI] [PubMed] [Google Scholar]
- Winberg J. O., Thatcher D. R., McKinley-McKee J. S. Alcohol dehydrogenase from the fruitfly Drosophila melanogaster. Substrate specificity of the alleloenzymes AdhS and AdhUF. Biochim Biophys Acta. 1982 May 21;704(1):7–16. doi: 10.1016/0167-4838(82)90125-x. [DOI] [PubMed] [Google Scholar]


