Abstract
Aim: A new series of 1,2,3-triazole-hydrazone derivatives were developed to evaluate their anti-Alzheimer's activity.
Materials & methods: All compounds were screened toward cholinesterases via the modified Ellman's method. The toxicity assay on SH-SY5Y cells was performed using the MTT assay, and the expression levels of GSK-3α, GSK-3β, DYRK1 and CDK5 were assessed in the presence of compounds 6m and 6p.
Results:6m and 6p; acting as mixed-type inhibitors, exhibited promising acetylcholinesterase and butyrylcholinesterase inhibitory activity, respectively. 6m demonstrated no toxicity under tested concentrations on the SH-SY5Y cells and positively impacted neurodegenerative pathways. Notably, 6m displayed a significant downregulation in mRNA levels of GSK-3α, GSK-3β and CDK5.
Conclusion: The target compounds could be considered in developing anti-Alzheimer's disease agents.
Keywords: : 1,2,3-Triazoles; AChE; Alzheimer's; BChE; CDK5; GSK-3α; GSK-3β; Hydrazones
Graphical Abstract
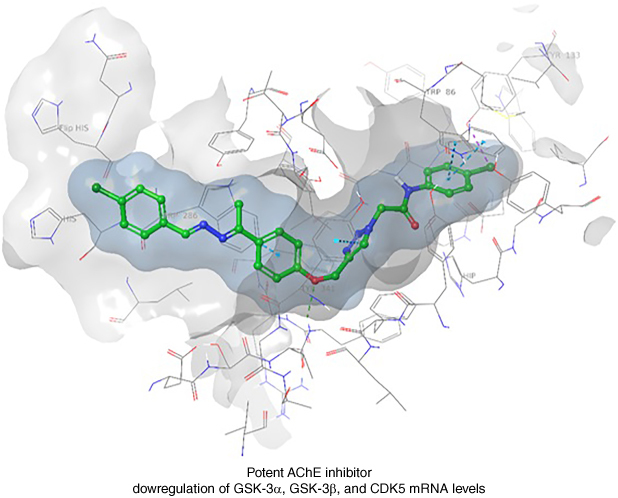
Plain language summary
Highlights.
A new series of 1,2,3-triazole-hydrazone hybrids were synthesized and evaluated for their anti- Alzheimer's activity.
Synthesized compounds were screened for cholinesterase inhibitory activity to select potent compounds for further biological assessment.
Compounds 6m and 6p exhibited promising inhibitory activity, with IC50 values of 5.23 and 3.39 μM against AChE and BChE, respectively, as mixed-type inhibitors.
Compounds 6m demonstrated no toxicity under tested concentrations on the SH-SY5Y cell line and positively impacted neurodegenerative pathways.
Compound 6m displayed a significant downregulation in mRNA levels of GSK-3α, GSK-3β and CDK5, suggesting a potential indirect role in mitigating toxic neurofibrillary tangle and amyloid β (Aβ) aggregate formation.
1. Background
Alzheimer's disease (AD) is characterized by a progressive and irreversible decline in cognitive function, particularly affecting memory, reasoning and language skills. As the most common cause of dementia, AD imposes a substantial burden on individuals, families and healthcare systems [1,2]. While the exact etiology of AD remains complex and multifaceted, research has increasingly spotlighted the key role of the accumulation of beta-amyloid (Aβ) plaques and neurofibrillary tau tangles, oxidative stress, neuroinflammation and cholinergic dysfunction in the pathophysiology of the disease [3–5].
According to the cholinergic hypothesis, the degeneration of cholinergic neurons and deficits in the neurotransmitter acetylcholine (ACh) play a pivotal role in the cognitive decline observed in patients with AD [6], particularly in older patients [7]. Acetylcholinesterase (AChE), primarily localized at cholinergic synapses, accelerates the breakdown of ACh and reduces its level in the synaptic cleft. In the late stage of AD, the level of AChE declines and butyrylcholinesterase (BChE) is considered to play the most dominant role in the hydrolysis of ACh [8]. AChE inhibitors such as donepezil, galantamine and rivastigmine have been employed to elevate ACh levels and alleviate cognitive symptoms temporarily, in the treatment of AD. Among the complex pathological changes in AD, protein kinases [9] play a significant role in triggering the abnormal phosphorylation of tau proteins. This process contributes to the formation of intracellular neuronal fiber tangles, neuronal loss, the deposition of extracellular Aβ, the creation of amyloid plaques and disruptions in synaptic function [6,10].
Glycogen synthase kinase 3 (GSK3) is a serine/threonine kinase that exists in two isoforms: GSK-3α and GSK-3β. Both isoforms, especially GSK-3α, play significant roles in various cellular processes, and their dysregulation has been implicated in the pathogenesis of AD [11,12]. These isoforms are involved in the hyperphosphorylation of tau protein, contributing to the formation of neurofibrillary tangles and dysregulation of amyloid precursor protein (APP) processing, affecting the production of Aβ plaques. Additionally, GSK3 is associated with apoptotic pathways that lead to neuronal cell death. The challenge lies in balancing its role in vital cellular processes and disease progress [13].
Cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine/threonine protein kinase crucial for brain development, neuronal survival, synaptic plasticity, and microtubule regulation [14]. However, Cdk5 becomes hyperactive under pathological stimuli, leading to the aberrant hyperphosphorylation of cytoskeletal proteins, including microtubule- and actin-associated proteins, as well as APP, tau and neurofilament, which leads to synaptic damage, mitochondrial dysfunction, cell cycle reactivation and neuronal apoptosis [15,16].
The intricate interplay between these molecular processes underscores the complexity of AD, posing challenges and opportunities for therapeutic interventions to preserve cholinergic function and alleviate cognitive decline. In this study, we have introduced a novel set of 1,2,3-triazole-hydrazone hybrids to assess their potential against AChE and BChE as an available strategy for the symptomatic treatment of AD. Additionally, kinetic and molecular docking studies were conducted to analyze the efficacy of the most promising analogs. Moreover, the neurotoxicity of the most potent derivatives on the SH-SY5Y cell line was examined. Furthermore, the post-treatment impact of the most potent analogs on the levels of GSK3α, GSK3β, Cdk5 and dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1) on the SH-SY5Y cell line was determined.
2. Methods & materials
2.1. Synthesis of 1,2,3-triazoles-hydrazone derivatives 6
A mixture of 1-(4-(prop-2-yn-1-yloxy)phenyl)ethan-1-one 1 (0.5 mmol) and in situ prepared azide derivative 2 (1 mmol) was stirred in the presence of catalytic amounts of CuSO4.5H2O and sodium ascorbate in H2O/t-BuOH (1:1) at room temperature (or in DMF at 50°C) for 24 h [17–20]. After completion of the reaction (checked by TLC), it was poured into ice cold water and the precipitates were filtered off to obtain compound 3. The mixture of the latter compound (1 mmol) and hydrazine hydrate (6 mmol) in MeOH (10 ml) was heated at reflux for 6 h. After completion of the reaction (checked by TLC), the mixture was cooled down to room temperature and the precipitates were filtered off to give compound 4. Finally, compound 4 (1 mmol) and desired aldehyde derivative 5 (1 mmol) reacted in the presence of catalytic amounts of glacial acetic acid in EtOH (10 ml) under reflux conditions for 6 h. After completion of the reaction (checked by TLC), the solvent was reduced under vacuum, the precipitates were filtered off and washed with cold ethanol to give compound 6. They were mostly pure; however, they could be recrystallized from EtOH.
2.1.1. 1-Benzyl-4-[(4-{1-[2-(phenylmethylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazole (6a)
Yield: 60%, mp = 197–199°C. IR (KBr): 3070, 1606, 1583, 1494, 1385, 1247 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.49 (s, 1H, =CH), 8.31 (s, 1H, triazole), 7.92–7.87 (m, 4H, H3, H5, H2″, H6″), 7.50–7.49 (m, 3H, H3″, H4″, H5″), 7.38–7.32 (m, 5H, H2′, H3′, H4′, H5′, H6′), 7.11 (d, J = 8.8 Hz, 2H, H2, H6), 5.62 (s, 2H, CH2), 5.21 (s, 2H, CH2), 2.45 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 163.8, 160.4, 157.1, 135.2, 133.8, 131.4, 131.0, 129.3, 129.2, 128.8, 128.6, 128.5, 128.4, 127.5, 125.2, 115.0, 60.1, 53.3, 15.6 ppm. MS (m/z, %): 91 (100), 144 (43), 238 (33), 332 (32), 409 (M+·, 22). Anal. calcd. for C25H23N5O: C, 73.33; H, 5.66; N, 17.10. Found: C, 73.56; H, 5.87; N, 16.90.
2.1.2. 1-[(2-Chlorophenyl)methyl]-4-[(4-{1-[2-(phenylmethylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazole (6b)
Yield: 88%, mp = 152–154°C. IR (KBr): 3070, 1605, 1503, 1441, 1380, 1245 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.47 (s, 1H, =CH), 8.27 (s, 1H, triazole), 7.88 (d, J = 7.3 Hz, 2H, H3, H5), 7.86–7.84 (m, 2H, H2″, H6″), 7.51 (d, J = 7.7 Hz, 1H, H3′), 7.48–7.47 (m, 3H, H3″, H4″, H5″), 7.40–7.34 (m, 2H, H4′, H5′), 7.21 (d, J = 7.5 Hz, 1H, H6′), 7.10 (d, J = 7.3 Hz, 2H, H2, H6), 5.71 (s, 2H, CH2), 5.21 (s, 2H, CH2), 2.44 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 163.8, 160.1, 158.0, 134.9, 133.7, 133.1, 132.4, 131.0, 130.8, 130.7, 130.1, 129.8, 129.3, 128.8, 128.5, 128.2, 125.7, 115.0, 61.3, 50.7, 15.8 ppm. MS (m/z, %): 125 (100), 178 (46), 238 (57), 366 (32), 443 (M+·, 24), 445 ([M+2]+·, 8). Anal. calcd. for C25H22ClN5O: C, 67.64; H, 5.00; N, 15.78. Found: C, 67.39; H, 5.28; N, 15.51.
2.1.3. 1-[(4-Chlorophenyl)methyl]-4-[(4-{1-[2-(phenylmethylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazole (6c)
Yield: 77%, mp = 126–128°C. IR (KBr): 3072, 1607, 1508, 1492, 1410, 1278 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.49 (s, 1H, =CH), 8.32 (s, 1H, triazole), 7.91–7.87 (m, 4H, H3, H5, H2″, H6″), 7.50–7.44 (m, 5H, H3′, H5′, H3″, H4″, H5″), 7.35 (d, J = 8.2 Hz, H2′, H6′), 7.11 (d, J = 8.5 Hz, H2, H6), 5.63 (s, 2H, CH2), 5.22 (s, 2H, CH2), 2.45 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 163.8, 160.2, 158.0, 143.3, 135.5, 135.0, 133.4, 131.3, 130.8, 130.4, 129.3, 129.2, 128.8, 128.5, 125.3, 115.0, 61.7, 52.5, 15.1 ppm. MS (m/z, %): 125 (100), 161 (42), 238 (58), 366 (24), 443 (M+·, 24), 445 ([M+2]+·, 8). Anal. calcd. for C25H22ClN5O: C, 67.64; H, 5.00; N, 15.78. Found: C, 67.80; H, 4.82; N, 15.94.
2.1.4. 1-[(4-Fluorophenyl)methyl]-4-[(4-{1-[2-(phenylmethylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazole (6d)
Yield: 90%, mp = 140–142°C. IR (KBr): 3079, 1600, 1507, 1447 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.50 (s, 1H, =CH), 8.31 (s, 1H, triazole), 7.91–7.87 (m, 4H, H3, H5, H2″, H6″), 7.50–7.48 (m, 2H, H2′, H6′), 7.42–7.39 (m, 2H, H3′, H5′), 7.22 (t, J = 8.8 Hz, 2H, H3″, H5″), 7.12–7.09 (m, 3H, H2, H6, H4″), 5.62 (s, 2H, CH2), 5.21 (s, 2H, CH2), 2.45 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 163.8, 162.4 (d, JC-F = 243.2 Hz), 160.2, 158.0, 143.3, 135.0, 132.7, 131.3, 130.8 (d, JC-F = 8.6 Hz), 129.3, 128.8, 128.5, 128.4, 125.2, 116.1 (d, JC-F = 85.8 Hz), 115.0, 61.7, 52.5, 15.1 ppm. MS (m/z, %): 91 (100), 144 (45), 238 (30), 427 (M+·, 15). Anal. calcd. for C25H22FN5O: C, 70.24; H, 5.19; N, 16.38. Found: C, 70.44; H, 5.34; N, 16.56.
2.1.5. N-Phenyl-2-{4-[(4-{1-[2-(phenylmethylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazol-1-yl}acetamide (6e)
Yield: 45%, mp = 210–213°C. IR (KBr): 3415, 3035, 1672, 1609, 1557, 1499, 1363, 1253 cm-1. 1H-NMR (500 MHz, DMSO-d6): 10.47 (s, 1H, NH), 8.48 (s, 1H, =CH), 8.28 (s, 1H, triazole), 7.91 (d, J = 6.8 Hz, 2H, H3, H5), 7.87 (d, J = 7.5, 2H, H2″, H6″), 7.58 (d, J = 8.4 Hz, 2H, H2′, H6′), 7.49–7.48 (m, 3H, H4″, H3″, H5″), 7.32 (t, J = 7.1 Hz, 2H, H3′, H5′), 7.14 (d, J = 6.8 Hz, 2H, H2, H6), 7.08 (t, J = 8.8 Hz, 1H, H4′), 5.35 (s, 2H, CH2), 5.25 (s, 2H, CH2), 2.45 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 164.6, 163.8, 160.3, 157.9, 142.7, 138.9, 135.0, 131.3, 130.8, 129.4, 129.3, 128.8, 128.5, 126.8, 124.2, 119.7, 115.0, 61.6, 53.1, 15.1 ppm. MS (m/z, %): 106 (60), 161 (68), 187 (24), 238 (100), 375 (51), 452 (M+·, 25). Anal. calcd. for C26H24N6O2: C, 69.01; H, 5.35; N, 18.57. Found: C, 69.25; H, 5.58; N, 18.78.
2.1.6. N-(4-Chlorophenyl)-2-{4-[(4-{1-[2-(phenylmethylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazol-1-yl}acetamide (6f)
Yield: 39%, mp = 233–235°C. IR (KBr): 3350, 1680, 2825, 1606 cm-1. 1H-HNMR (500 MHz, DMSO-d6): 10.62 (s, 1H, NH), 8.48 (s, 1H, =CH), 8.28 (s, 1H, triazole), 7.91 (d, J = 8.8 Hz, 2H, H3, H5), 7.87–7.85 (m, 2H, H2″, H6″), 7.60 (d, J = 8.9 Hz, 2H, H2′, H6′), 7.49–7.48 (m, 3H, H3″, H5″, H4″), 7.38 (d, J = 8.9 Hz, 2H, H3′, H5′), 7.13 (d, J = 8.8, 2H, H2, H6), 5.36 (s, 2H, CH2), 5.25 (s, 2H, CH2), 2.45 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 164.7, 163.8, 160.3, 157.9, 142.7, 137.5, 135.0, 134.0, 131.3, 130.8, 129.3, 128.8, 128.5, 127.9, 126.8, 121.3, 115.0, 61.6, 52.7, 15.1 ppm. MS (m/z, %): 106 (76), 134 (50), 168 (60), 187 (52), 231 (25), 272 (100), 375 (50), 486 (M+·, 21), 488 ([M+2]+·, 7). Anal. calcd. for C26H23ClN6O2: C, 64.13; H, 4.76; N, 17.26. Found: C, 64.45; H, 4.51; N, 17.52.
2.1.7. N-Benzyl-2-{4-[(4-{1-[2-(phenylmethylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazol-1-yl}acetamide (6g)
Yield: 69%, mp = 198–200°C. IR (KBr): 3304, 3081, 1656, 1604, 15502, 1449, 1562, 1245 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.84 (t, J = 5.8, 1H, NH), 8.47 (s, 1H, =CH), 8.22 (s, 1H, triazole), 7.90 (d, J = 8.8 Hz, 2H, H3, H5), 7.87–7.85 (m, 2H, H2″, H6″), 7.48 (m, 3H, H3″, H4″, H5″), 7.32 (t, J = 7.5 Hz, 2H, H3′, H5′), 7.28–7.23 (m, 3H, H2′, H4′, H6′), 7.12 (d, J = 8.8 Hz, 2H, H2, H6), 5.22 (s, 2H, CH2), 5.18 (s, 2H, CH2), 4.32 (d, J = 5.8 Hz, 2H, CH2), 2.44 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 165.9, 163.8, 160.3, 157.9, 142.6, 139.2, 134.9, 131.3, 130.7, 129.3, 128.9, 128.8, 128.5, 127.9, 127.5, 126.7, 115.0, 61.6, 52.1, 42.8, 15.1 ppm. MS (m/z, %): 91 (100), 161 (35), 201 (18), 238 (62), 389 (30), 466 (M+·, 20). Anal. calcd. for C27H26N6O2: C, 69.51; H, 5.62; N, 18.01. Found: C, 69.78; H, 5.41; N, 17.81.
2.1.8. 1-Benzyl-4-{[4-(1-{2-[(4-chlorophenyl)methylidene]hydrazin-1-ylidene}ethyl)phenoxy]methyl}-1H-1,2,3-triazole (6h)
Yield: 43%, mp = 140–142°C. IR (KBr): 3072, 1602, 1541, 1490, 1407, 1247 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.48 (s, 1H, =CH), 8.30 (s, 1H, triazole), 7.89–7.85 (m, 4H, H3, H5, H2″, H6″), 7.53 (d, J = 8.5 Hz, 2H, H3″, H5″), 7.35–7.30 (m, 5H, H2′, H3′, H4′, H5′, H6′), 7.09 (d, J = 8.7 Hz, 2H, H2, H6), 5.60 (s, 2H, CH2), 5.20 (s, 2H, CH2), 2.43 (s, 3H, CH3) ppm.13C-NMR (125 MHz, DMSO-d6): 164.1, 160.3, 156.9, 143.2, 136.4, 135.8, 134.5, 130.7, 130.1, 129.4, 129.2, 128.8, 128.6, 128.4, 125.2, 115.0, 61.6, 52.6, 15.1 ppm. MS (m/z, %): 91 (100), 144 (58), 272 (2), 332 (25), 443 (M+·, 17). Anal. calcd. for C25H22ClN5O: C, 67.64; H, 5.00; N, 15.78. Found: C, 67.33; H, 4.85; N, 15.51.
2.1.9. 1-[(2-Chlorophenyl)methyl]-4-{[4-(1-{2-[(4-chlorophenyl)methylidene]hydrazin-1-ylidene}ethyl)phenoxy]methyl}-1H-1,2,3-triazole (6i)
Yield: 67%, mp = 147–149°C. IR (KBr): 3040, 1598, 1509, 1481, 1366, 1251 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.48 (s, 1H, =CH), 8.27 (s, 1H, triazole), 7.89–7.87 (m, 4H, H3, H5, H2″, H6″), 7.55 (d, J = 8.5 Hz, 2H, H3″, H5″), 7.51 (d, J = 7.7, 1H, H3′), 7.40–7.34 (m, 2H, H4′, H5′), 7.21 (d, J = 7.4 Hz, 1H, H6′), 7.10 (d, J = 8.9 Hz, 2H, H2, H6), 5.71 (s, 2H, CH2), 5.21 (s, 2H, CH2), 2.43 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 164.0, 160.2, 156.9, 143.0, 135.6, 133.7, 133.1, 133.0, 131.0, 130.7, 130.2, 130.1, 129.8, 129.4, 128.9, 128.2, 125.7, 115.0, 61.6, 51.1, 15.1 ppm. MS (m/z, %): 125 (100), 178 (67), 272 (42), 366 (26), 477 (M+·, 18), 479 (([M+2]+·, 12), 481 (([M+4]+·, 2). Anal. calcd. for C25H21Cl2N5O: C, 62.77; H, 4.42; N, 14.64. Found: C, 62.51; H, 4.70; N, 14.81.
2.1.10. 1-[(4-Chlorophenyl)methyl]-4-{[4-(1-{2-[(4-chlorophenyl)methylidene]hydrazin-1-ylidene}ethyl)phenoxy]methyl}-1H-1,2,3-triazole (6j)
Yield: 85%, mp = 175–177°C. IR (KBr): 3080, 1598, 1527, 1516, 1361, 1257 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.47 (s, 1H, =CH), 8.29 (s, 1H, triazole), 7.85 (d, J = 9.0, 2H, H3, H5), 7.38–7.30 (m, 8H, H2′, H3′, H5′, H6′, H2″, H3″, H5″, H6″), 7.08 (d, J = 9.0 Hz, 2H, H2, H6), 5.61 (s, 2H, CH2), 5.19 (s, 2H, CH2), 2.26 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 163.7, 159.8, 158.0, 143.3, 136.5, 134.6, 131.4, 130.6, 129.8, 129.7, 129.2, 128.6, 128.5, 128.4, 124.2, 115.0, 61.7, 53.3, 14.9 ppm. MS (m/z, %): 103 (21), 130 (33), 176 (100), 251 (25), 298 (70), 477 (M+·, 9), 479 (([M+2]+·, 6), 481 (([M+4]+·, 1). Anal. calcd. for C25H21Cl2N5O: C, 62.77; H, 4.42; N, 14.64. Found: C, 62.90; H, 4.26; N, 14.33.
2.1.11. 4-{[4-(1-{2-[(4-Chlorophenyl)methylidene]hydrazin-1-ylidene}ethyl)phenoxy]methyl}-1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazole (6k)
Yield: 95%, mp = 137–139°C. IR (KBr): 3083, 1603, 1506, 1453, 1403, 1255 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.48 (s, 1H, =CH), 8.29 (s, 1H, triazole), 7.89–7.87 (m, 4H, H3, H5, H2″, H6″), 7.55 (d, J = 8.1 Hz, 2H, H3″, H5″), 7.40–7.37 (m, 2H, H2′, H6′), 7.20 (t, J = 8.5 Hz, 2H, H3′, H5′), 7.09 (d, J = 8.8 Hz, 2H, H2, H6), 5.59 (s, 2H, CH2), 5.19 (s, 2H, CH2), 2.43 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 164.2, 162.4 (d, JC-F = 249.4 Hz), 160.3, 156.9, 143.3, 135.8, 133.6, 132.7, 130.8 (d, JC-F = 8.6 Hz), 130.7, 130.1, 129.4, 128.9, 125.1, 116.1 (d, JC-F = 21.45 Hz), 115.0, 61.6, 52.5, 15.1 ppm. MS (m/z, %): 109 (100), 162 (39), 272 (27), 350 (12), 461 (M+·, 15), 463 ([M+2]+·, 5). Anal. calcd. for C25H21ClFN5O: C, 65.01; H, 4.58; N, 15.16. Found: C, 64.87; H, 4.70; N, 15.30.
2.1.12. 2-(4-{[4-(1-{2-[(4-Chlorophenyl)methylidene]hydrazin-1-ylidene}ethyl)phenoxy]methyl}-1H-1,2,3-triazol-1-yl)-N-phenylacetamide (6l)
Yield: 92%, mp = 236–238°C. IR (KBr): 3350, 3080, 1668, 1606, 1562, 1447, 1255 cm-1. 1H-NMR (500 MHz, DMSO-d6): 10.49 (s, 1H, NH), 8.50 (s, 1H, =CH), 8.30 (s, 1H, triazole), 7.93–7.89 (m, 4H, H3, H5, H2″, H6″), 7.60–7.55 (m, 4H, H2′, H6′, H3″, H5″), 7.34–7.32 (m, 2H, H3′, H5′), 7.16–7.09 (m, 3H, H2, H6, H4′), 5.37 (s, 2H, CH2), 5.26 (s, 2H, CH2), 2.46 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 164.6, 164.2, 160.4, 156.9, 142.7, 138.9, 135.8, 133.9, 130.7, 130.2, 129.5, 129.4, 128.9, 126.9, 124.3, 119.7, 115.0, 61.6, 52.7, 15.1 ppm. MS (m/z, %): 106 (73), 134 (52), 161 (57), 187 (52), 272 (100), 350 (12), 375 (50), 486 (M+·, 24), 488 ([M+2]+·, 8). Anal. calcd. for C26H23ClN6O2: C, 64.13; H, 4.76; N, 17.26. Found: C, 64.44; H, 4.50; N, 17.55.
2.1.13. N-(4-Chlorophenyl)-2-(4-{[4-(1-{2-[(4-chlorophenyl)methylidene]hydrazin-1-ylidene}ethyl)phenoxy]methyl}-1H-1,2,3-triazol-1-yl)acetamide (6m)
Yield: 67%, mp = 212–215°C. IR (KBr): 3415, 3060, 1675, 1605, 1513, 1490, 1253 cm-1. 1H-NMR (500 MHz, DMSO-d6): 10.63 (s, 1H, NH), 8.50 (s, 1H, CH), 8.29 (s, 1H, triazole), 7.93–7.88 (m, 4H, H3, H5, H2″, H6″), 7.62 (d, J = 8.6 Hz, 2H, H2′, H6′), 7.55 (d, J = 8.2 Hz, 2H, H3″, H5″), 7.39 (d, J = 8.6 Hz, 2H, H3′, H5′), 7.14 (d, J = 8.2 Hz, 2H, H2, H6), 5.38 (s, 2H, CH2), 5.26 (s, 2H, CH2), 2.46 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 164.8, 164.2, 160.3, 156.9, 142.8, 137.8, 135.8, 133.9, 130.2, 129.4, 129.3, 128.9, 128.5, 127.9, 126.9, 121.2, 115.0, 61.7, 52.7, 15.1 ppm. MS (m/z, %): 127 (87), 161 (62), 231 (27), 272 (100), 409 (16), 521 (M+·, 9), 523 ([M+2]+·, 3). Anal. calcd. for C26H22Cl2N6O2: C, 59.89; H, 4.25; N, 16.12. Found: C, 60.11; H, 4.48; N, 16.37.
2.1.14. N-Benzyl-2-(4-{[4-(1-{2-[(4-chlorophenyl)methylidene]hydrazin-1-ylidene}ethyl)phenoxy]methyl}-1H-1,2,3-triazol-1-yl)acetamide (6n)
Yield: 88%, mp = 215–217°C. IR (KBr): 3301, 3080, 1656, 1602, 1564, 1492, 1452, 1249 cm-1. 1H-NMR (500 MHz, DMSO-d6): 8.83 (t, J = 5.8 Hz, 1H, NH), 8.48 (s, 1H, CH), 8.22 (s, 1H, triazole), 7.91–7.87 (m, 4H, H3, H5, H2″, H6″), 7.55 (d, J = 8.5 Hz, 2H, H3″, H5″), 7.32 (d, J = 7.6 Hz, 2H, H3′, H5′), 7.28–7.23 (m, 3H, H2′, H4′, H6′), 7.12 (d, J = 8.9 Hz, 2H, H2, H6), 5.22 (s, 2H, CH2), 5.18 (s, 2H, CH2), 4.32 (d, J = 5.9 Hz, 2H, CH2), 2.44 (s, 3H, CH3) ppm.13C-NMR (125 MHz, DMSO-d6): 165.9, 164.2, 160.3, 156.9, 142.6, 139.1, 135.7, 133.9, 130.6, 130.2, 129.4, 128.9, 128.8, 127.9, 127.5, 126.7, 115.0, 61.6, 52.1, 42.8, 15.1 ppm. MS (m/z, %): 91 (100), 161 (22), 201 (23), 231 (10), 272 (41), 389 (21), 500 (M+·, 9), 502 ([M+2]+·, 3). Anal. calcd. for C27H25ClN6O2: C, 64.73; H, 5.03; N, 16.78. Found: C, 64.56; H, 4.87; N, 16.52.
2.1.15. 2-{4-[(4-{1-[2-({4-[(1-Benzyl-1H-1,2,3-triazol-4-yl)methoxy]-3-methoxyphenyl}methylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazol-1-yl}-N-phenylacetamide (6o)
Yield: 35%, mp = 167–169°C. IR (KBr): 3250, 1685, 2950, 1606, 1537 cm-1. 1H-NMR (500 MHz, DMSO-d6) (presence of two isomers): 10.47 (s, 1H, NH), 8.42 (s, 1H, CH), 8.29 (s, 1H, triazole), 8.28 (s, 1H, triazole), 7.89 (d, J = 8.6 Hz, 2H, H3, H5), 7.57 (d, J = 8.0 Hz, 2H, H2′, H6′), 7.49 (s, 1H, H2″), 7.36–7.30 (m, 11H, H3′, H5′, H6″, H2‴, H3‴, H4‴, H5‴, H6‴), 7.23 (d, J = 8.2 Hz, 1H, H5″), 7.12 (d, J = 8.6 Hz, 2H, H2, H6), 7.07 (t, J = 8.0 Hz, 1H, H4′), 5.61 (s, 2H, CH2), 5.35 (s, 2H, CH2), 5.24 (s, 2H, CH2), 5.18 (s, 2H, CH2), 3.78 (s, 3H, OCH3), 2.45 (s, 3H, CH3) ppm.13C-NMR (125 MHz, DMSO-d6): 164.6, 163.4, 160.2, 158.0, 150.4, 146.6, 138.8, 136.4, 133.7, 130.9, 129.4, 129.2, 128.7, 128.6, 128.5, 128.4, 126.9, 125.5, 124.2, 123.1, 119.7, 117.4, 114.5, 113.7, 110.2, 62.0, 61.5, 55.9, 53.3, 52.7, 15.7 ppm. MS (m/z, %): 91 (100), 144 (37), 284 (37), 455 (24), 669 (M+·, 3). Anal. calcd. for C37H35N9O4: C, 66.35; H, 5.27; N, 18.82. Found: C, 66.54; H, 5.42; N, 18.61.
2.1.16. 2-{4-[(4-{1-[2-({4-[(1-Benzyl-1H-1,2,3-triazol-4-yl)methoxy]-3-methoxyphenyl}methylidene)hydrazin-1-ylidene]ethyl}phenoxy)methyl]-1H-1,2,3-triazol-1-yl}-N-(4-chlorophenyl)acetamide (6p)
Yield: 28%, mp = 183–185°C. IR (KBr): 3278, 1685, 2850, 1615, 1535 cm-1. 1H-NMR (500 MHz, DMSO-d6) (presence of two isomers): 10.61 (s, 1H, NH), 8.41 (s, 1H, CH), 8.29 (s, 1H, triazole), 8.27 (s, 1H, triazole), 7.89 (d, J = 8.8 Hz, 2H, H3, H5), 7.59 (d, J = 7.1 Hz, 2H, H2′, H6′), 7.49 (s, 1H, H2″), 7.38–7.30 (m, 10H, H3′, H5′, H6″, H2‴, H3‴, H4‴, H5‴, H6‴), 7.23 (d, J = 8.5 Hz, 1H, H5″), 7.12 (d, J = 8.8 Hz, 2H, H2, H6), 5.61 (s, 2H, CH2), 5.36 (s, 2H, CH2), 5.24 (s, 2H, CH2), 5.18 (s, 2H, CH2), 3.78 (s, 3H, OCH3), 2.45 (s, 3H, CH3) ppm. 13C-NMR (125 MHz, DMSO-d6): 164.8, 163.5, 160.1, 158.0, 150.4, 149.7, 143.4, 143.0, 137.8, 136.4, 130.9, 129.3, 129.2, 128.7, 128.6, 128.4, 128.2, 127.6, 126.8, 125.4, 123.1, 121.2, 115.0, 113.7, 110.2, 62.1, 61.6, 55.8, 53.3, 52.7, 15.0 ppm. MS (m/z, %): 91 (100), 127 (47), 284 (48), 455 (26), 704 (M+·, 1). Anal. calcd. for C37H34ClN9O4: C, 63.11; H, 4.87; N, 17.90. Found: C, 63.40; H, 5.14; N, 17.71.
2.2. High-performance liquid chromatography analysis
The analysis was achieved on an YL9100 HPLC system (Korea) equipped with a UV detector and RP column (Teknokroma, Barcelona, Spain; C18, 5 μm, 150 × 4.6 mm) using the following program: methanol (solvent A) and water, a gradient of 0–100% solvent A in 11 min, 1 min at 0%, to 50% within 3 min, to 100% at 6 min, to 0 within 5 min (total run time 11 min); flow rate, 1 ml/min; detection, 254 nm; injection volume, 20 μl [21].
2.3. Cholinesterase inhibitory assay
All substrates and enzymes, and reagents including acetylthiocholine iodide (ATCI), butyrylthiocholine iodide (BTCI), butyrylcholinesterase (BChE; E.C. 3.1.1.8, from horse serum), acetylcholinesterase (AChE; E.C. 3.1.1.7, Type V-S, lyophilized powder, from electric eel, 1000 unit) and 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB), potassium hydroxide, sodium hydrogen carbonate, potassium dihydrogen phosphate and potassium hydrogen phosphate were obtained from Sigma-Aldrich (MI, USA). The assay was performed according to the modified Ellman's method [22–27] and for this purpose, compound 6 was dissolved in DMSO and diluted in methanol. Each well held 25 μl of the prepared sample (four distinct concentrations of each drug were evaluated in triplicate), 50 μl of potassium phosphate buffer (0.1 M, pH 8) and 25 μl of the enzyme (final concentration of 0.22 U/ml). After a 15-min preincubation at room temperature, 125 μl of DTNB (3 mM in buffer) was added to each well. Spectrophotometric analysis was used to characterize the hydrolysis of ATCI catalyzed by AChE at 405 nm, after which the substrate (ATCI, 3 mM in water) was added and the absorbance was measured 405 nm after 15 min. Using inhibition curves (log inhibitor concentration vs. percent of inhibition), the IC50 values were visually calculated. The BChE inhibition assay was also conducted using the same methodology.
2.4. Kinetic studies
The kinetic study was performed according to our previous report [28]. Briefly, using four distinct inhibitor concentrations, the kinetic study of inhibition of AChE and BChE by compounds 6m and 6p was conducted. In this regard, compound 6m was applied at the concentrations of 0, 2, 10 and 20 μM. Plotting 1/V against 1/[S] at various concentrations of the substrate (acetylthiocholine iodide: 187.5, 750, 1500 and 3000 μM) provided the Lineweaver–Burk reciprocal plot. Similarly, the inhibitory activity toward BChE was studied by compound 6p at the concentrations of 0, 1.5, 6 and 12 μM along with the corresponding substrate (butyrylthiocholine iodide: 187.5, 750, 1500, 3000 μM).
2.5. Docking
The molecular docking approach was performed using induced-fit molecular docking of the Schrodinger and the x-ray structures of AChE (Protein Data Bank [PDB] ID: 4EY7) and BChE (PDB ID: 4BDS), downloaded from the protein data database (PDB) according to previously reported procedures [29–32].
2.6. Toxicity assay on SH-SY5Y
Toxicity assay on SH-SY5Y cells was done according to a previously published protocol [33,34].
2.7. RNA extraction & real-time PCR
RNA was extracted from the cells using RNX-plus reagent (Cinagen, Tehran, Iran) following the manufacturer's instructions and reported protocol (Supplementary Table S1) [35].
2.8. Prediction of absorption, distribution, metabolism & excretion descriptors
The absorption, distribution, metabolism, excretion and toxicity (ADMET) properties were calculated according to online servers www.swissadme.ch/, https://preadmet.bmdrc.kr and https://biosig.lab.uq.edu.au/pkcsm/.
2.9. Statistical analysis
The GraphPad Prism software was used to perform statistical analysis. Data comparisons were done by one-way analysis of variance (ANOVA) with Tukey's multiple comparisons as the post hoc test. p-values <0.05 were considered statistically significant.
3. Results
All synthesized compounds 6 were recrystallized from EtOH and their purity was confirmed by TLC (supporting information) and HPLC analysis (for compounds 6m and 6p). Their structure was also characterized using NMR, IR spectroscopy and mass spectrometry.
The in vitro enzyme inhibitory assay was performed on AChE (from electric eels) and BChE (from horse serum), compared with donepezil as the positive control (Table 1).
Table 1.
ChE inhibitory activity of 1,2,3-triazoles-hydrazones 6.

| |||||
|---|---|---|---|---|---|
| Entry | Compound 6 | Ar | X | IC50 (μM)† | |
| AChE‡ | BChE§ | ||||
| 1 | 6a |

|

|
54.60 ± 0.91****¶
(#R2 = 0.91) |
>100 |
| 2 | 6b |

|

|
>100 | >100 |
| 3 | 6c |

|

|
>100 | >100 |
| 4 | 6d |

|

|
34.95 ± 0.85**** (R2 = 0.90) |
57.36 ± 0.96**** (R2 = 0.90) |
| 5 | 6e |

|

|
>100 | >100 |
| 6 | 6f |

|

|
>100 | >100 |
| 7 | 6g |

|

|
>100 | >100 |
| 8 | 6h |

|

|
>100 | >100 |
| 9 | 6i |

|

|
>100 | >100 |
| 10 | 6j |

|

|
39.67 ± 0.65**** (R2 = 0.96) |
28.20 ± 0.37**** (R2 = 0.96) |
| 11 | 6k |

|

|
>100 | >100 |
| 12 | 6l |

|

|
16.06 ± 0.50**** (R2 = 0.96) |
26.49 ± 0.74**** (R2 = 0.93) |
| 13 | 6m |

|

|
5.23 ± 0.11**** (R2 = 0.98) |
59.22 ± 0.69**** (R2 = 0.98) |
| 14 | 6n |

|

|
16.25 ± 0.46**** (R2 = 0.98) |
12.37 ± 0.51**** (R2 = 1.00) |
| 15 | 6o |

|

|
16.57 ± 0.65**** (R2 = 0.97) |
4.36 ± 0.14**** (R2 = 0.98) |
| 16 | 6p |

|

|
11.47 ± 0.44**** (R2 = 0.95) |
3.39 ± 0.13** (R2 = 0.91) |
| 17 | Donepezil | 0.03 ± 0.00 (R2 = 0.99) |
0.95 ± 0.11 (R2 = 0.99) |
||
Data are expressed as mean ± SD (three independent experiments).
The AChE from electric eels.
The BChE from horse serum.
R2 = Coefficients of determination.
Mean comparison of each compound with mean of donepezil through one-way analysis of variance (ANOVA) experiment with Tukey post hoc analysis confirmed the p-value < 0.01 and 0.0001.
Compounds 6m and 6p were the most potent AChE and BChE inhibitors with IC50 values of 5.23 and 3.39 μM, respectively. A kinetic study on the inhibition of AChE and BChE was studied by compounds 6m and 6p (Supplementary Table S2). Graphical analysis of both compounds' reciprocal Lineweaver–Burk plot described a mixed-type inhibition pattern (Figure 1). Also, Ki and Kis values were calculated for compound 6m as 4.30 and 21.44 μM, respectively. Moreover, these values for compound 6p were obtained as 3.67 and 0.70 μM, respectively.
Figure 1.
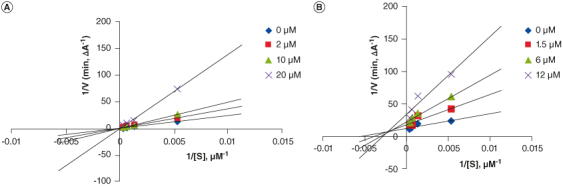
Kinetic study. (A) Lineaweaver-Burk plot for the inhibition of AChE by compound 6m. (B) Lineaweaver–Burk plot for the inhibition of AChE by compound 6p.
Docking studies of compounds 6m (Figure 2) and 6p (Figure 3) were studied in the AChE and BChE active sites. Desired H-bonding and pi–pi stacking interactions were constructed with related amino acid residues in the catalytic anionic site (CAS) and peripheral anionic site (PAS) of the enzymes that confirmed dual-binding site inhibition of those enzymes by compounds 6m and 6p, respectively.
Figure 2.
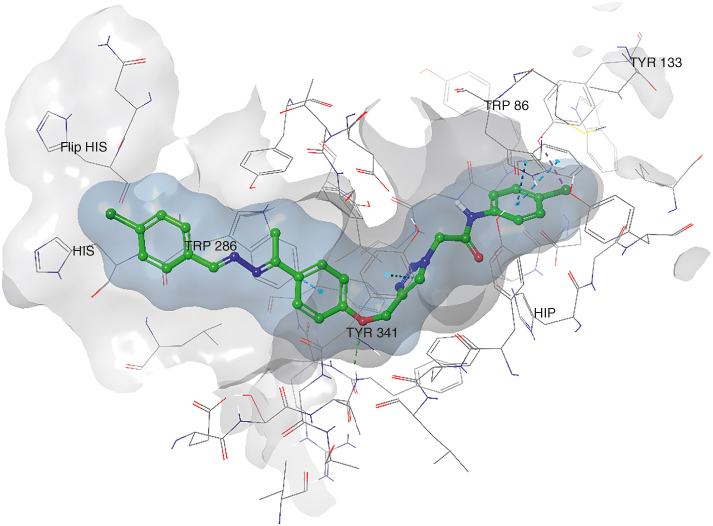
Binding position of compound 6m in the AChE active site.
Figure 3.
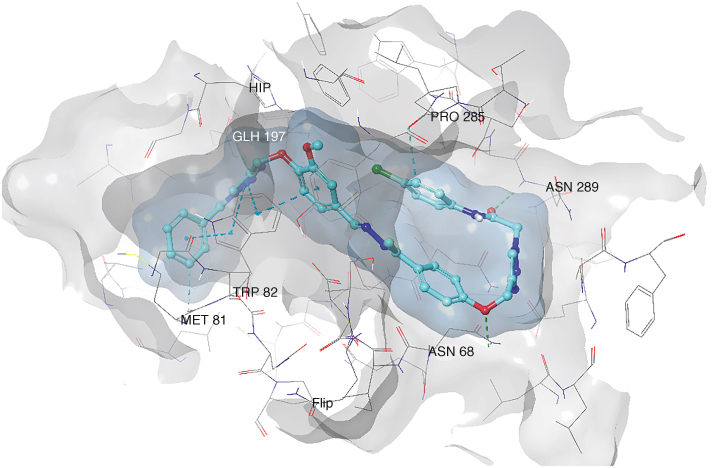
Binding position of compound 6p in the BChE active site.
The toxicity of compounds 6m and 6p was evaluated on the SH-SY5Y cell line (Figure 4). Compound 6m was found not toxic and compound 6p exhibited a dose-dependent response.
Figure 4.
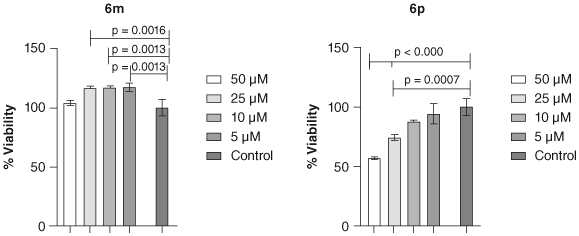
Cytotoxicity of 6m and 6p after 72 h exposure determined by MTT assay. Data represents the mean ± SEM.
The levels of GSK-3α, GSK-3β, DYRK1 and CDK5 were also assessed following the treatment of compounds 6m at 25 μm and 6p at 10 μm for a 72-h exposure as shown in Figure 5.
Figure 5.
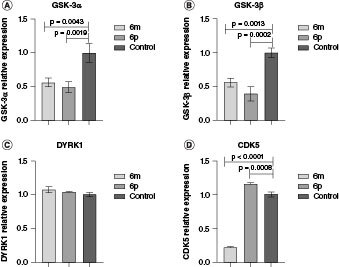
Gene expression measurements. GSK-3α (A), GSK-3β (B), DYRK1 (C), and CDK5 (D) gene expression on SH-SY5Y cells after 72 h treatment with 6m (25 μM) or 6p (10 μM).
A list of ADMET properties of synthesized compounds was provided and corresponding properties indicated the derivatives were druglike. (Supplementary Table S3).
4. Discussion
4.1. Designing
Heterocyclic rings play a prominent role in developing anti-AD agents [36] and at this juncture, aryl-1,2,3-triazoles have emerged as an effective scaffold for inhibiting ChEs [37–40].
The exploration of 1,2,3-triazole-isoxazole derivatives demonstrated higher potency against AChE than BChE (e.g. compound A; Figure 6) [22]. The heightened efficacy may be attributed to the dual inhibition mechanism due to the presence of the isoxazole moiety that targeted the CAS and the aryl-1,2,3-triazole directed toward the PAS of the AChE.
Figure 6.
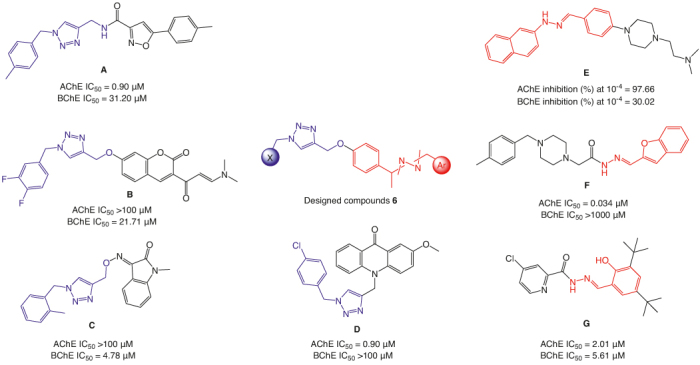
Design of target 1,2,3-triazoles-hydrazone hybrids 6a–p.
The AChE active site contains a long gorge with an overall length of approximately 20 Å including two main sites: CAS and PAS. The CAS which forms the catalytic triad, Ser200, Glu327 and His440 residues, is situated at the bottom of the gorge and responsible for the hydrolysis of AChE inside the triad. The PAS consisting of Tyr70, Tyr121 and Trp279 residues is located near the entrance of the gorge [41].
In another study, aryl-1,2,3-triazole derivatives containing dimethylaminoacrylyl–chromenone moiety revealed a shift in potency, with high efficacy against BChE and no significant activity against AChE (e.g. compound B; Figure 6) [42]. This discrepancy may be linked to the larger active site of BChE compared with AChE, coupled with the substantial size of the synthesized compounds. Additionally, aryl-1,2,3-triazole possessing methylindolinone moiety (e.g. compound C; Figure 6) was found to be a potent candidate against BChE, with the most effective analog recording an IC50 value of 4.78 μM [43]. Notably, this potency surpassed the reference drug donepezil, which exhibited an IC50 of 5.19 μM in a mixed mode of inhibition. Also, our previous study revealed that the 4-chloroaryl group connected to the 1,2,3-triazole ring (compound D; Figure 6) induced more potent AChE inhibitory activity than BChE as compared with other groups such as phenyl and 4-nitroaryl moieties [44]. Thus, it was considered in synthesizing various derivatives and exploring their structure–activity relationships (SARs).
On the other hand, hydrazine derivatives have been significant organic intermediates in developing novel compounds as far as the aryl hydrazones have been documented as promising scaffolds for designing anti-AD agents. Compounds E [45] and F (Figure 6) [46] were identified as potent inhibitors of both AChE and BChE. Molecular docking interactions have further confirmed binding affinity between them and the active sites of both enzymes. Moreover, recent discoveries include hydrazide-hydrazone imine derivatives (e.g. compound F; Figure 6) with inhibitory effects on both enzymes. Notably, compound G (Figure 6) demonstrated AChE inhibition as a mixed-type inhibitor with a Ki value of 0.68 μM [47].
The current study exploited the potency of 1,2,3-triazole and aryl hydrazone moieties via molecular hybridization strategies (6; Figure 6). The initial focus was placed on the substitution patterns of the 1,2,3-triazole ring at the X position, resulting in derivatives 6a–g. Subsequently, the derivative scope was expanded by changing substitutions connected to the heterocyclic scaffold or aromatic moiety (Ar) to afford analogs 6h–p. Comprehensive assessments were conducted on all derivatives to explore their potential as AChE and BChE inhibitors. Additionally, kinetic studies and in silico evaluations were executed to elucidate the inhibitory mechanisms of the most potent derivatives against each enzyme. Furthermore, toxicity assessments against the SH-SY5Y cell line were performed, offering valuable insights into the safety profile of these derivatives. Furthermore, the impact of the most potent analogs on the GSK3α, GSK3β, Cdk5 and DYRK expression levels in the SH-SY5Y cell line was undertaken. This study illustrates an approach to leverage the potency of 1,2,3- triazole and aryl hydrazone moieties to develop potential anti-AD agents.
4.2. Chemistry
The synthetic route for the preparation of 1,2,3-triazoles-hydrazone hybrids 6 has been demonstrated in Figure 7. Initially, 1-(4-(prop-2-yn-1-yloxy)phenyl)ethan-1-one 1 was prepared by the reaction of acetophenone and propargyl bromide in the presence of potassium carbonate and potassium iodide in DMF at 80°C [48]. Aizde derivatives 2 were also prepared by the reaction of benzyl chloride/bromide derivatives with sodium azide in the presence of triethylamine in H2O/t-BuOH. However, some derivatives were prepared by the reaction of 2-chloro-N-arylacetamide/N-benzyl-2-chloroacetamide in DMF. Subsequently, the reaction of compounds 1 and 2 continued for 24–48 h in the presence of catalytic amounts of sodium ascorbate and CuSO4.5H2O to give compound 3 that tolerated the reaction with hydrazine hydrate in refluxing methanol for 6 h to yield compound 4. Finally, the reaction of compound 4 and different aromatic aldehyde derivatives 5 in the presence of catalytic amounts of acetic acid in refluxing EtOH led to the formation of compound 6 as shown in Figure 7.
Figure 7.
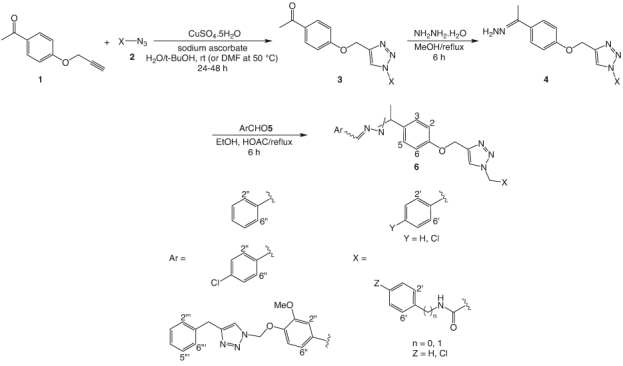
Synthesis of 1,2,3-triazoles-hydrazone derivatives 6.
4.3. AChE inhibitory activity of derivatives 6a–p
As reported in Table 1, the potency of designed compounds was lower than donepezil. In the case of derivatives 6a–g, the effect of phenyl substitution at Ar was explored to understand the impact of the X group. Notably, 6a bearing benzyl moiety at X, exhibited an IC50 value of 54.60 ± 0.9 μM. Substituting the benzyl ring with a chlorine atom at the ortho (6b) or para (6c) positions demonstrated detrimental to AChE activities (IC50 >100 μM). However, replacing p-chlorine with p-fluoro (6d) resulted in improved potency (IC50 = 34.95 ± 0.85 μM), underscoring the significance of the substitution size in activity.
To further validate this hypothesis, diverse bulky derivatives were introduced into the X position. Derivatives 6e (X = phenylacetamide) and 6f (X = 4-chloroarylacetamide) exhibited inferior potency, and benzylacetamide substitution at the X position (6g) showed no significant activity under the tested concentrations (IC50 >100 μM). Subsequent modifications involved synthesizing derivatives bearing 4-chloroaryl, which provided increased lipophilicity, electron-rich regions and more spacious structures at the Ar position (6h–p). Among derivatives with various benzyl substitutions at the X position, only 6j bearing 4-chlorobenzyl, demonstrated activity with an IC50 of 39.67 ± 0.65 μM.
An increase in the AChE inhibitory activity was observed in compound 6l, characterized by the incorporation of phenyl acetamide, resulting in an IC50 value of 16.06 ± 0.50 μM. Notable enhancement in potency was observed in compounds 6m upon introducing the 4-Cl group into phenylacetamide, a strong electron-withdrawing group. The replacement of phenylacetamide (6l) with benzylacetamide (6n), which increased in length, did not significantly alter potency, maintaining an IC50 of 16.25 ± 0.46 μM.
Turning attention to the substitution of 1-benzyl-4-((2-methoxyphenoxy)methyl)-1H-1,2,3-triazole at the Ar position, both synthesized derivatives 6o (X = phenylacetamide) and 6p (X = chlorophenylacetamide) exhibited good potency with IC50 values of 16.57 ± 0.65 and 11.47 ± 0.44 μM, respectively. These findings underscore the impact of structural modifications on AChE inhibitory activity and provide valuable insights for the rational design of novel AChE inhibitors.
4.4. BChE inhibitory activity of derivatives 6a–p
The initial screening of 6a–g where Ar = phenyl, highlighted the potency of 6d (para-fluorobenzyl) as the only active compound, demonstrating an IC50 value of 57.36 ± 0.96 μM. Intriguingly, the remaining derivatives in this series were inactive against BChE.
To enhance the potency against BChE, modifications were performed considering the bulkier nature of the BChE active site compared with AChE. Synthesizing derivatives with more spacious substituents at the Ar position revealed insightful trends. Compound 6h possessing Ar = 4-chloroaryl and X = benzyl, exhibited no activity. Also, the introduction of chlorine into the ortho position of the aryl ring (6i) failed to improve potency. In contrast, the relocation of the chlorine to the para position (6j) significantly increased anti-BChE activity (IC50 = 28.20 ± 0.37 μM). This suggested that the para position is pivotal in influencing BChE inhibitory potency.
Further exploration led to derivatives 6l–n, where increased bulkiness at X was emphasized. Among them, compound 6n incorporating benzylacetamide emerged as one of the potent inhibitors with an IC50 of 12.37 ± 0.51 μM, indicating the significance of optimal spatial characteristics for effective BChE inhibition. The benzylacetamide moiety was hypothesized to provide an optimum situation for rotation and occupation of the BChE active site, unlike the more rigid phenylacetamide moiety in 6l.
Good activity was observed in derivatives 6o and 6p, featuring X = benzyl-4-((2-methoxyphenoxy)methyl)-1H-1,2,3-triazole at the Ar. These compounds recorded IC50 values of 4.36 ± 0.14 and 3.39 ± 0.13 μM, respectively, affirming that an increase in bulkiness at the Ar significantly enhanced BChE inhibitory activity.
In summary, the SAR analysis indicated that modifications leading to increased bulkiness at the Ar position significantly enhanced BChE inhibitory potency. These findings provided valuable insights for the rational design of novel BChE inhibitors, with implications for therapeutic interventions targeting cholinesterase enzymes.
4.5. Kinetic study
The kinetic study was conducted to investigate the mechanism of inhibition by compounds 6m and 6p against AChE and BChE, respectively (Supplementary Table S2). Graphical analysis of the reciprocal Lineweaver–Burk plot of compound 6m described a mixed-type inhibition pattern (Figure 1; A) as Km and Vm increased by increasing the inhibitor concentration. In this case, Ki (for competitive inhibition) and Kis (for uncompetitive inhibition) values [49] were calculated as 4.30 and 21.44 μM, respectively.
A kinetic study of the inhibition of BChE also revealed a mixed-type inhibition pattern for compound 6p (Figure 1; B) with calculated Ki and Kis values of 3.67 and 0.70 μM, respectively. Compounds 6m and 6p may bind to the related enzyme whether or not the enzyme has already bound the substrate.
4.6. Docking
The AChE PAS is situated at the active gate entrance, governing various activities, managing diverse activities such as facilitating the entry of substrates, ligands, and inhibitors and engaging in interactions with Aβ (specifically Tyr70, Asp74, Tyr124, Trp286 and Tyr341 residues of the enzyme). The AChE CAS encompasses the anionic binding site (Trp86, Glu202 and Phe338), the esteratic binding site housing the catalytic triad (Ser203, His447 and Glu334), and the acyl binding site (Phe295 and Phe299), all crucial for the AChE functional sites. Likewise, BChE also features PAS and CAS, with its binding site approximately 20 Å larger than that of AChE. This increased size accommodates larger inhibitors, allowing them to occupy the active site. BChE's catalytic triad consists of Ser198, His438 and Glu325, with Asp70 and Tyr332 contributing to the PAS of the BChE.
Initially, the docking verification process, comparing co-crystallized and docking ligands, recorded a root mean square deviation (RMSD) value of less than 2 Å, confirming the accuracy of the docking protocol. Subsequent molecular docking studies of compound 6m (Figure 2); identified as a potent AChE inhibitor, revealed a glide score of -9.285. The 4-Cl-arylacetamide moiety of 6m engaged in two pi–pi stacking interactions with Trp86 and formed a halogen bond with Tyr133 in the anionic binding site located within the CAS region. Additionally, a hydrogen bonding interaction was observed with the crucial residue Phe295 of the acyl binding site. Furthermore, the 1,2,3-triazole ring and the aryl moiety established a pi–pi stacking interaction with Tyr341 and Trp286 in the PAS region.
Compound 6p, a potent BChE inhibitor, exhibited a glide score of -11.263. The benzyl group connected to the 1,2,3-triazole ring engaged in three pi–pi interactions with Trp82 and formed a hydrogen bonding interaction with Glu197 in the CAS pocket. Also, the ethyl-phenoxy moiety displayed pi-pi stacking interactions with Trp82. On the opposite side of the molecule, the acetamide group established a hydrogen bonding interaction with crucial Asn289 amino acid, and the phenoxy attached to the 1,2,3-triazole interacted with Asn68 in the PAS region (Figure 3).
4.7. Cell viability
The toxicity of compounds 6m and 6p as the most potent AChE and BChE inhibitors, was evaluated against the SH-SY5Y cell line. As shown in Figure 4, compound 6m demonstrated no toxicity under the tested concentrations, indicating its favorable safety profile.
In contrast, compound 6p exhibited a dose-dependent response, with viabilities of 56.96% and 74.19% observed at 50 and 25 μM, respectively. Notably, no significant toxicity was noted at the lower dose, suggesting a dose-dependent cytotoxic effect. These findings highlight the importance of efficacy and safety profiles in developing potential therapeutics.
4.8. Evaluating the protein expression
The applied concentrations were selected based on their non-toxic effect on the cell line. The 72-h exposure of 6m on SH-SY5Y demonstrated a 44% reduction in GSK-3α and a 45% reduction in GSK-3β mRNA levels. In the case of 6p, reductions of 51% and 61% in the levels of GSK-3α and GSK-3β were observed, respectively. These outcomes confirmed that derivatives 6m and 6p interfere with tau protein hyperphosphorylation and impede neurofibrillary tangle formation. However, both 6m and 6p did not affect the expression level of DYRK1, a gene associated with the formation of neurofibrillary tangles. Notably, 6m exhibited an approximately 80% reduction in CDK5, while 6p had no significant effect on CDK5 compared with the control (Figure 5).
4.9. In silico absorption, distribution, metabolism, excretion & toxicity evaluation
Evaluation of ADMET properties has been a strong tool in drug discovery research to avoid facing bad prospects in the future [50]. In this respect, related descriptors were provided in Supplementary Table S3. The synthesized compounds generally possessed desirable pharmacokinetic properties to be drug molecule candidates.
5. Conclusion
Given the global burden of AD and its profound impact on cognitive function and quality of life, the development of potent and multifaceted anti-AD agents is required. In response to this, a series of 1,2,3-triazole-hydrazone derivatives were designed and synthesized to explore their potential as inhibitors for both AChE and BChE. Compounds 6m and 6p, the most potent analogs against AChE and BChE, exhibited IC50 values of 5.23 and 3.39 μM, respectively. Their role as mixed-type inhibitors with Ki values of 4.30 and 3.67 μM, respectively, was shown through kinetic mechanistic exploration, corroborated by in silico studies confirming their high potency through interactions with the PAS and CAS pockets of the ChEs. Additionally, compound 6m demonstrated a notable decrease in GSK-3α, GSK-3β and CDK5 mRNA levels, implying its indirect influence on reducing toxic neurofibrillary tangle and Aβ aggregate formation. In summary, 6m stands out as a highly promising lead compound with potent anti-AD properties, worthy of further refinement and optimization in future investigations.
Supplementary Material
Funding Statement
The authors acknowledged the support from the National Institute for Medical Research Development with project No. 996147.
Supplemental material
Supplementary data for this article can be accessed at https://doi.org/10.1080/17568919.2024.2359894
Author contributions
D Shareghi-Boroujeni synthesized compounds. A Iraji performed docking studies, wrote the manuscript and supervised biological assays. M Dara studied and analyzed the gene expression. MH Hashempur contributed to the biological assay. S Zare performed MTT assay. R Hariri conducted ChE inhibitory assay. T Akbarzadeh supervised ChE inhibitory assay. M Saeedi designed the project, wrote the manuscript, and supervised all phases.
Financial disclosure
The authors acknowledged the support from the National Institute for Medical Research Development with project No. 996147. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Reference
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Korczyn AD, Grinberg LT. Is Alzheimer disease a disease? Nat Rev Neurol. 2024;20(4):245–251. doi: 10.1038/s41582-024-00940-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Recent reviews dealing with an overview of Alzheimer's disease.
- 2.Clifford K, Moreno M, Kloske CM. Navigating late-stage dementia: a perspective from the Alzheimer's Association. Alzheimers Dement (Amst). 2024;16(1):e12530. doi: 10.1002/dad2.12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajah Kumaran K, Yunusa S, Perimal E, et al. Insights into the pathophysiology of Alzheimer's disease and potential therapeutic targets: a current perspective. J Alzheimers Dis. 2023;91(2):507–530. doi: 10.3233/JAD-220666 [DOI] [PubMed] [Google Scholar]
- 4.Monteiro AR, Barbosa DJ, Remião F, et al. Alzheimer's disease: insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem Pharmacol. 2023;211:115522. doi: 10.1016/j.bcp.2023.115522 [DOI] [PubMed] [Google Scholar]
- 5.Akhtar A, Singh S, Kaushik R, et al. Types of memory, dementia, Alzheimer's disease, and their various pathological cascades as targets for potential pharmacological drugs. Ageing Res Rev. 2024;96:102289. doi: 10.1016/j.arr.2024.102289 [DOI] [PubMed] [Google Scholar]
- 6.Oliyaei N, Moosavi-Nasab M, Tanideh N, et al. Multiple roles of fucoxanthin and astaxanthin against Alzheimer's disease: their pharmacological potential and therapeutic insights. Brain Res Bull. 2023;193:11–21. doi: 10.1016/j.brainresbull.2022.11.018 [DOI] [PubMed] [Google Scholar]
- 7.Zuliani G, Zuin M, Romagnoli T, et al. Acetyl-cholinesterase-inhibitors reconsidered. A narrative review of post-marketing studies on Alzheimer's disease. Aging Clin Exp Res. 2024;36(1):1–11. doi: 10.1007/s40520-023-02675-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The importance of the cholinergic theory in Alzheimer's disease,
- 8.Nordberg A, Ballard C, Bullock R, et al. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer's disease. Prim Care Companion CNS Disord. 2013;5(2):26731. doi: 10.4088/PCC.12r01412 [DOI] [PMC free article] [PubMed] [Google Scholar]; • The importance of inhibition of BChE in the treatment of Alzheimer's disease.
- 9.Li Z, Yin B, Zhang S, et al. Targeting protein kinases for the treatment of Alzheimer's disease: recent progress and future perspectives. Eur J Med Chem. 2023;261:115817. doi: 10.1016/j.ejmech.2023.115817 [DOI] [PubMed] [Google Scholar]
- 10.Abyadeh M, Gupta V, Paulo JA, et al. Amyloid-beta and tau protein beyond Alzheimer's disease. Neural Regen Res. 2024;19(6):1262–1276. doi: 10.4103/1673-5374.386406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beurel E, Grieco SF, Jope RS, et al. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Tam KY. Dual-target inhibitors of cholinesterase and GSK-3β to modulate Alzheimer's disease. Drug Discov Today. 2024;29(4):103914. doi: 10.1016/j.drudis.2024.103914 [DOI] [PubMed] [Google Scholar]
- 13.Lauretti E, Dincer O, Praticò D. Glycogen synthase kinase-3 signaling in Alzheimer's disease. Acta Mol Cell Res. 2020;1867(5):118664. doi: 10.1016/j.bbamcr.2020.118664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau L-F, Seymour PA, Sanner MA, et al. Cdk5 as a drug target for the treatment of Alzheimer's disease. J Mol Neurosci. 2002;19(3):267–273. doi: 10.1385/JMN:19:3:267 [DOI] [PubMed] [Google Scholar]
- 15.Liu SL, Wang C, Jiang T, et al. The role of Cdk5 in Alzheimer's disease. Mol Neurobiol. 2016;53(7):4328–4342. doi: 10.1007/s12035-015-9369-x [DOI] [PubMed] [Google Scholar]
- 16.Allnutt AB, Waters AK, Kesari S, et al. Physiological and pathological roles of Cdk5: potential directions for therapeutic targeting in neurodegenerative disease. ACS Chem Neuroscience. 2020;11(9):1218–1230. doi: 10.1021/acschemneuro.0c00096 [DOI] [PubMed] [Google Scholar]
- 17.Mahdavi M, Saeedi M, Karimi M, et al. Synthesis of novel 1,2,3-triazole derivatives of 2,3-dihydroquinazolin-4(1H)-one. Monatsh fur Chem. 2016;147(12):2151–2156. doi: 10.1007/s00706-016-1739-1 [DOI] [Google Scholar]
- 18.Farid SM, Iraji A, Mojtabavi S, et al. Quinazolinone-1,2,3-triazole-acetamide conjugates as potent α-glucosidase inhibitors: synthesis, enzyme inhibition, kinetic analysis, and molecular docking study. RSC Med Chem. 2023;14(3):520–533. doi: 10.1039/D2MD00297C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi-Khanaposhtani M, Fahimi K, Karimpour-Razkenari E, et al. Design, synthesis and cytotoxicity of novel coumarin-1,2,3-triazole-1,2,4-oxadiazole hybrids as potent anti-breast cancer agents. Lett Drug Des Discov. 2019;16(7):818–824. doi: 10.2174/1570180815666180627121006 [DOI] [Google Scholar]
- 20.Saeedi M, Hariri R, Iraji A, et al. Novel N′-substituted benzylidene benzohydrazides linked to 1,2,3-triazoles: potent α-glucosidase inhibitors. Sci Rep. 2023;13(1):8960. doi: 10.1038/s41598-023-36046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iraji A, Shareghi-Brojeni D, Mojtabavi S, et al. Cyanoacetohydrazide linked to 1,2,3-triazole derivatives: a new class of α-glucosidase inhibitors. Sci Rep. 2022;12(1):8647. doi: 10.1038/s41598-022-11771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najafi Z, Mahdavi M, Saeedi M, et al. 1,2,3-Triazole-Isoxazole based acetylcholinesterase inhibitors: synthesis, biological evaluation and docking study. Lett Drug Des Discov. 2017;14(1):58–65. doi: 10.2174/1570180813666160628085515 [DOI] [Google Scholar]
- 23.Işık M, Demir Y, Durgun M, et al. Molecular docking and investigation of 4-(benzylideneamino)-and 4-(benzylamino)-benzenesulfonamide derivatives as potent AChE inhibitors. Chem Pap. 2020;74(5):1395–1405. doi: 10.1007/s11696-019-00988-3 [DOI] [Google Scholar]
- 24.Sever B, Türkeş C, Altıntop MD, et al. Novel metabolic enzyme inhibitors designed through the molecular hybridization of thiazole and pyrazoline scaffolds. Arch Pharm. 2021;354(12):2100294. doi: 10.1002/ardp.202100294 [DOI] [PubMed] [Google Scholar]
- 25.Güleç Ö, Türkeş C, Arslan M, et al. Novel beta-lactam substituted benzenesulfonamides: in vitro enzyme inhibition, cytotoxic activity and in silico interactions. J Biomol Struct Dyn. In press. doi: 10.1080/07391102.2023.2240889 www.tandfonline.com/doi/full [DOI] [PubMed] [Google Scholar]
- 26.Saeedi M, Rastegari A, Hariri R, et al. Design and synthesis of novel arylisoxazole-chromenone carboxamides: investigation of biological activities associated with Alzheimer's disease. Chem Biodivers. 2020;17(5):1900746. doi: 10.1002/cbdv.201900746 [DOI] [PubMed] [Google Scholar]
- 27.Saeedi M, Babaie K, Karimpour-Razkenari E, et al. In vitro cholinesterase inhibitory activity of some plants used in Iranian traditional medicine. Nat Prod Res. 2017;31(22):2690–2694. doi: 10.1080/14786419.2017.1290620 [DOI] [PubMed] [Google Scholar]
- 28.Saeedi M, Mohtadi-Haghighi D, Mirfazli SS, et al. Design and synthesis of selective acetylcholinesterase inhibitors: arylisoxazole-phenylpiperazine derivatives. Chem Biodivers. 2019;16(2):e1800433. doi: 10.1002/cbdv.201800433 [DOI] [PubMed] [Google Scholar]
- 29.Pourtaher H, Hasaninejad A, Iraji A. Design, synthesis, in silico and biological evaluations of novel polysubstituted pyrroles as selective acetylcholinesterase inhibitors against Alzheimer's disease. Sci Rep. 2022;12(1):15236. doi: 10.1038/s41598-022-18224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durgun M, Türkeş C, Işık M, et al. Synthesis, characterisation, biological evaluation and in silico studies of sulphonamide Schiff bases. J Enzyme Inhib Med Chem. 2020;35(1):950–962. doi: 10.1080/14756366.2020.1746784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durgun M, Akocak S, Lolak N, et al. Design and synthesis of pyrazole carboxamide derivatives as selective cholinesterase and carbonic anhydrase inhibitors: molecular docking and biological evaluation. Chem Biodivers. 2024;21(2):202301824. doi: 10.1002/cbdv.202301824 [DOI] [PubMed] [Google Scholar]
- 32.Efeoglu C, Selcuk O, Demir B, et al. New naphthoquinone thiazole hybrids as carbonic anhydrase and cholinesterase inhibitors: synthesis, crystal structure, molecular docking, and acid dissociation constant. J Mol Struct. 2024;1301:137365. doi: 10.1016/j.molstruc.2023.137365 [DOI] [Google Scholar]
- 33.Pourtaher H, Hasaninejad A, Zare S, et al. The anti-Alzheimer potential of novel spiroindolin-1,2-diazepine derivatives as targeted cholinesterase inhibitors with modified substituents. Sci Rep. 2023;13(1):11952. doi: 10.1038/s41598-023-38236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Güleç Ö, Türkeş C, Arslan M, et al. Cytotoxic effect, enzyme inhibition, and in silico studies of some novel N-substituted sulfonyl amides incorporating 1,3,4-oxadiazol structural motif. Mol Divers. 2022;26(5):2825–2845. doi: 10.1007/s11030-022-10422-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noori M, Dastyafteh N, Safapoor S, et al. Phenyl-quinoline derivatives as lead structure of cholinesterase inhibitors with potency to reduce the GSK-3β level targeting Alzheimer's disease. Int J Biol Macromol. 2023;253:127392. doi: 10.1016/j.ijbiomac.2023.127392 [DOI] [PubMed] [Google Scholar]
- 36.Patel A, Shah D, Patel Y, et al. A review on recent development of novel heterocycles as acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Curr Drug Targets. 2023;24(3):225–246. doi: 10.2174/1389450124666221213114500 [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Singh K, Kaur J, et al. Pathogenesis of Alzheimer's disease and diversity of 1,2,3-triazole scaffold in drug development: design strategies, structural insights, and therapeutic Potential. ACS Chem Neurosci. 2023;14(18):3291–3317. doi: 10.1021/acschemneuro.3c00393 [DOI] [PubMed] [Google Scholar]; •• The potency of aryl-1,2,3-triazoles in the inhibition of ChEs.
- 38.Faraji L, Shahkarami S, Nadri H, et al. Synthesis of novel benzimidazole and benzothiazole derivatives bearing a 1,2,3-triazole ring system and their acetylcholinesterase inhibitory activity. J Chem Res. 2017;41(1):30–35. doi: 10.3184/174751917X14836231670 [DOI] [Google Scholar]
- 39.Khan SA, Akhtar MJ, Gogoi U, et al. An overview of 1,2,3-triazole-containing hybrids and their potential anticholinesterase activities. Pharmaceuticals. 2016;16(2):179. doi: 10.3390/ph16020179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rayatzadeh A, Saeedi M, Mahdavi M, et al. Synthesis and evaluation of novel oxoisoindoline derivatives as acetylcholinesterase inhibitors. Monatsh Fur Chem. 2015;146(4):637–643. doi: 10.1007/s00706-014-1334-2 [DOI] [Google Scholar]
- 41.Luque FJ, Muñoz-Torrero D. Acetylcholinesterase: a versatile template to coin potent modulators of multiple therapeutic targets. Acc Chem Res. 2024;57(4):450–467. doi: 10.1021/acs.accounts.3c00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karimi Askarani H, Iraji A, Rastegari A, et al. Design and synthesis of multi-target directed 1,2,3-triazole-dimethylaminoacryloyl-chromenone derivatives with potential use in Alzheimer's disease. BMC Chem. 2020;14(1):64. doi: 10.1186/s13065-020-00715-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saeedi M, Maleki A, Iraji A, et al. Synthesis and bio-evaluation of new multifunctional methylindolinone-1,2,3-triazole hybrids as anti-Alzheimer's agents. J Mol Struct. 2021;1229:129828. doi: 10.1016/j.molstruc.2020.129828 [DOI] [Google Scholar]
- 44.Mohammadi-Khanaposhtani M, Saeedi M, Zafarghandi NS, et al. Potent acetylcholinesterase inhibitors: design, synthesis, biological evaluation, and docking study of acridone linked to 1, 2, 3-triazole derivatives. Eur J Med Chem. 2015;92:799–806. doi: 10.1016/j.ejmech.2015.01.044 [DOI] [PubMed] [Google Scholar]
- 45.Çevik UA, Osmaniye D, Sağlik BN, et al. Multifunctional quinoxaline-hydrazone derivatives with acetylcholinesterase and monoamine oxidases inhibitory activities as potential agents against Alzheimer's disease. Med Chem Res. 2020;29(6):1000–1011. doi: 10.1007/s00044-020-02541-4 [DOI] [Google Scholar]
- 46.Osmaniye D, Ahmad I, Sağlık BN, et al. Design, synthesis and molecular docking and ADME studies of novel hydrazone derivatives for AChE inhibitory, BBB permeability and antioxidant effects. J Biomol Struct Dyn. 2023;41(18):9022–9038. doi: 10.1080/07391102.2022.2139762 [DOI] [PubMed] [Google Scholar]
- 47.Güngör SA, Design D. Synthesis, in silico and in vitro studies of hydrazide-hydrazone imine derivatives as potential cholinesterase inhibitors. Chem Biol Drug Des. 2023;102(4):676–691. doi: 10.1111/cbdd.14274 [DOI] [PubMed] [Google Scholar]
- 48.Shareghi-Boroujeni D, Iraji A, Mojtabavi S, et al. Synthesis, in vitro evaluation, and molecular docking studies of novel hydrazineylideneindolinone linked to phenoxymethyl-1,2,3-triazole derivatives as potential α-glucosidase inhibitors. Bioorg Chem. 2021;111:104869. doi: 10.1016/j.bioorg.2021.104869 [DOI] [PubMed] [Google Scholar]
- 49.Copeland RA. Enzymes: A practical introduction to structure, mechanism, and data analysis. 3rd ed. NJ, USA: Wiley Press; 2023. [Google Scholar]
- 50.Dulsat J, López-Nieto B, Estrada-Tejedor R, et al. Evaluation of free online ADMET tools for academic or small biotech environments. Molecules. 2023;8(2):776. doi: 10.3390/molecules28020776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


