Abstract
Aim: To determine the mechanism of Calcitonin gene-related peptide (CGRP) in bone healing.
Materials & methods: Alkaline phosphatase (ALP) activity and inflammatory-factor levels were detected using ELISA. Osteogenic differentiation was assessed using Alizarin red staining technique. The interaction between histone deacetylase 6 (HDAC6) and A-kinase anchoring protein 12 (AKAP12) was investigated through Co- immunoprecipitation.
Results: CGRP treatment promoted rat bone marrow-derived macrophages (BMDMs) M2 polarization. CGRP facilitated osteogenic differentiation by enhancing M2 polarization of BMDMs. Mechanistically, CGRP promoted AKAP12 acetylation to activate the extracellular regulated protein kinases pathway by HDAC6 inhibition.
Conclusion: CGRP promoted M2 polarization of rat BMDMs and facilitated osteogenic differentiation through the HDAC6/AKAP12/extracellular regulated protein kinases signaling pathway, thereby promoting bone healing.
Keywords: : CGRP, HDAC6, M2 polarization, nonunion fracture, osteogenic differentiation
Graphical Abstract

Plain language summary
Article highlights.
Diverse concentrations of calcitonin gene-related peptide (CGRP) induced M2 polarization in rat macrophages.
CGRP promoted osteogenic differentiation after co-culture of rat macrophages and rat osteoblasts.
With the increase in CGRP concentration, there was a gradual downregulation of histone deacetylase 6 expression.
CGRP promoted M2 polarization of rat bone marrow-derived macrophages and facilitated osteogenic differentiation through histone deacetylase 6/A-kinase anchoring protein 12/extracellular regulated protein kinases signaling pathway, thereby promoting bone healing.
The present findings offer a theoretical basis for clinical interventions in fracture healing.
1. Background
Nonunion is defined as the absence of signs of healing are observed after 6 months or longer following a fracture and it is one of the most common and challenging complications in fracture treatment [1,2]. Nonunion leads to patient discomfort, functional impairment, psychological stress and diminished life quality, additionally. Furthermore, it imposes a substantial financial burden on the healthcare system [3,4]. The immune system alterations at the fracture site, especially the dynamic regulation mechanism of macrophage polarization, exert a significant influence on fracture healing and osteogenic differentiation. Previous studies have demonstrated that M1 macrophages are capable of promoting an inflammatory response and the recruitment of osteoprogenitor cells, while M2 macrophages have the ability to secrete various growth factors and anti-inflammatory factors, thereby facilitating the differentiation of osteoprogenitor cells into osteoblasts [5,6]. Hence, it is crucial to investigate the role and mechanism through which macrophages contribute to the differentiation of osteoblasts.
CGRP, a 37-amino acid neuropeptide predominantly synthesized and secreted by neurons, elicits potent vasodilatory and analgesic effects [7]. In recent years, an increasing body of research has elucidated that CGRP is involved in bone metabolism, osteogenic differentiation, macrophage polarization and other processes, which holds significant implications for bone repair and bone disease treatment. CGRP is one of the most abundant neuropeptides in bone and exerts its effects on the proliferation and differentiation of bone cells through binding to its receptor, which consists of receptor activity-modifying protein 1 (RAMP1) and calcitonin receptor-like receptor (CRLR), leading to the activation of downstream signaling pathways including cyclic adenosine monophosphate (cAMP), cAMP-response element binding protein (CREB), mitogen-activated protein kinase (MAPK), etc [8]. With aging, the content of CGRP in bone gradually decreases, resulting in detrimental alterations to bone metabolism, such as reduced osteoblasts, increased osteoclasts and weakened angiogenesis [8]. CGRP can also exert its anabolic effect on bone cells by activating the canonical Wnt signaling pathway, which inhibites osteoblast cell apoptosis and promotes local bone regeneration [9]. Inhibition of CGRP negatively impacted fracture healing in mice [10]. The M2 macrophages play a pivotal role in promoting the process of bone healing [11]. Research has shown that CGRP specifically targets M2 macrophages in the pathogenesis of pulmonary fibrosis [12]. The deficiency of CGRP exacerbates postoperative lymphedema by inhibiting the accumulation of M2 macrophages [13]. Co-localization analysis demonstrated that the presence of CGRP with macrophages, while the inhibitory effects of α-CGRP on pro-inflammatory cytokines and M1 markers were observed, along with its promotion of anti-inflammatory cytokines, signal transducers and M2 markers [14]. Several studies have demonstrated that CGRP can suppress the production and function of M1 macrophages while promoting the proportion and activity of M2 macrophages in osteogenesis [15,16] as well as hypertrophic scar formation [17]. The effects of CGRP confer it with anti-inflammatory and immunomodulatory functions. It is hypothesized that CGRP may modulate osteogenic differentiation by influencing macrophage polarization; however, the precise underlying mechanism remains elusive.
HDAC6 is a histone deacetylase involved in histone deacetylation, as well as playing a significant role in osteogenic differentiation and macrophage polarization. Research findings suggest that HDAC6 can inhibit osteogenic differentiation by inactivating RUNX family transcription factor 2 (Runx2) promoter in senile osteoporosis [18]. In studies related to aortic valve calcification, the inhibition of HDAC6 facilitated osteogenic differentiation in valvular interstitial cells via the endoplasmic reticulum stress/ATF4 pathway [19]. In previous studies on tooth regeneration, it has been observed that pharmacological inhibition of HDAC6 deacetylase activity can expedite the senescence process in periodontal ligament stem cells, thereby reducing their capacity for osteogenic differentiation [20]. Furthermore, the antibody chip analysis demonstrated that HDAC6 facilitated the release of sIL-6R to augment M2 polarization of macrophages [21]. Tubastatin A inhibition of HDAC6 can prevent the progression of peritoneal fibrosis by blocking M2 macrophage polarization [22]. Moreover, the deacetylation of AKAP12 by HDAC6 can increase its ubiquitination level, thereby promoting AKAP12 degradation via proteasome [23]. Next-generation sequencing reveled that AKAP12 is potentially implicated in the regulation of osteoblasts of rheumatoid arthritis [24]. In the investigation of colitis, a reduced proportion of M2 macrophages was observed in the inflamed colon of AKAP12 deficient mice compared with their wild-type counterparts, indicating that AKAP12 plays a regulatory role in modulating macrophage polarization toward an M2 phenotype [25]. In a murine model of collagen-induced arthritis, AKAP12-silent mice demonstrated increased clinical arthritis scores and significant ankle swelling, accompanied by reduced expression of M2-polarized macrophages and their products (IL-10 and arginase-1) [26]. In conclusion, HDAC6 may play a role in osteogenic differentiation via regulating M2 macrophage polarization by regulating AKAP12 deacetylation.
Therefore, the current study aims to investigate whether CGRP regulates M2 macrophage polarization and osteogenic differentiation by deacetylating AKAP12 and activating the extracellular regulated protein kinases (ERK) pathway via inhibiting HDAC6 expression. Our study offers a theoretical foundation for the treatment of fracture nonunion by targeting CGRP.
2. Materials & methods
2.1. Cell isolation, culture & treatment
Three male Sprague-Dawley rats (6–8 weeks,170–200 g) were housed in a barrier facility. Bone marrow cells were isolated from the femur and tibia bones, then cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum. Furthermore, recombinant rat granulocyte-macrophage colony-stimulating factor at a concentration of 10 ng/ml (400-23, PeproTech, Suzhou, China) was added to the culture for a duration of 7 days as previously described [27]. Osteoblasts derived from the calvariae of 1-day-old Sprague-Dawley rats were obtained using a series of enzymatic digestion steps, following a previously established protocol [28]. Briefly, the calvariae were finely minced and then placed in an enzymatic solution consisting of 0.1% collagenase (17100017, Gibco, NY, USA) and 0.05% trypsin-EDTA (25300054, Gibco), followed by gentle shaking at room temperature for 20 minutes. This process was repeated six-times to yield a total of six digests. The cells obtained from the final four to six digestions were cultured separately. The co-culture method was described previously [29]. Briefly, macrophages were seeded in 12-well plates with a cell density of 1.0 × 105 cells per well. Following treatment with IL-4 (400-04, PeproTech) and IL-13 (400-16, PeproTech) at a concentration of 20 ng/ml for 24 h to induce M2 polarization, the macrophages were cultured in fresh medium for another 48 h to ensure complete polarization. Subsequently, rat osteoblasts were seeded in each well with a cell density of 2.0 × 105 cells per well. The co-culture medium was composed of DMEM with 1 μg/ml L-ascorbic acid, 1% antibiotic/antimycotic, 10% Fetal bovine serum and 10 mM β-glycerophosphate. CGRP treatment was divided into low, medium and high groups, with concentrations of 10, 50 and 100 ng/ml respectively for a duration of 4 h [9]. All procedures performed during these experiments were approved by Ethical Committee in Qiqihar Medical University (QMU-AECC-2021-81).
2.2. Cell transfection
The lentivirus packaging vector (1 μg/ml) and the lentiviral expression vector Lv-HDAC6 (1 μg/ml, HanBio, Shanghai, China) were co-transfected into 293T cells using Lipofectamin 2000 (11668500, Invitrogen, CA, USA). The virus particles were present in the filtered and centrifuged supernatant, which was collected 48 h after cell culture. The virus was obtained during the exponential growth phase and its titer was measured. Trypsinization was employed to dissociate the cells upon reaching the logarithmic growth phase. The resulting cell suspension, with a concentration of of 5 × 104 cells/ml, was carefully dispensed into each well of a 6-well plate at a volume of 2 ml per well. Each lentivirus (virus titer of 1 × 108 TU/ml, MOI = 10) was added to the cell culture medium for 48 h and stable cell lines were selected using puromycin (UC0E03, Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 2 μg/ml for a duration of 2 weeks.
2.3. Osteogenic differentiation induction
The rat macrophages and osteoblasts were co-cultured to promote osteogenic differentiation in the above experiment. The method of inducing osteogenic differentiation was as described previously [18]. Briefly, the cells were s treated with dexamethasone at a concentration of 100 nM, β-glycerophosphate disodium at a concentration of 10 mM and ascorbic acid at a concentration of 50 μg/ml. The in vitro treatment with tubastatin A (SML0044, Sigma-Aldrich) dissolved in DMSO at a concentration of 8 μM for a duration of 7–21 days. For the control group, vehicle controls were treated solely with culture medium containing DMSO.
2.4. Flow cytometry
Rat bone marrow-derived macrophages (BMDMs) were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD206 antibody (MA5–16870, Thermo Fisher, MA, USA) and incubated in the dark at 4°C for 30 min. Subsequently, 2 ml of phosphate-buffered saline (PBS) solution (P4417, Sigma-Aldrich) was added to the BMDMs, which were then centrifuged at 4°C, 1500 g for 10 min. The supernatant was removed and the cells were fixed with a solution of 2% paraformaldehyde (30525-89-4, Sigma-Aldrich) in PBS. They were stored in the dark at 4°C and analyzed using FACS Aria II flow cytometer (BD Bioscience, NJ, USA) within 24 h.
2.5. ELISA
The cell supernatant was obtained through centrifugation and the levels of IL-10, TGF-β and IL-6 in the supernatants were quantified using ELISA kits (Bosterbio) following the manufacturer's instructions.
2.6. Alizarin red staining
After 21 days of osteogenic differentiation induction, the cells to be stained were washed with PBS for three-times and fixed with 4% paraformaldehyde at 4°C for 10 min. After fixation, the cells were rinsed with PBS and then incubation in 40 mM alizarin red (pH 4.2, TMS-008, Sigma-Aldrich) at a temperature of 37°C for a duration of 30 min. Subsequently, cells were subjected to another round of PBS washing prior to imaging. The decalcification process was carried out overnight at 4°C using a 0.1 M HCl solution. Following this, a volume of 20 μl from the sample was transferred to a tube containing 1 ml of methyl thymol blue solution and an additional 1 ml of alkaline solution. The absorbance measurement was performed at a wavelength of 610 nm.
2.7. ALP activity assay
The ALP activity assay was performed using methods that have been previously documented [30]. Briefly, after 7 days of osteogenic induction, RIPA lysis buffer (P0013C, Beyotime biotechnology) was used to lyse 1 million cells for a duration of 5 min. The lysate was subsequently centrifuged at 4°C for 3 min to obtain the supernatant. ALP activity was thendetermined by colorimetric analysis using ALP activity detection kit (21101ES60, Yeasen, Shanghai, China).
2.8. Co-immunoprecipitation
The cells were lysed by incubation in a cell lysis buffer (containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100 and a protease inhibitor cocktail) for a duration of 30 min at a temperature of 4°C with continuous rotation. Subsequently, the resulting lysates were centrifuged at 17000 g for a period of 20 min at the same temperature. After reserving a portion of the cell extracts for input analysis, the remaining supernatants were transferred to new microcentrifuge tubes and incubated overnight with acetylated lysine, HDAC6 (7612, Cell Signaling Technology, MA, USA) and AKAP12 (PA1–29372, Invitrogen) antibodies on a rotator at 4°C. The following day, 40 μl of protein A/G agarose beads were added and incubated at a temperature of 4°C of 1–3 h. Immunoprecipitation of HA- or flag-tagged proteins was performed using anti-HA or anti-flag affinity gel according to the manufacturer's instructions. The proteins bound to the beads were subjected to four rounds of washing with cell lysis buffer before being eluted using 2× sample buffer for subsequent analysis.
2.9. Quantitative real time polymerase chain reaction (qRT-PCR)
After 7 days of osteogenic differentiation induction, markers of osteogenic differentiation were detected. The total RNA was isolated and its concentration and purity were determined using the Nanodrop2000 micro UV spectrophotometer (Thermo Fisher). Subsequently, cDNA synthesis was performed according to the manufacturer's protocol for the PrimeScript RT reagent Kit (RR047A, Takara, Osaka, Japan). For RT-qPCR analysis, the Fast SYBR Green PCR kit (RR820A, Takara) and ABI PRISM 7300 RT-PCR system (Applied Biosystems, MA, USA) were utilized. Each sample was tested in triplicate. Actin beta (β-actin) served as an internal reference and the relative gene expression was analyzed by the 2-ΔΔCt method. The primer sequences were shown in the Table 1.
Table 1.
The primer sequences used in the study.
| Gene | 5′-3′ |
|---|---|
| Arg-1 | F, CTGGACCCAGTATTCACCCC, |
| R, ATGATTACCTTCCCGTTTCGT; | |
| CD206 | F, AACTTCATCTGCCAGCGACA, |
| R, CGTGCCTCTTTCCAGGTCTT; | |
| CCL17 | F, GAAGCTGGTGACGTGGTTCA, |
| R, TCACATGCTTGTCTTTGGGGT; | |
| Fizz1 | F, GCTGGGATGACTGCTACTGG, |
| R, GCCCAGTCAACGATTAAGCAC; | |
| RUNX2 | F, CATGGCCGGGAATGATGAGA, |
| F, GGGAGGATTTGTGAAGACCGT; | |
| COL1A1 | F, AACAAGGGAGGAGAGAGTGC, |
| R, AGTCTCTTGCTTCCTCCCAC; | |
| ALP | F, CCTTCTTCCGTCAGTACCGT, |
| R, AGCTGGTTCATCCCGATTGT; | |
| OPN | F, GAGGAGAAGGCGCATTACAG, |
| R, ACAGAATCCTCGCTCTCTGC; | |
| BMP-2 | F, CGGAAGCGTCTTAAGTCCAG, |
| R, GCTAAGCTCAGTGGGGACAC; | |
| MCP-1 | F, TAGCATCCACGTGCTGTCTC, |
| R, CAGCCGACTCATTGGGATCA; | |
| β-actin | F, CTCATGCCATCCTGCGTCTG, |
| R, GGCAGTGGCCATCTCTTGCT. |
β-actin: Actin beta; AKAP12: A-kinase anchoring protein 12; ALP: Alkaline phosphatase; Arg-1: Arginase 1; BMDM: Bone marrow-derived macrophage; BMP-2: Bone morphogenetic protein 2; cAMP: Cyclic adenosine monophosphate; CCL17: C-C motif chemokine ligand 17; CD206: Mannose receptor C-type 1; CGRP: Calcitonin gene-related peptide; Co-IP: Co-immunoprecipitation; COL1A1: Collagen type I alpha 1 chain; CREB: CAMP-response element binding protein; CRLR: Composed of calcitonin receptor-like receptor; ERK: Extracellular regulated protein kinases; Fizz1: Resistin like alpha; HDAC6: Histone deacetylase 6; IP: Immunoprecipitation; MCP-1: Macrophage cationic peptide 1; MAPK, mitogen-activated protein kinase; OPN: Secreted phosphoprotein 1; RAMP1: Receptor activity-modifying protein 1; RUNX2: RUNX family transcription factor 2.
2.10. Western blot
After 7 days of osteogenic differentiation induction, markers of osteogenic differentiation were detected. The cells were harvested and disrupted using RIPA lysis buffer (P0013B, Beyotime, Shanghai, China) supplemented with 1% Phenylmethylsulfonyl fluorid (PMSF). The protein concentration in the lysates was quantified using the BCA method (P0012S, Beyotime). An appropriate volume of 5 × loading Buffer was added to the samples and subjected to boiling at 100°C for 10 min to ensure proper denaturation of proteins. The protein amount loaded on the gel was standardized to 50 μg. The subsequent steps involved the preparation of a separating gel and stacking gel for electrophoresis, followed by transfer of target protein bands onto a Polyvinylidene Fluoride (PVDF) membrane. Then, the PVDF membrane was placed in 5% skimmed milk powder and incubated overnight at 4°C with primary antibodies: HDAC6 (7612, 1:1000, Cell Signaling Technology); p-ERK1/2 (PA5–40294, 1:500, Invitrogen); ERK1/2 (MA5–15605, 1:1000, Invitrogen); Arg-1 (PA5–32267, 1:100, Invitrogen); CD206 (24595, 1:1000, Cell Signaling Technology); CCL17 (PA5–34515, 1:500, Invitrogen); Fizz1 (PA1–4257, 1:1000, Invitrogen); RUNX2 (PA5–87299, 1:1000, Invitrogen); COL1A1 (PA5–29569, 1:1000, Invitrogen); ALP (PA5–106391, 1:1000, Invitrogen); OPN (PA5–34579, 1:500, Invitrogen); BMP-2 (PA5–85956, 1:1000, Invitrogen); MCP-1 (PA5–34505, 1:500, Invitrogen); AKAP12 (PA1–29372, 1:1000, Invitrogen); GAPDH (5174, 1:1000, Cell Signaling Technology) following a blocking period of 1 h at room temperature. After being washed with Phosphate buffered solution and Tween-20 (PBST) at room temperature, Horseradish peroxidase (HRP)-labeled goat antirabbit/goat antimouse IgG (1:10000, BA1054/BA1050, bosterbio) secondary antibodies were incubated at room temperature for 1 h and subsequently washed with PBST six-times for 5 min each time. ECL reagent (AR1172, bosterbio, Wuhan, China) was evenly added to the membrane and then exposed in the imaging instrument (Amersham Imager 600, GE). Gray value analysis was conducted by Image J (National Institutes of Health, MD, USA) and the experiment was repeated three-times.
2.11. Statistical analysis
Statistical analysis of the data in this study was performed using SPSS software (version 21.0). The measurement data were presented as mean ± standard deviation and normality and homogeneity of variance tests were performed first. Student's t-test was used for intergroup comparison, whereas one-way analysis of variance was used for multiple group comparison. p < 0.05 indicated that the difference was statistically significant. All experiments were repeated three-times.
3. Results
3.1. CGRP promoted M2 polarization of rat macrophages
It has been reported that CGRP exerts a beneficial influence on fracture healing, which is likely mediated by its regulation of macrophage polarization [15,16]. To investigate the impact of CGRP treatment on macrophage polarization, rat macrophages were exposed to varying concentrations of CGRP. As the concentration of CGRP treatment increased, there was a gradual increase in the M2 polarization marker mannose receptor C-type 1 (CD206) in rat macrophages Additionally, the expression of arginase 1 (Arg-1), C-C motif chemokine ligand 17 (CCL17), resistin like alpha (Fizz1) was gradually upregulated both at protein and mRNA levels (Figure 1A–C). Subsequently, it was observed that the increase in CGRP treatment concentration led to a decrease in the levels of IL-10, TGF-β, IL-6 in rat macrophages (Figure 1D), thus confirming the anti-inflammatory effect of CGRP. Finally, as the concentration of CGRP treatment increased, there was a gradual downregulation of HDAC6 expression in rat macrophages, accompanied by an increase in levels of the ERK pathway related gene (p-ERK1/2)(Figure 1E). The concentration of 50 ng/ml demonstrated a statistically significant difference among the tested concentrations, thus this particular concentration was selected for subsequent experiments. The findings indicated that CGRP facilitated the M2 polarization of rat macrophages.
Figure 1.

Different concentrations of CGRP promoted M2 polarization of rat macrophages. (A) CGRP treatment was divided into low, medium and high groups, with concentrations of 10, 50 and 100 ng/ml and rat macrophages was treated for 4 h. Macrophage M2 polarization marker CD206 was detected by flow cytometry. (B) The mRNA levels of M2 marker genes Arg-1, CD206, CCL17 and Fizz1 were detected by qRT-PCR. (C) Protein levels of M2 marker genes Arg-1, CD206, CCL17 and Fizz1 were analyzed by western blot. (D) The levels of inflammatory cytokines IL-10, TGF-β and IL-6 were detected by ELISA. (E) The protein levels of HDAC6 and ERK pathways including p-ERK1/2 and ERK1/2 were analyzed by western blot. The results were average of three experiments and represented as mean ± SD.
*p < 0.05; **p < 0.01; ***p < 0.001.
Arg-1: Arginase 1; CCL17: C-C motif chemokine ligand 17; CD206: Mannose receptor C-type 1; CGRP: Calcitonin gene-related peptide; ERK: Extracellular regulated protein kinases; Fizz1: Resistin like alpha; HDAC6: Histone deacetylase 6; SD: Standard Deviation.
3.2. CGRP promoted osteogenic differentiation by promoting M2 polarization of macrophages
Studies have shown that M2-type macrophages are capable of secreting a variety of growth factors and anti-inflammatory factors, promoting osteoblast differentiation [5]. We co-cultured macrophages and osteoblasts to investigate the effect of CGRP on osteogenic differentiation. Our findings revealed that CGRP treatment significantly increased the calcified nodules, while adding CGRP receptor antagonist [CGRP (8–37)] significantly reduced the calcified nodules (Figure 2A). Meanwhile, the ALP activity exhibited a significant increase following CGRP treatment, whereas treatment with CGRP (8–37) (CGRP receptor antagonist) led to a significant reduction in ALP activity (Figure 2B). Additionally, the expression of osteogenic differentiation genes RUNX2, collagen type I alpha 1 chain (COL1A1), ALP, secreted phosphoprotein 1 (OPN), bone morphogenetic protein 2 (BMP-2) and osteogenic chemokine macrophage cationic peptide 1 (MCP-1) at mRNA and protein levels were upregulated following CGRP treatment, while adding CGRP (8–37) (CGRP receptor antagonist) reversed the above results (Figure 2C–D). In conclusion, these findings suggested that CGRP facilitated osteogenic differentiation through the promotion of M2 macrophage polarization.
Figure 2.

CGRP regulated the differentiation of rat osteoblasts by promoting the M2 polarization of rat macrophages. Cells were treated for 4 h with 50 ng/ml CGRP alone or in association with its receptor antagonist, CGRP receptor antagonist [CGRP (8–37)] (10 nM) that was added 30 min before the neuropeptide. (A) Alizarin red staining was used to detect the mineralization of osteoblast secreted extracellular matrix. (B) The ALP activity was detected by ELISA. (C) The mRNA levels of osteogenic differentiation marker genes (RUNX2, COL1A1, ALP, OCN, BMP-2 and MCP-1) were detected by qRT-PCR. (D) The protein levels of osteogenic differentiation marker genes (RUNX2, COL1A1, ALP, OCN, BMP-2 and MCP-1) were analyzed by western blot. The results were average of three experiments and represented as mean ± SD.
*p < 0.05; **p < 0.01; ***p < 0.001.
ALP: Alkaline phosphatase; BMP-2: Bone morphogenetic protein 2; CGRP: Calcitonin gene-related peptide; COL1A1: Collagen type I alpha 1 chain; MCP-1: Macrophage cationic peptide 1; RUNX2: RUNX family transcription factor 2.
3.3. CGRP promoted AKAP12 acetylation by inhibiting HDAC6 expression & activating the ERK pathway
Evidence has shown that HDAC6 exerts an inhibitory effect on osteogenic differentiation [18]. Meanwhile, HDAC6 is capable of interacting with AKAP12 and deacetylate AKAP12 at K526/K531 sites [23]. However, there is no available research regarding the involvement of AKAP12 in osteogenic differentiation. Subsequently, we further investigated the underlying mechanisms of CGRP and AKAP12 in the process of osteogenic differentiation. As the concentration of CGRP treatment increased, there was a gradual upregulation of AKAP12 expression observed (Figure 3A). Co-immunoprecipitation further confirmed the specific interaction between HDAC6 and AKAP12 (Figure 3B). After the overexpression of HDAC6, the protein expression of AKAP12 was suppressed (Figure 3C). These findings suggest that HDAC6 facilitates the degradation of AKAP12 through protein binding. Moreover, treatment with CGRP led to increased acetylation and protein expression levels of AKAP12, as well as activation of the ERK pathway, while HDAC6 protein expression decreased. However, overexpression of HDAC6 resulted in reduced acetylation and protein expression levels of AKAP12, as well as inhibition of the ERK pathway (Figure 3D–E). In conclusion, CGRP can promote AKAP12 acetylation by inhibiting HDAC6 expression and activating the ERK pathway.
Figure 3.

CGRP promoted AKAP12 acetylation by inhibiting HDAC6 expression and activating the ERK pathway. (A) The protein level of AKAP12 was analyzed by western blot. (B) The protein interaction of HDAC6 and AKAP12 was analyzed by Co-IP and western blot. (C) The protein levels of HDAC6 and AKAP12 of cells in Oe-NC group and Oe-HDAC6 group were analyzed by western blot. (D) The AKAP12 acetylation levels of cells in Control group, CGRP group, CGRP + Oe-NC group and CGRP + Oe-HDAC6 group were analyzed by IP and western blot. (E) The protein levels of ERK pathways including p-ERK1/2 and ERK1/2 were analyzed by western blot. The results were average of three experiments and represented as mean ± SD.
*p < 0.05; **p < 0.01; ***p < 0.001.
AKAP12: A-kinase anchoring protein 12; Co-IP: Co-immunoprecipitation; ERK: Extracellular regulated protein kinases; HDAC6: Histone deacetylase 6.
3.4. CGRP promoted M2 polarization of macrophages by regulating HDAC6 expression
Subsequent experiments were then conducted to investigate the potential role of CGRP in macrophage polarization through the regulation of HDAC6 expression. Results revealed that the M2 polarization marker CD206 was gradually decreased and the expression levels of Arg-1, CD206, CCL17 and Fizz1 were progressively downregulated in rat macrophages following overexpression of HDAC6 compared with CGRP treatment group (Figure 4A–C), thereby reversing the impact of CGRP treatment. Simultaneously, after overexpression of HDAC6, the levels of IL-10, TGF-β and IL-6 in rat macrophages were significantly increased compared with those in the CGRP treatment group (Figure 4D), attenuating the treatment effect of CGRP. These findings indicated that CGRP could regulate M2 polarization of rat macrophages by regulating HDAC6 expression.
Figure 4.

CGRP regulated M2 polarization of rat macrophages through HDAC6. Cells treated with CGRP was transfected with the lentiviral expression vector Lv-HDAC6. (A) Macrophage M2 polarization marker CD206 were detected by flow cytometry. (B) The mRNA levels of M2 marker genes (Arg-1, CD206, CCL17 and Fizz1) were detected by qRT-PCR. (C) Protein levels of M2 marker genes (Arg-1, CD206, CCL17 and Fizz1) were analyzed by western blot. (D) The levels of inflammatory cytokines (IL-10, TGF-β and IL-6) were detected by ELISA. The results were average of three experiments and represented as mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001.
Arg-1: Arginase 1; CCL17: C-C motif chemokine ligand 17; CD206: Mannose receptor C-type 1; Fizz1: Resistin like alpha.
3.5. CGRP promoted osteogenic differentiation by promoting M2 polarization of macrophages via regulating HDAC6 expression
Subsequent experiments were carried out to investigate whether CGRP promotes osteogenic differentiation by increasing M2 polarization of macrophages through HDAC6. Alizarin red staining assays revealed that, compared with the CGRP treatment group, overexpression of HDAC6 led to a decrease in both calcified nodules and ALP activity (Figure 5A–B). At the same time, overexpression of HDAC6 downregulated the expression levels of osteogenic differentiation genes (RUNX2, COL1A1, ALP, OCN, BMP-2) and osteogenic chemokine MCP-1 (Figure 5C–D), thereby counteracting the effect of CGRP treatment. In conclusion, these data indicate that CGRP promotes osteogenic differentiation by promoting M2 polarization of macrophages by HDAC6.
Figure 5.
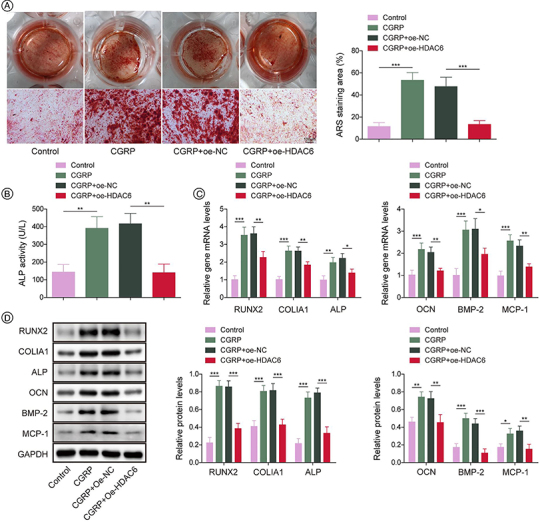
CGRP promoted osteogenic differentiation by facilitating M2 polarization of macrophages through HDAC6 modulation. (A) Alizarin red staining showing the mineralization of osteoblast secreted extracellular matrix. (B) The ALP activity of cells was detected by ELISA. (C) The mRNA levels of osteogenic differentiation marker genes (RUNX2, COL1A1, ALP, OCN, BMP-2 and MCP-1) were detected by qRT-PCR. (D) The protein levels of osteogenic differentiation marker genes (RUNX2, COL1A1, ALP, OCN, BMP-2 and MCP-1) were analyzed by western blot. The results were average of three experiments and represented as mean ± SD.
*p < 0.05; **p < 0.01; ***p < 0.001.
ALP: Alkaline phosphatase; BMP-2: Bone morphogenetic protein 2; COL1A1: Collagen type I alpha 1 chain; HDAC6: Histone deacetylase 6; MCP-1: Macrophage cationic peptide 1; RUNX2: RUNX family transcription factor 2.
4. Discussion
Nonunion occurs when the osseous consolidation process fails, which can be attributed to inadequate immobilization, unsuccessful surgical intervention, insufficient biological response or infection following an extended period of impaired healing [31]. The modulation of fracture healing is significantly influenced by the dynamic regulation of macrophage polarization, as demonstrated in previous studies [5,6]. In this study, we investigated the regulatory mechanism of CGRP on osteogenic differentiation by modulating M2 polarization of rat BMDMs for the first time. Subsequently, we identified that CGRP activates the ERK pathway by promoting AKAP12 acetylation through the inhibition of HDAC6 expression.
Previous studies have suggested that CGRP has the ability to enhance M2 polarization in mouse macrophages obtained from neural and bone marrow origins [14,16] and that M2 macrophages are the target cells of CGRP in pulmonary fibrosis [12]. Consistent with these studies, our study demonstrates that CGRP can promote the differentiation of rat bone marrow-derived M2 macrophages, increasing the expression of M2 polarization markers. Furthermore, the downregulation of proinflammatory factors provides further evidence for the anti-inflammatory properties of CGRP. It is widely recognized that M2 macrophages can secrete various growth factors and anti-inflammatory factors, promoting the differentiation of osteoprogenitor cells into osteoblasts [5,6]. We have presented evidence that the facilitation of osteogenic differentiation can be observed through alizarin red staining and detection of osteogenic differentiation factors in the presence of CGRP. Our study has established the potential therapeutic value of CGRP as a target for nonunion fracture.
Subsequently, we further investigated the regulatory mechanism of CGRP on M2 polarization in rat BMDMs. Notably, our study revealed that the expression of HDAC6 in rat macrophages was downregulated after CGRP treatment. Studies have reported that the involvement of HDAC6 in the deacetylation process of α-tubulin is implicated in the pathogenesis of of Charcot-Marie-Tooth disease [32]. Meanwhile, HDAC6 interacts with isocitrate dehydrogenase 1 (IDH1) to deacetylate lysine 233 on IDH1, thereby promoting the proliferation of hematopoietic stem and progenitor cells (HSPCs) within the bone marrow [33]. Interestingly, HDAC6 has been shown to interact with AKAP12 and promote AKAP12 degradation through the proteasome pathway [23]. Consistently, our findings demonstrated the interaction between HDAC6 and AKAP12, with overexpression of HDAC6 leading to a downregulation of AKAP12 expression. Significantly, treatment with CGRP led to an increase in acetylation and protein expression levels of AKAP12, accompanied by a decrease in HDAC6 protein expression. Moreover, the effects of CGRP were attenuated by overexpression of HDAC6. There is evidence indicating that ERK pathway plays a crucial role in osteogenesis. KIAA1199 modulates osteogenesis by suppressing the production of osteopontin via integrin-mediated AKT and ERK-MAPK intracellular signaling [34]. Additionally, studies have indicated that downregulation of Cdk5 expression results in an upregulation of Erk phosphorylation, leading to an enhanced expression of osteoblast-specific genes [35]. In this study, we observed that CGRP facilitated AKAP12 acetylation by suppressing HDAC6 expression and leading to the activation of the ERK pathway. Therefore, the regulation of AKAP12 deacetylation by HDAC6 represents a mechanism through which CGRP exerts its biological effects.
Given that impaired bone regeneration affects a large proportion of fracture patients, it is crucial from both clinical and scientific perspectives to gain a deep understanding of the mechanism underlying bone healing and identify the molecular mediators that influence bone regeneration. Previous research has demonstrated the importance of αCGRP, a key molecule in bone synthesis and metabolism, in maintaining normal bone function [36]. This finding offers a new molecular insight into the role of CGRP in regulating bone healing, thus holding significant research value in the field of regenerative medicine. Enhanced expression of M2 markers in macrophages by CGRP has been demonstrated in previous studies [37]. Additionally, M2 macrophages have been shown to elevate growth factor expression, thereby promoting bone formation [38]. Nevertheless, the specific molecular mechanisms through which CGRP regulates M2 macrophage polarization and controls osteogenic differentiation remain to be elucidated. This study delved deeply into the mechanism of CGRP promoted bone healing by modulating HDAC6-mediated AKAP12 acetylation and M2 macrophage polarization. Unlike previous studies on the role of CGRP in bone metabolism, this paper focuses more on the detailed mechanisms of its role in bone healing process, especially its contribution to macrophage polarization and osteogenesis, revealing the specific molecular mechanisms by which CGRP affects bone metabolism. In the field of neurology, all CGRP-targeted therapies for acute migraine management and prevention have consistently demonstrated efficacy, providing robust support for the pivotal role of CGRP in migraine pathophysiology [39,40]. Furthermore, these treatments exhibit favorable tolerability and safety profiles, with no specific warnings or precautions related to adverse effects apart from potential allergic reactions and injection site responses [41,42]. This study offers theoretical underpinning for targeted CGRP therapy in nonunion fractures, holding promise for potential clinical benefits in patients with this condition.
5. Conclusion
The nonunion of fractures is a common and challenging complication in fracture management. CGRP, an abundant neuropeptide in bone, may lead to adverse changes in bone metabolism when its levels are reduced. By inducing the polarization of rat macrophages, our study demonstrated that different concentrations of CGRP induced the polarization of rat macrophages toward M2 phenotype and co-culture with rat osteoblasts resulted in enhanced osteogenic differentiation. Furthermore, we observed that CGRP had concentration-dependent effects on HDAC6 expression. Subsequent investigation revealed that CGRP promoted AKAP12 acetylation by inhibiting HDAC6 expression and activates ERK pathway. Overexpression of HDAC6 attenuated the promoting effect of CGRP on osteogenic differentiation. In conclusion, our findings suggest that CGRP regulates macrophage M2 polarization through HDAC6/AKAP12 signaling pathway to promote osteogenic differentiation. This study provides insights into the mechanism by which CGRP modulates HDAC6-mediated AKAP12 acetylation on the ERK pathway and M2 macrophage polarization, offering a theoretical basis for clinical interventions in fracture healing.
Funding Statement
This work was supported by Heilongjiang Provincial Health Commission (20210404070196).
Author contributions
WC and XJ designed this study. WC, LM, WS, WX, HG and JX collected the materials and performed the experiments. WC analyzed the data and wrote the manuscript. XJ revised the manuscript. All authors read and approved the final version of the manuscript.
Financial disclosure
This work was supported by Heilongjiang Provincial Health Commission (20210404070196). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval (QMU-AECC-2021-81) and/or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
- 1.Ozkan S, Nolte PA, van den Bekerom MPJ, et al. Diagnosis and management of long-bone nonunions: a nationwide survey. Eur J Trauma Emerg Surg. 2019;45:3–11. doi: 10.1007/s00068-018-0905-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saul D, Menger MM, Ehnert S, et al. Bone healing gone wrong: pathological fracture healing and non-unions-overview of basic and clinical aspects and systematic review of risk factors. Bioengineering (Basel). 2023;10:85. doi: 10.3390/bioengineering10010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmal H, Brix M, Bue M, et al. Nonunion - consensus from the 4th annual meeting of the Danish Orthopaedic Trauma Society. EFORT Open Rev. 2020;5:46–57. doi: 10.1302/2058-5241.5.190037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeckey C, Mommsen P, Andruszkow H, et al. The aseptic femoral and tibial shaft non-union in healthy patients - an analysis of the health-related quality of life and the socioeconomic outcome. Open Orthop J. 2011;5:193–197. doi: 10.2174/1874325001105010193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Li J, Xie X, et al. Macrophage-osteoclast associations: origin, polarization and subgroups. Front Immunol. 2021;12:778078. doi: 10.3389/fimmu.2021.778078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Shen A, Wang Z. Enhanced osteogenic differentiation of BMSCs and M2-phenotype polarization of macrophages on a titanium surface modified with graphene oxide for potential implant applications. RSC Adv. 2020;10:16537–16550. doi: 10.1039/c9ra10563h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amara SG, Jonas V, Rosenfeld MG, et al. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0 [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Wang J, Chen X, et al. The effects of calcitonin gene-related peptide on bone homeostasis and regeneration. Curr Osteoporos Rep. 2020;18:621–632. doi: 10.1007/s11914-020-00624-0 [DOI] [PubMed] [Google Scholar]
- 9.Mrak E, Guidobono F, Moro G, et al. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by beta-catenin stabilization. J Cell Physiol. 2010;225:701–708. doi: 10.1002/jcp.22266 [DOI] [PubMed] [Google Scholar]
- 10.Wee NKY, Novak S, Ghosh D, et al. Inhibition of CGRP signaling impairs fracture healing in mice. J Orthop Res. 2023;41:1228–1239. doi: 10.1002/jor.25474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajarinen J, Lin T, Gibon E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi: 10.1016/j.biomaterials.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartopo AB, Emoto N, Vignon-Zellweger N, et al. Endothelin-converting enzyme-1 gene ablation attenuates pulmonary fibrosis via CGRP-cAMP/EPAC1 pathway. Am J Respir Cell Mol Biol. 2013;48:465–476. doi: 10.1165/rcmb.2012-0354OC [DOI] [PubMed] [Google Scholar]
- 13.Matsui S, Tanaka M, Kamiyoshi A, et al. Endogenous calcitonin gene-related peptide deficiency exacerbates postoperative lymphedema by suppressing lymphatic capillary formation and M2 macrophage accumulation. Am J Pathol. 2019;189:2487–2502. doi: 10.1016/j.ajpath.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 14.Yuan K, Zheng J, Shen X, et al. Sensory nerves promote corneal inflammation resolution via CGRP mediated transformation of macrophages to the M2 phenotype through the PI3K/AKT signaling pathway. Int Immunopharmacol. 2022;102:108426. doi: 10.1016/j.intimp.2021.108426 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Wu B, Yuan Y, et al. CGRP-modulated M2 macrophages regulate osteogenesis of MC3T3-E1 via Yap1. Arch Biochem Biophys. 2021;697:108697. doi: 10.1016/j.abb.2020.108697 [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y, Jiang Y, Wang B, et al. Deficiency of calcitonin gene-related peptide affects macrophage polarization in osseointegration. Front Physiol. 2020;11:733. doi: 10.3389/fphys.2020.00733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Hua T, Weng X, et al. Calcitonin gene-related peptide alleviates hypertrophic scar formation by inhibiting the inflammation. Arch Dermatol Res. 2022;314:53–60. doi: 10.1007/s00403-020-02179-7 [DOI] [PubMed] [Google Scholar]
- 18.Ma C, Gao J, Liang J, et al. HDAC6 inactivates Runx2 promoter to block osteogenesis of bone marrow stromal cells in age-related bone loss of mice. Stem Cell Res Ther. 2021;12:484. doi: 10.1186/s13287-021-02545-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Z, Li F, Jia L, et al. Histone deacetylase 6 reduction promotes aortic valve calcification via an endoplasmic reticulum stress-mediated osteogenic pathway. J Thorac Cardiovasc Surg. 2019;158:408–417 e2. doi: 10.1016/j.jtcvs.2018.10.136 [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Ma Y, Zhu Y, et al. Declined expression of histone deacetylase 6 contributes to periodontal ligament stem cell aging. J Periodontol. 2017;88:e12–e23. doi: 10.1902/jop.2016.160338 [DOI] [PubMed] [Google Scholar]
- 21.Xu G, Niu L, Wang Y, et al. HDAC6-dependent deacetylation of TAK1 enhances sIL-6R release to promote macrophage M2 polarization in colon cancer. Cell Death Dis. 2022;13:888. doi: 10.1038/s41419-022-05335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Li J, Chen H, et al. Pharmacologic inhibition of histone deacetylase 6 prevents the progression of chlorhexidine gluconate-induced peritoneal fibrosis by blockade of M2 macrophage polarization. Front Immunol. 2022;13:899140. doi: 10.3389/fimmu.2022.899140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Y, Gao J, Xu G, et al. HDAC6-dependent deacetylation of AKAP12 dictates its ubiquitination and promotes colon cancer metastasis. Cancer Lett. 2022;549:215911. doi: 10.1016/j.canlet.2022.215911 [DOI] [PubMed] [Google Scholar]
- 24.Chen YJ, Chang WA, Hsu YL, et al. Deduction of novel genes potentially involved in osteoblasts of rheumatoid arthritis using next-generation sequencing and bioinformatic approaches. Int J Mol Sci. 2017;18:2396. doi: 10.3390/ijms18112396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang JM, Lee HS, Seo JH, et al. Structural environment built by AKAP12+ colon mesenchymal cells drives M2 macrophages during inflammation recovery. Sci Rep. 2017;7:42723. doi: 10.1038/srep42723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni Y, Cao J, Yuan J, et al. Loss of AKAP12 aggravates rheumatoid arthritis-like symptoms and cardiac damage in collagen-induced arthritis mice. Exp Anim. 2023;72:242–252. doi: 10.1538/expanim.22-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Chen Y, Hong W, et al. Chronic exposure to biomass ambient particulate matter triggers alveolar macrophage polarization and activation in the rat lung. J Cell Mol Med. 2022;26:1156–1168. doi: 10.1111/jcmm.17169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizuya T, Yokose S, Hori M, et al. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest. 1997;99:2961–2970. doi: 10.1172/JCI119491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye W, Wang J, Lin D, et al. The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int J Biol Macromol. 2020;146:25–35. doi: 10.1016/j.ijbiomac.2019.12.028 [DOI] [PubMed] [Google Scholar]
- 30.Zhu D, Zhu Z, Qi H. NEAT1/microRNA 339-5p/SPI1 axis feedback loop contributes to osteogenic differentiation in acute suppurative osteomyelitis in children. J Inflamm Res. 2023;16:2675–2687. doi: 10.2147/JIR.S410339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildemann B, Ignatius A, Leung F, et al. Non-union bone fractures. Nat Rev Dis Primers. 2021;7:57. doi: 10.1038/s41572-021-00289-8 [DOI] [PubMed] [Google Scholar]
- 32.Benoy V, Van Helleputte L, Prior R, et al. HDAC6 is a therapeutic target in mutant GARS-induced Charcot-Marie-Tooth disease. Brain. 2018;141:673–687. doi: 10.1093/brain/awx375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Liu Y, Yin H, et al. HDAC6 deacetylates IDH1 to promote the homeostasis of hematopoietic stem and progenitor cells. EMBO Rep. 2023;24:e56009. doi: 10.15252/embr.202256009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Shi K, Ditzel N, et al. KIAA1199 deficiency enhances skeletal stem cell differentiation to osteoblasts and promotes bone regeneration. Nat Commun. 2023;14:2016. doi: 10.1038/s41467-023-37651-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad M, Kruger BT, Kroll T, et al. Inhibition of Cdk5 increases osteoblast differentiation and bone mass and improves fracture healing. Bone Res. 2022;10:33. doi: 10.1038/s41413-022-00195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schinke T, Liese S, Priemel M, et al. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res. 2004;19:2049–2056. doi: 10.1359/JBMR.040915 [DOI] [PubMed] [Google Scholar]
- 37.Duan JX, Zhou Y, Zhou AY, et al. Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro. Mol Immunol. 2017;91:105–113. doi: 10.1016/j.molimm.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Bose T, Unger RE, et al. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017;369:273–286. doi: 10.1007/s00441-017-2598-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: The ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887–1898. doi: 10.1001/jama.2019.16711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott LJ. Ubrogepant: first approval. Drugs. 2020;80:323–328. doi: 10.1007/s40265-020-01264-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bussiere JL, Davies R, Dean C, et al. Nonclinical safety evaluation of erenumab, a CGRP receptor inhibitor for the prevention of migraine. Regul Toxicol Pharmacol. 2019;106:224–238. doi: 10.1016/j.yrtph.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 42.Yuan H, Spare NM, Silberstein SD. Targeting CGRP for the prevention of migraine and cluster headache: a narrative review. Headache. 2019;59(Suppl. 2):20–32. doi: 10.1111/head.13583 [DOI] [PubMed] [Google Scholar]


