Abstract
Background
Increased length of stay after surgery is associated with increased healthcare utilization and adverse patient outcomes. While enhanced recovery after surgery (ERAS) protocols have been shown to reduce length of stay after colorectal surgery in trial settings, their effectiveness in real-world settings is more uncertain. The aim of this study was to assess the impact of ERAS protocol implementation on length of stay after colorectal surgery, using real-world data.
Methods
In 2012, ERAS protocols were introduced at 15 Ontario hospitals as part of the iERAS study. A cohort of patients undergoing colorectal surgery treated at these hospitals between 2008 and 2019 was created using health administrative data. Mean length of stay was computed for the intervals before and after ERAS implementation. Interrupted time series analyses were performed for predefined subgroups, namely all colorectal surgery, colorectal surgery without complications, right-sided colorectal surgery, and left-sided colorectal surgery. Sensitivity analyses were then conducted using adjusted length of stay, accounting for length of stay predictors, including: patient age, sex, marginalization, co-morbidities, and diagnosis; surgeon volume of cases, years in practice, and colorectal surgery expertise; hospital volume; and other contextual factors, including procedure type and timing, surgical approach, and in-hospital complications.
Results
A total of 32 612 patients underwent colorectal surgery during the study interval. ERAS implementation led to a decrease in length of stay of 1.05 days (13.7%). Larger decreases in length of stay were seen with more complex surgeries, with a level change of 1.17 days (15.6%) noted for the subgroup of patients undergoing left-sided colorectal surgery. The observed decreases in length of stay were durable for the length of the study interval in all analyses. When adjusting for predictors of length of stay, the effect of ERAS implementation on length of stay was larger (reduction of 1.46 days).
Conclusion
Introducing formal ERAS protocols reduces length of stay after colorectal surgery significantly, independent of temporal trends toward decreasing length of stay. These effects are durable, demonstrating that ERAS protocol implementation is an effective hospital-level intervention to reduce length of stay after colorectal surgery.
ERAS protocol implementation is associated with a prompt and durable reduction in length of stay after colorectal surgery, independent of secular trends toward decreasing length of stay over time. This association is stronger when accounting for other determinants of length of stay and in cohorts with more complex surgeries. These results are attributable to protocol implementation, rather than specific protocol elements.
Introduction
Increased length of stay (LOS) in hospital after surgery has been associated with many adverse patient outcomes, including increased rates of thromboembolic disease1–3, deconditioning4, iatrogenic complications5, and nosocomial infections6. Additionally, on a system level, increased LOS after surgery leads to consumption of finite hospital resources and can result in bed-blocking and delays in care for other patients and impaired patient flow in the hospital.
Enhanced recovery after surgery (ERAS) protocols, or fast-track surgery protocols, refer to a bundle of preoperative, intraoperative, and postoperative interventions aimed at improving patient outcomes after surgery7. Although the specific elements of these protocols vary, based on the specific implementing site, ERAS protocols have been demonstrated in controlled trials to reduce LOS after colorectal surgery (CRS)7–16. However, outside of these controlled settings, the surgeon influences how these protocols are applied to an individual patient and plays an important role in the patient’s LOS17. Consequently, the results seen in the trials may not be realized in practice.
To date, the impact of ERAS protocol implementation on LOS after CRS has not been thoroughly explored in the real-world setting, with the existing data providing conflicting results and limited by methodological issues, including, importantly, lack of accounting for temporal trends in LOS. Additionally, the durability of the effect of ERAS protocol implementation on LOS has not been investigated. These aspects are important to understand when considering the benefits of implementing hospital-level protocols.
The aim of this study was to assess the magnitude and durability of the impact of ERAS protocol implementation on LOS after CRS, while accounting for temporal trends in LOS.
Methods
In 2012, the iERAS project was conducted to study the impact of introducing a comprehensive package of evidence-based ERAS protocols at 15 teaching hospitals in Ontario, Canada18. It included patients undergoing elective CRS and assessed their outcomes after introduction of ERAS protocols to their hospital. A full description of the study and the specifics of the ERAS protocols have been previously described18. Briefly, protocols comprised preoperative interventions (for example preoperative counselling and appropriate bowel preparation), intraoperative interventions (for example goal-directed fluid therapy), and postoperative interventions (for example early mobilization and feeding). The protocols were implemented at the hospital level. Of note, the protocols did not include specific discharge criteria, but were, instead, centred around patient care items.
Study population and data sources
Patients were included in the study cohort if they were 18 years or older, underwent elective CRS between 2008 and 2019 at one of the 15 iERAS hospitals, and when no concomitant surgeries were performed, other than a diverting stoma. Patients were excluded if they were preadmitted to hospital before CRS, were emergency patients, or died during the index hospitalization. The surgeon billing codes used to identify patients undergoing CRS at the 15 iERAS hospitals are listed in Table S1.
Ontario health administrative databases were used to create the study cohort. Health administrative data were accessed through the Institute for Clinical Evaluative Sciences (ICES), where population-based health data sets are stored and linked between patients19. These databases contain records for all instances of publicly funded healthcare delivery in Ontario. As Ontario has a single-payer government-funded healthcare system, and these databases are linked to physician billings, these databases have been shown to have close to 100% capture of events, including surgical procedures20. The only procedures missing from the cohort would be the rare CRS performed on patients without provincial healthcare coverage. ICES databases, including the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), the Corporate Provider Database (CPDB), the Institution Information System (INST), the Ontario Health Insurance Plan (OHIP), the Ontario Marginalization Index (ONMARG), and the Registered Persons Database (RPDB), were linked, using well-established methodology21–23. This linkage methodology and the integrity of the component databases have been validated and have been used widely in peer-reviewed studies20–26. Database details are available in Table S2.
Outcomes of interest
Two outcomes were analysed: crude LOS and adjusted LOS. Mean values for crude LOS were calculated at 1-month intervals for different CRS performed at ERAS hospitals for 5 years before and after ERAS was implemented; 1-month intervals were selected to ensure enough data points to confirm the linearity of pre- and post-implementation trends in LOS (a necessary assumption underlying the interrupted time series method)27.
Adjusted LOS was also computed using multivariable negative binomial regression to account for patient, surgeon, hospital, and other contextual factors listed in Table 1. Surgeon factors included surgeon experience, based on volume of cases, years in practice, and expertise in CRS (that is greater than 50% of their overall cases eligible for the study). Surgeon and hospital volume were categorized by assigning surgeons and hospitals to quartiles, based on their CRS volumes; CRS was determined by assigning surgeons and hospitals to quartiles, based on their volume of CRS relative to all surgeons or hospitals in Ontario. Patient factors included age, sex, marginalization, based on the Ontario Marginalization Index, co-morbidities, based on the Johns Hopkins Adjusted Clinical Group (ACG) score, and diagnosis. Specific details about the surgical procedure included procedure type, in-hospital complications that included anastomotic leak, reoperation, sepsis, cardiac arrest, myocardial infarction, blood transfusion, deep-vein thrombosis, pulmonary embolus, and stroke, surgical approach (open versus laparoscopic), creation of a diverting stoma, extensive lysis of adhesions, reoperative surgery, and timing of surgery during the week (that is early (Monday to Wednesday) versus late (Thursday to Sunday)).
Table 1.
Baseline and demographic characteristics for patients undergoing colorectal surgery at hospitals where ERAS protocols were implemented during the study interval
| All patients | Pre-implementation | Post-implementation | P | |
|---|---|---|---|---|
| Surgeon and hospital factors | ||||
| Years in practice, mean(s.d.) | 21.9(9.6) | 22.6(9.6) | 21.4(9.5) | <0.0001 |
| Surgeon volume quartile | <0.0001 | |||
| Lowest | 503 (1.5) | 247 (1.9) | 256 (1.3) | |
| Medium low | 1975 (6.1) | 903 (6.8) | 1072 (5.5) | |
| Medium high | 3378 (10.4) | 1566 (11.8) | 1812 (9.4) | |
| High | 26 756 (82.0) | 10 514 (79.5) | 16 242 (83.8) | |
| Colorectal specialists | 17 956 (55.1) | 6751 (51.0) | 11 205 (57.8) | <0.0001 |
| Surgeon sex, male | 24 900 (76.4) | 10 177 (76.9) | 14 723 (75.9) | 0.06 |
| Hospital volume quartile | <0.0001 | |||
| Lowest | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Medium low | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Medium high | 3217 (9.9) | 1531 (11.6) | 1686 (8.7) | |
| High | 29 395 (90.1) | 11 699 (88.4) | 17 696 (91.3) | |
| Patient factors | ||||
| Age (years), mean(s.d.) | 59.7(15.8) | 59.4(16.0) | 59.8(15.7) | 0.02 |
| Patient sex, male | 16 989 (52.1) | 6961 (47.4) | 10 028 (51.7) | 0.12 |
| Ontario Marginalization Index*, mean(s.d.) | 3.09(0.8) | 3.13(0.8) | 3.06(0.8) | <0.0001 |
| Co-morbidities (Johns Hopkins Adjusted Clinical Group (ACG) score)† | 0.001 | |||
| 0–2 | 171 (0.5) | 71 (0.5) | 100 (0.5) | |
| 3 | 9264 (28.4) | 3883 (29.4) | 5381 (27.7) | |
| 4 | 10 802 (33.1) | 4413 (33.4) | 6389 (33.0) | |
| 5 | 12 375 (38.0) | 4863 (36.8) | 7512 (38.8) | |
| Diagnosis | 0.01 | |||
| Colorectal cancer | 19 578 (60.0) | 7936 (60.0) | 11 642 (60.1) | |
| Crohn’s disease | 2376 (7.3) | 1006 (7.6) | 1370 (7.0) | |
| Ulcerative colitis | 2055 (6.3) | 873 (6.6) | 1182 (6.1) | |
| Diverticular disease | 2110 (6.5) | 875 (6.6) | 1235 (6.4) | |
| Other | 6493 (19.9) | 2540 (19.2) | 3953 (20.4) | |
| Details of surgical procedure | ||||
| Surgery | <0.0001 | |||
| Ileostomy reversal | 6300 (19.3) | 2544 (19.2) | 3756 (19.4) | |
| Right hemicolectomy | 7539 (23.1) | 2999 (22.3) | 4540 (23.4) | |
| Anterior resection | 4691 (14.4) | 1184 (9.0) | 3507 (18.1) | |
| Abdominoperineal resection | 1997 (6.1) | 820 (6.2) | 1177 (6.1) | |
| Other surgeries | 12 085 (37.1) | 5683 (43.3) | 6402 (33.0) | |
| In-hospital complications‡ | 3654 (11.2) | 1653 (12.5) | 2001 (10.3) | <0.0001 |
| Laparoscopic surgery | 10 947 (33.6) | 3483 (26.3) | 7464 (38.5) | <0.0001 |
| Surgery with diverting ileostomy | 2658 (8.2) | 958 (7.2) | 1700 (8.8) | <0.0001 |
| Surgery with lysis of adhesions | 2632 (8.1) | 886 (6.7) | 1746 (9.0) | <0.0001 |
| Repeat bowel resection | 1602 (4.9) | 775 (5.9) | 827 (4.3) | <0.0001 |
| Surgery in early week (Monday to Wednesday) | 19 200 (58.9) | 7811 (59.0) | 11 389 (58.8) | 0.87 |
Values are n (%) unless otherwise indicated. Continuous and categorical variables were compared before and after ERAS implementation using independent sample t tests and chi-squared tests respectively. *Using validated methodology, census-based data were combined to approximate the relative marginalization that patients experience based on the residential instability, material deprivation, dependency, and ethnic concentration of dissemination areas in which patients live26,28. Higher scores indicate higher levels of marginalization. †Existing diagnoses associated with patients were used to predict the propensity of individual patients to utilize the healthcare system. This is a validated and commonly used proxy for patient co-morbidity in the Health Services Research literature25,29,30. Higher scores indicate higher levels of patient co-morbidity. ‡Included anastomotic leak, reoperation, sepsis, cardiac arrest, myocardial infarction, blood transfusion, deep-vein thrombosis, pulmonary embolus, and stroke. ERAS, enhanced recovery after surgery.
Statistics
Single-series interrupted time series analyses were conducted, comparing mean values for crude LOS for 1-month intervals spanning 5 years before and after ERAS implementation. A 6-month interval for implementation was selected a priori, such that the interval ‘before ERAS implementation’ ended 3 months before the implementation date of the ERAS protocols and the interval ‘after ERAS implementation’ started 3 months after ERAS implementation. Previous studies assessing the impact of protocol-based changes on post-surgical outcomes21 have utilized 3-month ‘buffer’ intervals before and after the intervention. This approach balances the need for secure exposure ascertainment with the ability to assess the impact of these protocols on LOS as close to the time of implementation as possible (start of the iERAS study).
The primary analysis was performed using mean values for crude LOS for four cohorts to explore the potential differential impact of ERAS protocol introduction in the context of different degrees of surgical complexity: all CRS within the study interval; only CRS with no postoperative complications; right-sided CRS (ileostomy reversals, ileocolic resections, and right hemicolectomies); and left-sided CRS. A sensitivity analysis was performed, repeating all analyses using adjusted LOS as the outcome. Crude LOS was used as the primary outcome, as it was directly observed data, in comparison with adjusted LOS, which was calculated and therefore used for the sensitivity analysis.
The two primary outputs of the interrupted time series were the ‘level change’ and the ‘slope change’ of the mean values for LOS. These parameters were calculated using crude LOS in the main analysis and adjusted LOS in the sensitivity analysis. The ‘level change’ was defined as the change in mean LOS before and after ERAS implementation and measures the immediate impact of ERAS protocol implementation. The pre-existing trend in LOS after CRS was quantified by the slope of the trend line for mean LOS before ERAS. Similarly, the slope of the trend line for mean LOS after ERAS defined the post-ERAS trend in LOS27. ‘Slope change’ refers to a change in the rate at which pre-existing LOS trends changed over time and assesses the extent to which ERAS protocol implementation led to ongoing decreases in LOS after CRS. Slope change was calculated as the difference in slopes of LOS before and after the introduction of ERAS protocols. The slope change and the level change for mean LOS after CRS were calculated using linear regression, along with 95% confidence intervals, and tests of statistical significance were conducted for each of the aforementioned parameters31.
The assumptions underlying linear regression were checked and verified for all analyses. The presence of autocorrelation in the model residuals was assessed. Where autocorrelation was detected, standard errors for the model were adjusted to avoid biased estimates resulting from inflated standard errors. Newey–West standard error adjustment was performed to account for autocorrelation in the data31. All analyses were conducted using SAS Enterprise version 7.4. All statistical tests conducted were two-sided and P < 0.050 was considered statistically significant.
Results
Cohort characteristics
A total of 32 612 patients underwent CRS at the 15 iERAS hospitals between 2008 and 2019, with 41% of patients undergoing surgery in the pre-intervention interval and 59% in the post-intervention interval (Fig. S1). Baseline cohort characteristics of the patients and surgeons are reported in Table 1.
Post-implementation group patients were older, with more co-morbidities. The post-ERAS implementation group had 4% more surgeries performed by high-volume surgeons, 8% more surgeries performed by colorectal specialists, 12% more laparoscopic procedures, and 2% fewer complications than the pre-ERAS implementation group. The median LOS for all patients in the cohort was 5 (interquartile range 4–8) days and the mean(s.d.) LOS was 6.88(6.91) days.
The median LOS for patients treated before and after the ERAS implementation interval was 6 (interquartile range 4–8) days and 5 (interquartile range 4–7) days respectively. The mean(s.d.) LOS for patients treated before and after the ERAS implementation interval was 7.66(7.97) days and 6.27(6.00) days respectively, corresponding to a difference in mean LOS of 1.39 days.
Impact of ERAS protocol implementation on length of stay after colorectal surgery (level change)
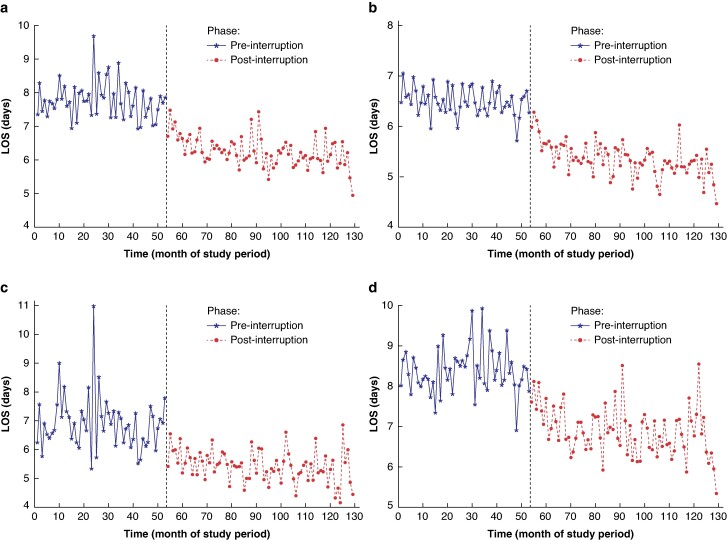
Table 2 shows the results of the interrupted time series analysing the impact of ERAS protocol implementation on LOS. For the cohort containing all patients undergoing CRS during the study interval, ERAS protocols were associated with a decrease in LOS of 1.05 (95% c.i. 0.72 to 1.38) days (P < 0.001) (Fig. 1). Decreases in LOS (level change decreases) were also seen for the other study cohorts (Fig. 1). The largest decrease in LOS was 1.17 (95% c.i. 0.71 to 1.63) days (P < 0.001) for the left-sided CRS group and the smallest decrease in LOS was 0.71 (95% c.i. 0.51 to 0.91) days (P < 0.001) for the CRS group without complications.
Table 2.
Impact of ERAS protocol implementation on length of stay after colorectal surgery (level change)
| Cohort/subgroup | Level change in crude length of stay (95% c.i.), P | Level change in adjusted length of stay (95% c.i.), P |
|---|---|---|
| All colorectal surgery | Decreased by 1.05 (0.72,1.38) days, <0.001 | Decreased by 1.46 (1.25,1.66) days, <0.001 |
| Colorectal surgery without complications | Decreased by 0.71 (0.51,0.91) days, <0.001 | Decreased by 1.02 (0.93,1.10) days, <0.001 |
| Right-sided colorectal surgery | Decreased by 1.01 (0.5,1.51) days, 0.0001 | Decreased by 1.38 (1.28,1.49) days, <0.0001 |
| Left-sided colorectal surgery | Decreased by 1.17 (0.71,1.63) days, <0.001 | Decreased by 1.31 (1.2,1.41) days, <0.001 |
ERAS, enhanced recovery after surgery.
Fig. 1.
Results of the interrupted time series analysis for the impact of the introduction of ERAS protocols on LOS after colorectal surgery
a All colorectal surgery. b Colorectal surgery without complications. c Right-sided colorectal surgery. d Left-sided colorectal surgery. Results are presented for ‘pre-interruption’ and ‘post-interruption’ intervals, corresponding to the pre-implementation and post-implementation phases respectively. LOS, length of stay; ERAS, enhanced recovery after surgery; CRS, colorectal surgery.
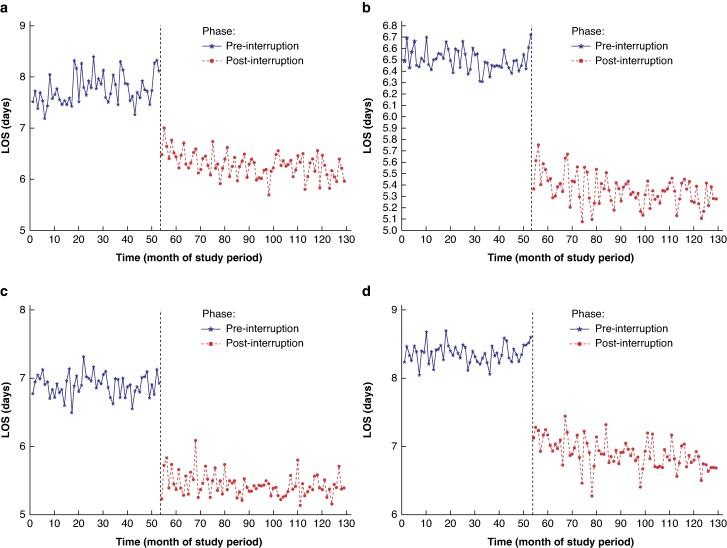
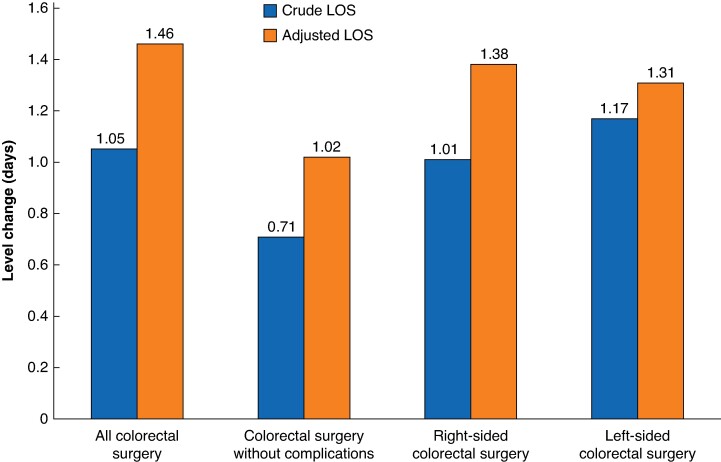
The effect of ERAS protocol implementation on adjusted LOS was greater (Table 2), with a decrease in adjusted LOS for all patients undergoing CRS of 1.46 (95% c.i. 1.25 to 1.66) days (P < 0.001) (Fig. 2). Decreases in adjusted LOS (level change decreases) were also seen for the other study cohorts (that is patients undergoing CRS without complications, patients undergoing right-sided CRS, and patients undergoing left-sided CRS) after ERAS protocol implementation (Fig. 2). The largest decrease in LOS was 1.38 (95% c.i. 1.28 to 1.49) days (P < 0.001) for the right-sided CRS group and the smallest decrease in LOS was 1.02 (95% c.i. 0.93 to 1.10) days (P < 0.001) for the CRS group without complications. The level changes observed in all analyses are depicted graphically in Fig. 3.
Fig. 2.
Results of the interrupted time series analysis for the impact of the introduction of ERAS protocols on adjusted LOS after colorectal surgery
a All colorectal surgery. b Colorectal surgery without complications. c Right-sided colorectal surgery. d Left-sided colorectal surgery. Results are presented for ‘pre-interruption’ and ‘post-interruption’ intervals, corresponding to the pre-implementation and post-implementation phases respectively. LOS, length of stay; ERAS, enhanced recovery after surgery.
Fig. 3.
Graph depicting the level change (decrease in LOS, measured in days) after ERAS protocol implementation for patients in each cohort
The blue bars represent the level change in crude LOS and the red bars represent the level change in adjusted LOS. LOS, length of stay; ERAS, enhanced recovery after surgery.
Impact of ERAS protocol implementation on rate of decrease in length of stay after colorectal surgery: slope change
In the primary analysis (using crude LOS), no significant slope change was noted for any of the cohorts, indicating that, although the introduction of ERAS protocols led to a prompt and sustained decrease in LOS, it did not significantly alter the rate at which LOS decreased over time compared with the temporal trends seen pre-intervention.
When analysing adjusted LOS for the entire study cohort, there was a small, but statistically significant, slope change (−0.011 (95% c.i. −0.017 to −0.004); P = 0.001). Similarly, for the subgroup of patients undergoing left-sided CRS, a significant slope change was noted (−0.006 (95% c.i. −0.009 to −0.003); P = 0.004). This indicates that, when adjusting for covariates, ERAS protocols were seen to slightly increase the rate at which LOS continued to decrease after the intervention, when compared with the temporal trends pre-intervention. No significant slope change was seen for the subgroup of patients undergoing CRS without complications or the subgroup of patients undergoing right-sided CRS. The slope change identified in each of these analyses is listed in Table 3.
Table 3.
Impact of ERAS protocol implementation on the rate of decrease in LOS over time (slope change)
| Cohort/subgroup | Slope change in crude length of stay (95% c.i.), P | Slope change in adjusted length of stay (95% c.i.), P |
|---|---|---|
| All colorectal surgery | −0.0006 (−0.015,0.004), 0.231 | −0.011 (−0.017,−0.004), 0.001 |
| Colorectal surgery without complications | −0.004 (−0.009,0.001), 0.131 | −0.001 (−0.004,0.001), 0.299 |
| Right-sided colorectal surgery | −0.008 (−0.016,0.014), 0.913 | −0.002 (−0.005,0.001), 0.255 |
| Left-sided colorectal surgery | −0.011 (−0.023,0.002), 0.903 | −0.006 (−0.009,−0.003), 0.0004 |
ERAS, enhanced recovery after surgery; LOS, length of stay.
Discussion
This study shows that the organized implementation of ERAS protocols at 15 hospitals is associated with a decrease in LOS of 1.05 days, independent of pre-existing temporal trends and an adjusted decrease in LOS. This decrease in LOS improves to 1.46 days after adjustment for important patient- and provider-level factors.
These results are similar to two previous population-based studies32,33 that used pre–post designs to assess the impact of ERAS implementation on LOS and found decreases of 0.932 and 1.933 days respectively. However, the pre–post design utilized by those studies precluded consideration of temporal trends toward decreasing LOS. This limited the ability of those studies to attribute changes in LOS to ERAS protocol implementation itself, exclusive of pre-existing trends toward decreasing LOS.
Another study in the literature34 considered the impact of pre-existing trends toward LOS after CRS. In that study, no significant change in LOS was found after ERAS implementation. In the present analysis, longer observation intervals before and after protocol implementation were maintained, a formal implementation interval was built into the analysis, and the consistency of the results for subgroups and the adjusted analysis was validated. In addition, it is possible that the processes used to implement the protocols across the hospitals differed between the two studies, contributing to the differences in observed effectiveness. Nevertheless, this study provides evidence that hospital-level ERAS protocol implementation can be an effective strategy for reducing LOS after CRS.
Previous randomized clinical trials (RCTs) have shown an association between ERAS interventions and decreased LOS after CRS7–16, of up to 2.26 days. In contrast, the present study, showing a smaller reduction in LOS of 1.05–1.46 days, was conducted in the ‘real world’ at a population level, outside the controlled setting of an RCT. As such, although the findings of the present study are less dramatic than the existing RCT evidence, these findings are likely more reflective of the effectiveness of ERAS protocol implementation and durability in the real-world setting. Additionally, the fact that these results were seen in a wide implementation at 15 hospitals, rather than a single centre, strengthens the results and would suggest that they have broad applicability to other settings. Accurate estimates of the effectiveness of ERAS protocol implementation are vital to CRS providers and healthcare system planners who are considering allocating resources to introduce formal ERAS protocols in their respective practice contexts.
It is notable that ERAS protocol implementation produced immediate decreases in LOS (level change), despite the individual elements of the ERAS protocols being well known. It is likely that individual ERAS elements were in use, to varying degrees, both before and after formal ERAS protocol implementation. The immediate decrease seen in LOS in this study therefore demonstrates that protocols implemented at the hospital level can yield significant benefits, even though there may have been a large degree of variation in how the ERAS protocols were operationalized at the individual surgeon-patient level.
In contrast to the immediate decrease in LOS, there was no material change in the rate at which LOS continued to change over time (slope change). Importantly, however, the effect was durable, with the data showing no regression towards pre-intervention LOS levels after the iERAS observation interval was complete. This long-term sustainability has not previously been shown in the literature and makes it unlikely that the Hawthorne effect (observer bias) was responsible for the observed effects. Instead, this finding lends further credence to ERAS protocol implementation as a catalyst for this lasting change.
Although this study demonstrates the effectiveness of ERAS protocol implementation as a means of reducing LOS after CRS, it does not elucidate the mechanism by which ERAS protocols bring about this effect. It is implausible that the effects are mediated purely by improved patient outcomes (for example lower complications), given the rapidity and durability of effect without significant ongoing change over time. However, it is possible that ERAS protocols may be effective due to their ability to change physician behavior by automating care processes (for example Foley catheter removal), forcing functions (for example changes to standard order sets), or, perhaps, by decreasing variation in care and leading to discussions between surgeons around best practices. The literature in this area is sparse and further focused study to elucidate the specific means by which ERAS protocols reduce LOS may help optimize their implementation.
This study is limited by the fact that compliance with ERAS protocol elements was variable between patients and at the surgeon level35. These differences in protocol adoption and compliance may have contributed to ongoing variation in LOS. Additionally, not all study patients at the iERAS hospitals may have been treated on the ERAS pathway by their surgeon. However, this particular study was not intended to assess the efficacy of the ERAS interventions themselves, but rather the effectiveness of ERAS protocol implementation at the hospital level with regard to individual patient outcomes. This reflects the real-world setting, whereby patients are not necessarily treated uniformly and protocol adherence may be suboptimal. Similarly, this study was not designed to assess which specific ERAS elements may be of greatest benefit. Importantly, incomplete adherence to ERAS protocols and the inclusion of patients who were not formally treated as part of an ERAS protocol would bias towards the null and therefore it is possible that initiatives to improve adherence could yield even greater reductions in LOS. Given the central nature of LOS as a study outcome, CRS patients who died in hospital were not included in the study. Had they been included, it would be challenging to analyse patients with short LOS due to their death in the early postoperative course. Given the low rates of in-hospital mortality after elective CRS, and the differences between these patients and the rest of the cohort, it is unlikely that significant bias was introduced. However, it must be noted that this study cannot comment on the efficacy of ERAS implementation as a tool to reduce mortality after CRS. Finally, this study included only patients treated at teaching hospitals with relatively high CRS volumes and, as such, the results may not be immediately generalizable to other settings that are not represented in the original iERAS study cohort.
This study shows that ERAS protocol implementation at a multi-hospital level is directly associated with a reduction in LOS after CRS of 1.46 days and that this effect is independent of temporal trends and durable over time. The study also demonstrates that protocols implemented at the hospital level can yield significant benefits, even though they may be operationalized at the individual surgeon-patient level. When scaled over the number of CRS performed every year, ERAS protocols have the potential to significantly improve the patient experience and to reduce health resource utilization. Future studies will be necessary to understand the mechanisms by which ERAS protocol implementation produces these effects and may help optimize implementation processes.
Supplementary Material
Acknowledgements
The authors thank the Toronto Community Health Profiles Partnership for providing access to the Ontario Marginalization Index.
Contributor Information
Zubair Bayat, Division of General Surgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Department of Surgery, Mount Sinai Hospital, Sinai Health System, Toronto, Ontario, Canada.
Anand Govindarajan, Division of General Surgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Department of Surgery, Mount Sinai Hospital, Sinai Health System, Toronto, Ontario, Canada; Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada.
J Charles Victor, Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada.
Erin D Kennedy, Division of General Surgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Department of Surgery, Mount Sinai Hospital, Sinai Health System, Toronto, Ontario, Canada.
Funding
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ontario Ministry of Long-Term Care (MLTC). Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI) and the Ontario MOH. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Author contributions
Zubair Bayat (Conceptualization, Data curation, Formal analysis, Validation, Investigation, Methodology, Project administration, Writing—original draft), Anand Govindarajan (Conceptualization, Investigation, Methodology, Supervision, Writing—review & editing), J. Charles Victor (Formal analysis, Validation, Writing—review & editing), and Erin D. Kennedy (Conceptualization, Investigation, Methodology, Supervision, Writing—review & editing)
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary data is available at BJS Open online.
Data availability
Ontario health administrative data are available through the Institute for Clinical Evaluative Sciences (ICES), the Canadian Institute for Health Information (CIHI), and the Ontario Ministry of Health (MOH) after appropriate approvals; anonymized data can be shared upon request.
References
- 1. DeWane MP, Davis KA, Schuster KM, Maung AA, Becher RD. Venous thromboembolism-related readmission in emergency general surgery patients: a role for prophylaxis on discharge? J Am Coll Surg 2018;226:1072–1077.e3 [DOI] [PubMed] [Google Scholar]
- 2. Iannuzzi JC, Young KC, Kim MJ, Gillespie DL, Monson JRT, Fleming FJ. Prediction of postdischarge venous thromboembolism using a risk assessment model. J Vasc Surg 2013;58:1014–1020.e1 [DOI] [PubMed] [Google Scholar]
- 3. Nielsen AW, Helm MC, Kindel T, Higgins R, Lak K, Helmen ZM et al. Perioperative bleeding and blood transfusion are major risk factors for venous thromboembolism following bariatric surgery. Surg Endosc 2018;32:2488–2495 [DOI] [PubMed] [Google Scholar]
- 4. Suesada MM, Martins MA, Carvalho CRF. Effect of short-term hospitalization on functional capacity in patients not restricted to bed. Am J Phys Med Rehabil 2007;86:455–462 [DOI] [PubMed] [Google Scholar]
- 5. Andrews LB, Stocking C, Krizek T, Gottlieb L, Krizek C, Vargish T et al. An alternative strategy for studying adverse events in medical care. Lancet 1997;349:309–313 [DOI] [PubMed] [Google Scholar]
- 6. Graves N, Weinhold D, Roberts JA. Correcting for bias when estimating the cost of hospital-acquired infection: an analysis of lower respiratory tract infections in non-surgical patients. Health Econ 2005;14:755–761 [DOI] [PubMed] [Google Scholar]
- 7. Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: a review. JAMA Surg 2017;152:292–298 [DOI] [PubMed] [Google Scholar]
- 8. Eskicioglu C, Forbes SS, Aarts M-A, Okrainec A, McLeod RS. Enhanced Recovery After Surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg 2009;13:2321–2329 [DOI] [PubMed] [Google Scholar]
- 9. Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014;38:1531–1541 [DOI] [PubMed] [Google Scholar]
- 10. Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172–188 [DOI] [PubMed] [Google Scholar]
- 11. Hedrick TL, Thiele RH, Hassinger TE, Donovan J, Reines HD, Damico E et al. Multicenter observational study examining the implementation of enhanced recovery within the Virginia Surgical Quality Collaborative in patients undergoing elective colectomy. J Am Coll Surg 2019;229:374–382.e3 [DOI] [PubMed] [Google Scholar]
- 12. Bednarski BK, Nickerson TP, You YN, Messick CA, Speer B, Gottumukkala V et al. Randomized clinical trial of accelerated enhanced recovery after minimally invasive colorectal cancer surgery (RecoverMI trial). Br J Surg 2019;106:1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868–875 [DOI] [PubMed] [Google Scholar]
- 14. Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J et al. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg 2012;36:407–414 [DOI] [PubMed] [Google Scholar]
- 15. Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum 2003;46:851–859 [DOI] [PubMed] [Google Scholar]
- 16. Lau CSM, Chamberlain RS. Enhanced Recovery After Surgery programs improve patient outcomes and recovery: a meta-analysis. World J Surg 2017;41:899–913 [DOI] [PubMed] [Google Scholar]
- 17. Bayat Z, Guidolin K, Elsolh B, De Castro C, Kennedy E, Govindarajan A. Impact of surgeon and hospital factors on length of stay after colorectal surgery systematic review. BJS Open 2022;6:zrac110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aarts M-A, Rotstein OD, Pearsall EA, Victor JC, Okrainec A, McKenzie M et al. Postoperative ERAS interventions have the greatest impact on optimal recovery: experience with implementation of ERAS across multiple hospitals. Ann Surg 2018;267:992–997 [DOI] [PubMed] [Google Scholar]
- 19. Institute for Clinical Evaluative Sciences (ICES) . ICES FAQs. https://www.ices.on.ca/About-ICES/FAQs (accessed 5 February 2021)
- 20. Simunovic M, Thériault M-E, Paszat L, Coates A, Whelan T, Holowaty E et al. Using administrative databases to measure waiting times for patients undergoing major cancer surgery in Ontario, 1993–2000. Can J Surg 2005;48:137–142 [PMC free article] [PubMed] [Google Scholar]
- 21. Urbach DR, Govindarajan A, Saskin R, Wilton AS, Baxter NN. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med 2014;370:1029–1038 [DOI] [PubMed] [Google Scholar]
- 22. Govindarajan A, Urbach DR, Kumar M, Li Q, Murray BJ, Juurlink D et al. Outcomes of daytime procedures performed by attending surgeons after night work. N Engl J Med 2015;373:845–853 [DOI] [PubMed] [Google Scholar]
- 23. Satkunasivam R, Klaassen Z, Ravi B, Fok K-H, Menser T, Kash B et al. Relation between surgeon age and postoperative outcomes: a population-based cohort study. CMAJ 2020;192:E385–E392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schull MJ, Azimaee M, Marra M, Cartagena RG, Vermeulen MJ, Ho M et al. ICES: data, discovery, better health. Int J Popul Data Sci 2020;4:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 2011;49:932–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matheson FI, Dunn JR, Smith KLW, Moineddin R, Glazier RH. Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health Rev Can Sante Publique 2012;103(Suppl 2):S12–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matheson FI, van Ingen T. 2016 Ontario Marginalization Index: User Guide. 2018. https://www.publichealthontario.ca/-/media/documents/O/2017/on-marg-userguide.pdf?la=en (accessed 5 February 2021)
- 29. Johns Hopkins Medicine . The Johns Hopkins ACG® System. https://www.hopkinsacg.org/wp-content/uploads/2020/11/Johns-Hopkins-ACG-System-Overview-v111820.pdf (accessed 8 February 2021)
- 30. Johns Hopkins Bloomberg School of Public Health . The Johns Hopkins ACG® System—Excerpt From Technical Reference Guide. https://www.hopkinsacg.org/document/acg-system-version-11-technical-reference-guide/ (accessed 8 February 2021)
- 31. Caswell J. Interrupted Time Series Analysis for Single Series and Comparative Designs: A Guide for Beginners with SAS Macro. 2017. https://www.linkedin.com/pulse/interrupted-time-series-analysis-single-comparative-designs-caswell-1/ (accessed 8 February 2021)
- 32. Nelson G, Wang X, Nelson A, Faris P, Lagendyk L, Wasylak T et al. Evaluation of the implementation of multiple Enhanced Recovery After Surgery pathways across a provincial health care system in Alberta, Canada. JAMA Netw Open 2021;4:e2119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Zelm R, Coeckelberghs E, Sermeus W, Wolthuis A, Bruyneel L, Panella M et al. Effects of implementing a care pathway for colorectal cancer surgery in ten European hospitals: an international multicenter pre–post-test study. Updat Surg 2020;72:61–71 [DOI] [PubMed] [Google Scholar]
- 34. AlBalawi Z, Gramlich L, Nelson G, Senior P, Youngson E, McAlister FA. The impact of the implementation of the Enhanced Recovery After Surgery (ERAS®) program in an entire health system: a natural experiment in Alberta, Canada. World J Surg 2018;42:2691–2700 [DOI] [PubMed] [Google Scholar]
- 35. Wood T, Aarts M-A, Okrainec A, Pearsall E, Victor JC, McKenzie M et al. Emergency room visits and readmissions following implementation of an Enhanced Recovery After Surgery (iERAS) program. J Gastrointest Surg 2018;22:259–266 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Ontario health administrative data are available through the Institute for Clinical Evaluative Sciences (ICES), the Canadian Institute for Health Information (CIHI), and the Ontario Ministry of Health (MOH) after appropriate approvals; anonymized data can be shared upon request.
References
- 1. DeWane MP, Davis KA, Schuster KM, Maung AA, Becher RD. Venous thromboembolism-related readmission in emergency general surgery patients: a role for prophylaxis on discharge? J Am Coll Surg 2018;226:1072–1077.e3 [DOI] [PubMed] [Google Scholar]
- 2. Iannuzzi JC, Young KC, Kim MJ, Gillespie DL, Monson JRT, Fleming FJ. Prediction of postdischarge venous thromboembolism using a risk assessment model. J Vasc Surg 2013;58:1014–1020.e1 [DOI] [PubMed] [Google Scholar]
- 3. Nielsen AW, Helm MC, Kindel T, Higgins R, Lak K, Helmen ZM et al. Perioperative bleeding and blood transfusion are major risk factors for venous thromboembolism following bariatric surgery. Surg Endosc 2018;32:2488–2495 [DOI] [PubMed] [Google Scholar]
- 4. Suesada MM, Martins MA, Carvalho CRF. Effect of short-term hospitalization on functional capacity in patients not restricted to bed. Am J Phys Med Rehabil 2007;86:455–462 [DOI] [PubMed] [Google Scholar]
- 5. Andrews LB, Stocking C, Krizek T, Gottlieb L, Krizek C, Vargish T et al. An alternative strategy for studying adverse events in medical care. Lancet 1997;349:309–313 [DOI] [PubMed] [Google Scholar]
- 6. Graves N, Weinhold D, Roberts JA. Correcting for bias when estimating the cost of hospital-acquired infection: an analysis of lower respiratory tract infections in non-surgical patients. Health Econ 2005;14:755–761 [DOI] [PubMed] [Google Scholar]
- 7. Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: a review. JAMA Surg 2017;152:292–298 [DOI] [PubMed] [Google Scholar]
- 8. Eskicioglu C, Forbes SS, Aarts M-A, Okrainec A, McLeod RS. Enhanced Recovery After Surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg 2009;13:2321–2329 [DOI] [PubMed] [Google Scholar]
- 9. Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014;38:1531–1541 [DOI] [PubMed] [Google Scholar]
- 10. Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172–188 [DOI] [PubMed] [Google Scholar]
- 11. Hedrick TL, Thiele RH, Hassinger TE, Donovan J, Reines HD, Damico E et al. Multicenter observational study examining the implementation of enhanced recovery within the Virginia Surgical Quality Collaborative in patients undergoing elective colectomy. J Am Coll Surg 2019;229:374–382.e3 [DOI] [PubMed] [Google Scholar]
- 12. Bednarski BK, Nickerson TP, You YN, Messick CA, Speer B, Gottumukkala V et al. Randomized clinical trial of accelerated enhanced recovery after minimally invasive colorectal cancer surgery (RecoverMI trial). Br J Surg 2019;106:1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868–875 [DOI] [PubMed] [Google Scholar]
- 14. Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J et al. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg 2012;36:407–414 [DOI] [PubMed] [Google Scholar]
- 15. Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum 2003;46:851–859 [DOI] [PubMed] [Google Scholar]
- 16. Lau CSM, Chamberlain RS. Enhanced Recovery After Surgery programs improve patient outcomes and recovery: a meta-analysis. World J Surg 2017;41:899–913 [DOI] [PubMed] [Google Scholar]
- 17. Bayat Z, Guidolin K, Elsolh B, De Castro C, Kennedy E, Govindarajan A. Impact of surgeon and hospital factors on length of stay after colorectal surgery systematic review. BJS Open 2022;6:zrac110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aarts M-A, Rotstein OD, Pearsall EA, Victor JC, Okrainec A, McKenzie M et al. Postoperative ERAS interventions have the greatest impact on optimal recovery: experience with implementation of ERAS across multiple hospitals. Ann Surg 2018;267:992–997 [DOI] [PubMed] [Google Scholar]
- 19. Institute for Clinical Evaluative Sciences (ICES) . ICES FAQs. https://www.ices.on.ca/About-ICES/FAQs (accessed 5 February 2021)
- 20. Simunovic M, Thériault M-E, Paszat L, Coates A, Whelan T, Holowaty E et al. Using administrative databases to measure waiting times for patients undergoing major cancer surgery in Ontario, 1993–2000. Can J Surg 2005;48:137–142 [PMC free article] [PubMed] [Google Scholar]
- 21. Urbach DR, Govindarajan A, Saskin R, Wilton AS, Baxter NN. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med 2014;370:1029–1038 [DOI] [PubMed] [Google Scholar]
- 22. Govindarajan A, Urbach DR, Kumar M, Li Q, Murray BJ, Juurlink D et al. Outcomes of daytime procedures performed by attending surgeons after night work. N Engl J Med 2015;373:845–853 [DOI] [PubMed] [Google Scholar]
- 23. Satkunasivam R, Klaassen Z, Ravi B, Fok K-H, Menser T, Kash B et al. Relation between surgeon age and postoperative outcomes: a population-based cohort study. CMAJ 2020;192:E385–E392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schull MJ, Azimaee M, Marra M, Cartagena RG, Vermeulen MJ, Ho M et al. ICES: data, discovery, better health. Int J Popul Data Sci 2020;4:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 2011;49:932–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matheson FI, Dunn JR, Smith KLW, Moineddin R, Glazier RH. Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health Rev Can Sante Publique 2012;103(Suppl 2):S12–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matheson FI, van Ingen T. 2016 Ontario Marginalization Index: User Guide. 2018. https://www.publichealthontario.ca/-/media/documents/O/2017/on-marg-userguide.pdf?la=en (accessed 5 February 2021)
- 29. Johns Hopkins Medicine . The Johns Hopkins ACG® System. https://www.hopkinsacg.org/wp-content/uploads/2020/11/Johns-Hopkins-ACG-System-Overview-v111820.pdf (accessed 8 February 2021)
- 30. Johns Hopkins Bloomberg School of Public Health . The Johns Hopkins ACG® System—Excerpt From Technical Reference Guide. https://www.hopkinsacg.org/document/acg-system-version-11-technical-reference-guide/ (accessed 8 February 2021)
- 31. Caswell J. Interrupted Time Series Analysis for Single Series and Comparative Designs: A Guide for Beginners with SAS Macro. 2017. https://www.linkedin.com/pulse/interrupted-time-series-analysis-single-comparative-designs-caswell-1/ (accessed 8 February 2021)
- 32. Nelson G, Wang X, Nelson A, Faris P, Lagendyk L, Wasylak T et al. Evaluation of the implementation of multiple Enhanced Recovery After Surgery pathways across a provincial health care system in Alberta, Canada. JAMA Netw Open 2021;4:e2119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Zelm R, Coeckelberghs E, Sermeus W, Wolthuis A, Bruyneel L, Panella M et al. Effects of implementing a care pathway for colorectal cancer surgery in ten European hospitals: an international multicenter pre–post-test study. Updat Surg 2020;72:61–71 [DOI] [PubMed] [Google Scholar]
- 34. AlBalawi Z, Gramlich L, Nelson G, Senior P, Youngson E, McAlister FA. The impact of the implementation of the Enhanced Recovery After Surgery (ERAS®) program in an entire health system: a natural experiment in Alberta, Canada. World J Surg 2018;42:2691–2700 [DOI] [PubMed] [Google Scholar]
- 35. Wood T, Aarts M-A, Okrainec A, Pearsall E, Victor JC, McKenzie M et al. Emergency room visits and readmissions following implementation of an Enhanced Recovery After Surgery (iERAS) program. J Gastrointest Surg 2018;22:259–266 [DOI] [PubMed] [Google Scholar]