ABSTRACT
Osteosarcoma (OS), a primary human malignant tumor that affects the bones, mostly arises in children and adolescents. Even though surgical resection followed by radiotherapy and chemotherapy has improved the survival rate up to 60%, the long-term positive effect for most patients with OS is not satisfactory. Hence, elucidating the specific mechanisms involved in the pathogenesis of OS is particularly important. Aurora-B, a serine/threonine kinase, plays a crucial role in centrosome regulation, spindle formation and chromosomal separation during mitosis. It has been found that Aurora-B overexpression is related to the occurrence and development of several malignant tumors, including OS. This article summarizes the role of Aurora-B in the invasion and metastasis of OS.
Keywords: : Aurora-B, biomarker, invasion, metastasis, osteosarcoma
Plain language summary
Article highlights.
Osteosarcoma (OS) seriously affects the health of children and adolescents. It is urgent to search for novel biomarkers of OS to develop effective treatment strategies.
Aurora-B influences the progress of mitosis, and has been found to be abnormally expressed in OS.
Biological functions of Aurora-B
Aurora-B plays an important role in mitosis, including chromatin condensation, bipolar orientation and segregation and cytoplasmic division.
The expression & role of Aurora-B in OS
Aurora-B is highly expressed in OS, and associates with tumor metastasis and worse prognosis of OS patients.
Several factors affect the expression of Aurora-B.
Related mechanisms
Let-7 cluster influences the development of OS by targeting Aurora-B.
The phosphorylation of NPM1Ser125 induced by Aurora-B promotes the malignant phenotype of OS through ERK/NF-κβ signaling pathway.
PI3K/Akt/NF-κB signaling pathway plays a key role in the occurrence of OS.
Autophagy activated by AMKP/mTOR/ULK1 signaling pathway could prevent OS progression.
Aurora-B as a potential drug target
Five inhibitors, including VX-680, ZM447439, AZD1152, HOI-07 and APIO-EE-7, show good promise in the treatment of OS.
Conclusion & future perspective
Aurora-B is a promising therapeutic target for OS, but more experiments in vivo and vitro with larger sample sizes are still needed for further validation.
1. Background
Growing from osteogenic mesenchymal stem cells, osteosarcoma (OS) is one of the most serious bone tumors in children and adolescents. It typically develops in the distal femur, proximal tibia and proximal humerus, which are long bones in the extremities [1,2]. With the use of neoadjuvant chemotherapy, the five-year survival rate of patients with OS has increased from 55 to 80%. However, due to the high recurrence rate of OS and multiple drug resistance, the prognosis of patients with OS is not very optimistic [3,4]. Considering the high recurrence, multidrug-resistance and metastasis of OS, there is an urgent need to identify novel biomarkers and elucidate their specific mechanisms in the development of OS, thereby developing more effective and precise treatment strategies for OS.
Aurora kinase family includes three members in mammalian cells, namely Aurora-A, Aurora-B and Aurora-C. Aurora kinase family, which is involved in multiple processes of mitosis, plays a crucial role in maintaining gene stability and regulating cell cycle [5,6]. Aurora-B, an important member of Aurora kinase family, can catalyze substrate phosphorylation by transferring ATP phosphate groups, regulating cell signal transduction, differentiation, growth and other biological processes [7]. Recently, a growing body of studies have demonstrated the abnormal expression of Aurora-B in malignant tumors, including OS, and related specific mechanisms have been gradually clarified with the progress of research. Treatment of OS with its small molecule inhibitors has taken a new turn, particularly when used in conjunction with chemotherapy medications. In this review, we first summarized the biological functions of Aurora-B and its relationship with OS, followed by the progress of inhibitors targeting Aurora-B.
2. Biological functions of Aurora-B
Aurora-B, encoded by the gene located in the 17p13, is an important member of chromosomal passenger complex (CPC). Its correct localization depends on the function of three other CPC members, namely Borealin, Survivin and INCENP [8,9]. It has been reported that Aurora-B plays a role in regulating chromatin condensation, bipolar orientation and segregation and cytoplasmic division during mitosis [10]. Aurora-B phosphorylates variant GENP-A on Ser7 and histone H3 on Ser10 in early G2, which causes chromatin to condense [11]. Accurate separation of chromatids requires correct connection between centromeres and microtubules. Aurora-B can phosphorylate the MCAK of the centromeres to inhibit microtubule depolymerization during mitotic spindle assembly. If the microtubules and centromeres are correctly connected, Aurora-B can maintain the stable state of correct connection by phosphorylating MCAK. Conversely, this role of Aurora-B will lost if incorrect connection exists, which may lead to the microtubule-centromere depolymerization and prevent the aggregation of the mitotic process [12–16]. At the end of mitosis, Aurora-B regulates cytokinesis by coordinating its action with Rho, an important kinase regulating cytokinesis [11,17]. Inhibition of the function of Aurora-B can seriously affect the formation of the central spindle during cytokinesis, resulting in cells being unable to divide normally and become polyploid cells. Consequently, when Aurora-B expression is abnormal, it will result in the formation of abnormal two-nucleon cells at the chromosomal separation stage from the two daughter cells that initially divide normally, which may promote carcinogenesis [18–20].
3. The expression & role of Aurora-B in OS
Overexpression of Aurora-B has been shown to cause multiple abnormalities in the process of mitosis, which may lead to cancer progression. It has been noted that a large number of malignant tumors express high levels of Aurora-B, such as lung, breast and bladder cancers, indicating that there may be a direct correlation between Aurora-B and the progression of tumors [21–23]. A recent study used tissue array analysis to profile the expression of Aurora-B in different tissues, and the authors identified that Aurora-B was overexpressed in OS compared with normal bone tissues [24]. In another study, Luo et al. [25] illustrated that the positive expression rate of Aurora-B in OS was significantly higher than that in osteochondroma. And the cells in mitotic stage had darker staining of Aurora-B, which indicated that Aurora-B was more strongly expressed in cells with active proliferation.
According to recent research, upregulation of Aurora-B was linked to an increase in tumor cell metastasis whereas downregulation of Aurora-B could prevent certain tumor cells from invasion and migration [26,27]. Liu et al. [28] reported that the positive rate of Aurora-B was markedly higher in OS with distant metastasis (78.6%) than in OS without distant metastasis (45.7%, p <0.05). Nevertheless, He et al. [29] discovered that OS cell proliferation, migration and invasive capabilities were inhibited when Aurora-B was knocked down, which also caused cell death and cell-cycle arrest at the G2 phase.
In addition to promoting the malignant phenotype, Aurora-B may affect the prognosis of patients with OS. Through Kaplan-Meier analysis, Wu et al. [24] showed that in OS patients, higher Aurora-B expression were inversely linked with both worse overall survival (p <0.01) and worse metastasis-free survival (p <0.01). Attempts have been made in another study to profile this connection and has similar results [30]. In conclusion, Aurora-B expression in OS is higher compared with other normal tissues, with a functional role of affecting the occurrence and development of OS. These suggest that Aurora-B as an oncogene may be a novel target for the therapy of this tumor.
Actually, the chromosome region where Aurora-B is located is prone to translocation, deletion and amplification, meaning the natural instability of the gene [16,31]. Hence, its abnormal expression or mutation will directly affect the level of Aurora-B. According to previous published papers, Aurora-B is regulated by transcription factors at the mRNA level and the abnormal process will affect its expression [32,33]. For example, it has been shown that the transcription factor, FoxM1, can regulate the expression of Aurora-B. In the late G2 phase of mitosis, the level of Aurora-B will increase if FoxM1 binds to the promoter region of Aurora-B, which may affect the progression of mitosis [34]. In addition, the abnormal degradation of Aurora-B is also one reason for its overexpression [35]. However, further studies are still needed to gain a deeper insight into the specific mechanisms underlying the overexpression of Aurora-B, as well as its potential association with OS.
4. Related mechanisms of Aurora-B in the invasion & metastasis of OS
4.1. Correlation between Aurora-B & let-7 cluster
MicroRNAs (miRNAs) are known as a class of tiny, non-coding RNA molecules with the length of around 22 nucleotides. By attaching to the 3′-untranslated regions (3′-UTRs) of target genes, mature miRNAs play important roles in the transcriptional and post-transcriptional control of gene expression [36,37]. Growing research has shown that miRNAs are crucial in controlling the expression of oncogenes and involve in cells growth, death and metastasis [38,39]. One of the miRNAs in the Caenorhabditis elegans genome that has been studied the most is let-7 cluster, which has been found to be strongly associated with the development of cancer [40–42]. Several studies have suggested the significant role of let-7 cluster in the progression of OS by targeting Aurora-B, including let-7a/g/i.
According to recent findings, compared with normal tissues, OS tissues had considerably lower levels of let-7a/g/i. As opposed to the cells in the group under negative control, let-7a/g/i up-regulated U2-OS and HOS cells showed considerably lower expression of Aurora-B mRNA and proteins, suggesting that let-7a/g/i could downregulate Aurora-B expression in human OS cells. Bioinformatical prediction and luciferase reporter assays revealed that let-7a/g/i had predicted binding sites in the 3′-UTR of Aurora-B, which mediated the suppression of Aurora-B. Moreover, at least partially by targeting Aurora-B in vitro, let-7a/g/i could suppress the malignant phenotype of OS cells [10,43–46]. These results indicated the potential role of let-7 cluster as a new biomarker for the diagnosis and treatment of OS by targeting Aurora-B.
In addition, Yu et al. [44] provided promising evidence that let-7a acted as a tumor inhibitor to inhibit NF-κβ/MMP signaling pathway by targeting Aurora-B in OS. NF-κβ, a member of the Rel family proteins, is essential in controlling protein production that mediates cell cycle/proliferation, anti-apoptosis and cytokine secretion [47]. MMP-2 and MMP-9 are two members of the matrix MMP family, which can degrade extracellular matrix and promote the invasion and metastasis of OS cells [48]. The activation of NF-κβ promotes tumor cell progression by increasing MMP-2 and MMP-9 proteins [49]. In this study, it was found that let-7a decreased the expression of NF-κβ, MMP-2 and MMP-9 proteins, and inhibited the malignant phenotype of OS by negatively regulating Aurora-B. These results suggest that targeting let-7a and Aurora-B/NF-κβ may be a potential treatment approach for the therapy of OS. Another study also confirmed this idea [46]. Research, however, on the role of let-7 cluster in the invasion and metastasis of OS is still at the preliminary stage, and more studies are needed to clarify the specific molecular mechanisms whereby let-7 cluster is involved in the progress of OS.
4.2. Correlation between Aurora-B & NPM1
NPM1 is a widely expressed nucleocytoplasmic shuttling protein, which is involved in DNA replication, transcription, ribosome assembly and chromatin remodeling. Post-translational modifications such as phosphorylation, acetylation and ubiquitination can localize NPM1 to different subcellular regions and perform different functions [50–52]. In recent years, accumulating studies have suggested that NPM1 is closely related to tumor progression.
Using the KinasePhos database, Pi et al. [53] found that NPM1 had serine and threonine phosphorylation sites, indicating a possible phosphorylation between Aurora-B and NPM1 since Aurora-B is a serine/threonine kinase. Further experiments in vitro showed that the expression of phosphorylated NPM1Ser125 protein was decreased while the expression of non-phosphorylated NPM1 protein was unchanged after downregulating Aurora-B, suggesting that Aurora-B was able to mediate the phosphorylation of NPM1. The malignant phenotype validation in vitro revealed that silencing Aurora-B could inhibit the invasion, proliferation and migration of OS and this effect could partially alleviated by overexpression of NPM1. It indicated that Aurora-B could enhance the malignant phenotype of OS by mediating NPM1 protein phosphorylation.
The results above were also confirmed in another study [54], and found that the phosphorylation of NPM1Ser125 induced by Aurora-B could activate the ERK/NF-κβ signaling, promoting the proliferation and metastasis of OS cells. ERK/NF-κβ signaling acts as an important factor in the malignant progression of tumors [55]. ERK1/2 is a highly conserved extracellular-regulated kinase, and its phosphorylation can induce the activation of NF-κβ in tumor cells. Then, the transcription of MMP-2 and MMP-9 initiates, enhancing the invasion, proliferation and migration of tumor cells by degradation of extracellular matrix [56]. This finding suggests that targeting the Aurora-B/NPM1/ERK/NF-κβ axis may be an innovative therapeutic option for the treatment of OS. However, more experiments are still necessary to investigate specific molecular mechanisms of the malignant progression of OS mediated by NPM1.
4.3. Correlation between Aurora-B & PI3K/Akt/NF-κB signaling pathway
PI3K/Akt is essential in both cell-extracellular matrix and cell-cell adhesion. Adhesion-dependent signals will be interrupted when lacks correct adhesion, leading to adhesion-related apoptosis [57]. The PI3K/Akt signaling has been proved an important role in the proliferation, invasion and migration of various tumors by influencing its downstream effect factors [58,59]. The phosphorylation of Akt has been considered to be a crucial regulatory factor of NF-κB activation. With the progress of research, the role of PI3K/Akt/NF-κB signaling pathway has been confirmed and attracted increasing attention in the occurrence and progression of OS [60,61].
Using immunohistochemistry to detect the expression of Aurora-B and p-Akt proteins in OS tissues from 24 patients with pulmonary metastatic disease, Zhu et al. [57] found a positive correlation between Aurora-B and p-Akt proteins (R = 0.726, p = 0.02), indicating a possible relationship between Aurora-B expression and Akt phosphorylation in OS. Further research showed that the levels of PI3K, p-PI3K (Tyr199) and p-Akt (Ser473) protein were significantly lower in cells with Aurora-B inhibition compared with the negative control group. Additionally, knockdown of Aurora-B significantly reduced the Akt phosphorylation, which in turn inhibited the expression of NF-κB. It indicated that inhibition of Aurora-B could prevent OS cells invasion and migration in vitro through PI3K/Akt/NF-κB pathway.
In another study, Pi et al. [62] found by western blot analysis that overexpression of Aurora-B increased the expression of PTK2, PI3K, Akt and NF-κB, thus speculating that Aurora-B could potentially enhance the malignant phenotype of OS cells through the activation of the PTK2/PI3K/Akt/NF-κB pathway. PTK2 is a cytosolic non-receptor tyrosine kinase that is involved in the oncogenesis and development of tumor cells. It is a significant regulator of integrin-regulated signaling [63,64]. Ren et al. [65] demonstrated that the malignant phenotype of OS cells was significantly inhibited by siRNA-based knockdown of PTK2. However, further research is needed to verify whether Aurora-B promotes the malignant phenotype of OS cells through activating the PTK2/PI3K/Akt/NF-κB signaling pathway.
4.4. The role of autophagy in Aurora-B-induced OS
Autophagy, also known as type II programmed death, is an evolutionarily extremely conserved process of intracellular self-digestion [66]. Autophagy is a complex biological behavior and plays a dual role in the progress of tumors [67]. In the early stages of carcinogenesis, autophagy acts as a tumor suppressor by reducing oxidative stress, preventing chromosomal instability and causing autophagic cell death. Later on, autophagy involves as a tumor activator, maintaining the homeostasis of tumor cells through anti-anoikis and metabolism-promoting activities. This ultimately leads to the spread and growth of the tumors [67,68]. In recent years, research on the function of autophagy in the emergence and development of tumors has gained significant attraction.
A study by Wu et al. [24] found that Aurora-B expression was negatively correlated with the level of autophagy-related protein LC3 in OS tissues. Additionally, the RFP-GFP-LC3 fusion assay demonstrated that autophagosomes and autolysosomes were higher in Aurora-B silenced cells than those in the negative control group. These findings showed that inhibiting Aurora-B could enhance OS cell autophagy. Another study also confirmed this connection and found that autophagy induced by Aurora-B knockdown could promote apoptosis of cells in OS [7]. Chloroquine, an autophagy inhibitor, could reverse this effect by inhibiting Aurora-B knockdown-induced autophagy.
Numerous signaling pathways, including mTOR and AMPK, play improtant roles in regulating autophgy. Recent studies revealed that the activation of autophagy depended on the alteration of AMKP/mTOR/ULK1 signaling pathway. AMPK is an essential stress and energy metabolism sensor that adversely controls mTOR, activating autophagy initiation factor ULK1 and enhancing autophagic flux [69–71]. Wu et al. [7] found that through activating ULK1Ser555 phosphorylation, silencing Aurora-B could cause autophagy, promoting apoptosis in OS 143B cells. But bioinformatics analysis proved that there was no direct docking molecular structures between Aurora-B and ULK1, indicating that unknown molecules may exist and involve in intermediate regulatory links. Further study revealed that in Aurora-B-knockdown cell lines 143B and HOS, there was an upregulation of AMPK phosphorylation and higher expression of ULK1, whereas the expression of mTOR downregulated [24]. These results indicated that, by inhibiting the mTOR/ULK1 signaling pathway, silencing Aurora-B could promote autophagy. In addition, 143B and HOS cell migration and invasion could be greatly inhibited by silencing Aurora-B, but this effect could be reversed by MHY1485 (a mTOR activator). This suggested that activating mTOR/ULK1 pathway could revert the impact of Aurora-B inhibition on OS cell invasion and migration. These results are similar to another study [30]. In conclusion, silencing of Aurora-B can induce autophagy of OS cells partly through Aurora-B/mTOR/ULK1 axis, and this signaling pathway may be an effective treatment target and an indicator of prognosis for OS (Figure 1).
Figure 1.
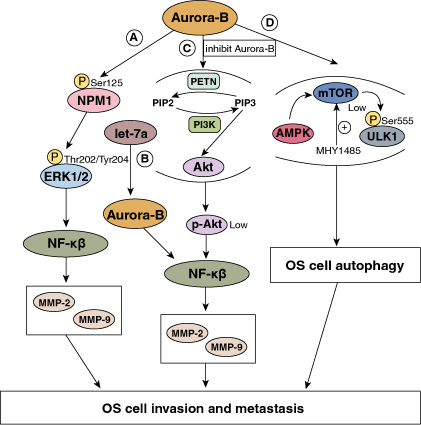
The role of Aurora-B in the invasion and metastasis of OS (By Figdraw). (A) Aurora-B mediates NPM1 phosphorylation, activating the ERK/NF-κβ signaling pathway. The activation of NF-κβ initiates MMP-2 and MMP-9 transcription, promoting the invasion and metastasis of OS. (B) Let-7a downregulates Aurora-B expression, inhibiting NF-κβ/MMP signaling pathway. The decrease of MMP-2 and MMP-9 proteins prevents the malignant progression of OS. (C) Inhibiting Aurora-B influences the PI3K/Akt signaling pathway, reducing the Akt phosphorylation. Low p-Akt inhibites the expression of NF-κB, which in turn prevents the progression of OS. PETN: pentaerythrityl tetranitrate (a compound dephosphorylating PIP3); PI3K: a kinase phosphorylating PIP2. (D) Silence of Aurora-B promotes AMPK phosphorylation and ULK1 expression, while inhibits mTOR expression. The alteration of AMKP/mTOR/ULK1 signaling pathway activates autophagy, preventing the development of OS. MHY1485: a mTOR activator.
5. Aurora-B as a potential drug target in OS
At present, the widely accepted chemotherapy drugs for OS include methotrexate, cisplatin, doxorubicin and ifosfamide, but they have large toxic effects on the hematopoietic system, digestive system, etc. In this regard, there is an urgent need to develop more effective drugs with less side effects for the treatment of OS [72]. Due to the role of Aurora-B in tumor cell mitosis, it is a promising target for tumor diagnosis and treatment. To date, a growing number of inhibitors targeting Aurora-B have been developed as potential therapeutic agents. Several of them have entered clinical trials, while many are in preclinical research or development stages.
VX-680 and ZM447439, the first generation inhibitors of Aurora kinases, can inhibit their activity by blocking the ATP-binding sites. Both of them have achieved clinical validation in preclinical and I/II phases, and presented efficacy in several tumors with limited collateral toxicity to normal human cells [72–76]. In the preclinical validation, Tavanti et al. [77] revealed that human OS cell lines were highly sensitive to VX-680 and ZM447439, both of which variably caused apoptosis and hyperploidy in most cell lines. And VX-680 in combination with conventional chemotherapeutic agents could improve the clinical efficacy. These suggested their worth for further preclinical and clinical validation. AZD1152, the second generation inhibitor of Aurora kinases, is converted to AZD1152-HQPA rapidly in plasma, inhibiting the activity of Aurora-B selectively [78,79]. Many studies have shown that it is effective against several human tumors, including hepatocellular carcinoma and breast cancer [80,81]. It has also been confirmed to effectively inhibit the proliferation of human U2-OS cells by inhibiting the expression of Aurora-B in vitro [72,73]. However, in phase I studies, AZD1152 showed dose-limiting toxicity for individuals with neutropenia. Furthermore, in clinical trials, limited efficacy of AZD1152 for tumors was discovered due to the use of different Aurora kinase inhibitors [82,83]. These may reduce the value of its clinical application. Nevertheless, further clinical evaluation is still needed to determine its clinical efficacy.
In recent years, more and more inhibitors have been developed to inhibit the malignant phenotype of OS by targeting Aurora-B. Zhao et al. [82] identified a small molecule inhibitor called HOI-07. This compound could inhibit Aurora-B activity in OS, reduce the growth of OS anchorage-independent cells and cause G2-M phase arrest, inducing apoptosis as a result. Importantly, it was observed in the patient-derived xenograft (PDX) mouse model that HOI-07 treatment significantly inhibited the growth of OS without any significant toxicity. Jin et al. [84] identified a novel compound called APIO-EE-7, which can interact with Aurora-B at the ATP-binding pocket, inducing OS cell apoptosis and limiting their proliferation and colony formation. Moreover, in OS metastasis mouse model, it was found that APIO-EE-7 could significantly reduce tumor incidence and volume. At present, these two inhibitors are still in the laboratory development stage. However, before entering clinical trials, comprehensive evaluation and preclinical validation are needed to analyze their toxic reactions and drug resistance.
In general, the evidence indicates that Aurora-B represents a new candidate therapeutic target for OS. In the future, more drugs targeting Aurora-B to treat OS need to be developed and demonstrated through further clinical trials.
6. Conclusion
Aurora-B, a key kinase in the process of mitosis, is highly expressed in OS compared with normal bone tissues and its abnormal expression is closely related to the malignant phenotype of OS, which influences the prognosis of patients with OS as a result. Due to the high malignancy, hidden onset and rapid progression of OS, it is difficult to diagnose and treat this tumor in the early stage. As a promising anti-tumor target, Aurora-B provides new ideas for the treatment of OS. At present, there are many studies to explore the specific molecular mechanisms of the malignant phenotype of OS mediated by Aurora-B, and inhibitors that prevent the progression of OS by targeting Aurora-B have also been discovered. However, several limitations are still exist. Most studies have only clarified the effect of Aurora-B on OS through experiments in vitro. However, plenty of studies indicate that the tumor microenvironment plays an essential role in the malignant progression of tumors [85,86]. But effect of this factor is unable to be taken into consideration in vitro experiments, which may bias the results. Although some scholars have researched the effect of silencing Aurora-B on the change of OS tumor body in nude mice, studies of the effect on the metastasis of tumor cells in vivo remain vacant [87]. Therefore, more experiments in vivo are needed to investigate the effect of Aurora-B on the development, invasion and metastasis of OS. In addition, the sample sizes of OS tissues were small in most studies, which may affect the accuracy of the results. Therefore, larger sample sizes are needed for further investigation to explore whether Aurora-B can be used as a new molecular target for the treatment of OS.
7. Future perspective
At present, the treatment of OS mainly focuses on surgery and chemotherapy. Exploration of selective targeted anti-tumor drugs has always been the direction and goal of efforts. As a novel biomarker, Aurora-B has attracted extensive attention. In the future, more experiments in vivo and in vitro are needed to further prove the role of Aurora-B in the invasion and metastasis of OS, while expanding the sample sizes and optimizing the experimental design schemes. Although nude mouse models can well simulate the microenvironment of OS growth and metastasis in vivo, they lack thymus and mature cellular immunity, which cannot completely simulate the real immune microenvironment in vivo. In this regard, using genetically engineered mouse models to simulate the microenvironment in vivo is feasible to better optimize the experiments. Moreover, more experiments are needed to explore the upstream regulatory mechanisms of Aurora-B, such as whether there are epigenetic regulatory mechanisms that influence the expression of Aurora-B in OS patients. In addition, developing more specific drugs targeting Aurora-B and reducing their toxic side effects is also important for achieving the purpose of precise and effective therapy for OS.
Funding Statement
This work was supported by the Natural Science Foundation of Jiangxi Province, P.R. China (No. 20232ACB206043); Science and Technology Project of Jiangxi Provincial Health Commission, P.R. China (No. 20201017); and “Double Thousand Plan” Talent Project of Jiangxi Province, P.R. China.
Author contributions
All authors contributed to the study conception and design. JL suggested the topic of the manuscript. SL conceived the outline of the manuscript by searching the literature, wrote the major parts of the manuscript and created the figures. YZ and JL reviewed and edited the manuscript. Data authentication is not applicable. All authors have read and approved the final manuscript.
Financial disclosure
This work was supported by the Natural Science Foundation of Jiangxi Province, P.R. China (No. 20232ACB206043); Science and Technology Project of Jiangxi Provincial Health Commission, P.R. China (No. 20201017); and “Double Thousand Plan” Talent Project of Jiangxi Province, P.R. China. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.de Azevedo JWV, de Medeiros Fernandes TAA, Fernandes JV Jr, et al. Biology and pathogenesis of human osteosarcoma. Oncol Lett. 2020;19(2):1099–1116. doi: 10.3892/ol.2019.11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camuzard O, Santucci-Darmanin S, Carle GF, et al. Role of autophagy in osteosarcoma. J Bone Oncol. 2019;16:100235. doi: 10.1016/j.jbo.2019.100235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Liu R, Wang W, et al. Advances in targeted therapy for osteosarcoma based on molecular classification. Pharmacol Res. 2021;169:105684. doi: 10.1016/j.phrs.2021.105684 [DOI] [PubMed] [Google Scholar]
- 4.Cersosimo F, Lonardi S, Bernardini G, et al. Tumor-associated macrophages in osteosarcoma: from mechanisms to therapy. Int J Mol Sci. 2020;21(15):5207. doi: 10.3390/ijms21155207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan M, Wang C, He B, et al. Aurora-A kinase: a potent oncogene and target for cancer therapy. Med Res Rev. 2016;36(6):1036–1079. doi: 10.1002/med.21399 [DOI] [PubMed] [Google Scholar]
- 6.Du R, Huang C, Liu K, et al. Targeting AURKA in cancer: molecular mechanisms and opportunities for cancer therapy. Mol Cancer. 2021;20(1):15. doi: 10.1186/s12943-020-01305-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Liu JM, Song HH, et al. [Aurora kinase-B silencing promotes apoptosis of osteosarcoma 143B cells by ULK1 phosphorylation-induced autophagy]. Journal of Southern Medical University. 2020;40(9):1273–1279. Chinese. doi: 10.12122/j.issn.1673-4254.2020.09.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gassmann R, Carvalho A, Henzing AJ, et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166(2):179–191. doi: 10.1083/jcb.200404001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy B, Han SJY, Fontan AN, et al. Aurora B phosphorylates Bub1 to promote spindle assembly checkpoint signaling. Curr Biol. 2022;32(1):237–247; e6. doi: 10.1016/j.cub.2021.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou YF, Liu JM, Chen XY, et al. [Let-7a/g/i targeted to Aurora-B in human osteosarcoma cells]. China Oncol. 2015;25(12):966–971. Chinese. doi: 10.3969/j.issn.1007-3969.2015.12.008 [DOI] [Google Scholar]
- 11.Kunitoku N, Sasayama T, Marumoto T, et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell. 2003;5(6):853–864. doi: 10.1016/s1534-5807(03)00364-2 [DOI] [PubMed] [Google Scholar]
- 12.Chen A, Wen S, Liu F, et al. CRISPR/Cas9 screening identifies a kinetochore-microtubule dependent mechanism for Aurora-A inhibitor resistance in breast cancer. Cancer Commun (Lond). 2021;41(2):121–139. doi: 10.1002/cac2.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosio C, Fimia GM, Loury R, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22(3):874–885. doi: 10.1128/mcb.22.3.874-885.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ems-McClung SC, Hainline SG, Devare J, et al. Aurora B inhibits MCAK activity through a phosphoconformational switch that reduces microtubule association. Curr Biol. 2013;23(24):2491–2499. doi: 10.1016/j.cub.2013.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lan W, Zhang X, Kline-Smith SL, et al. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14(4):273–286. doi: 10.1016/j.cub.2004.01.055 [DOI] [PubMed] [Google Scholar]
- 16.Luo Y. [The expression of aurora-B kinase in osteosarcoma] [master's thesis]. Nanchang (JX): Nanchang University; 2008. Chinese. [Google Scholar]
- 17.Inaba H, Yamakawa D, Tomono Y, et al. Regulation of keratin 5/14 intermediate filaments by CDK1, Aurora-B, and Rho-kinase. Biochem Biophys Res Commun. 2018;498(3):544–550. doi: 10.1016/j.bbrc.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 18.Goto H, Yasui Y, Kawajiri A, et al. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J Biol Chem. 2003;278(10):8526–8530. doi: 10.1074/jbc.M210892200 [DOI] [PubMed] [Google Scholar]
- 19.Hadders MA, Hindriksen S, Truong MA, et al. Untangling the contribution of Haspin and Bub1 to Aurora B function during mitosis. J Cell Biol. 2020;219(3):e201907087. doi: 10.1083/jcb.201907087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britigan EMC, Wan J, Sam DK, et al. Increased Aurora B expression reduces substrate phosphorylation and induces chromosomal instability. Front Cell Dev Biol. 2022;10:1018161. doi: 10.3389/fcell.2022.1018161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K, Yu HA, Yang S, et al. Targeting aurora B kinase prevents and overcomes resistance to EGFR inhibitors in lung cancer by enhancing BIM- and PUMA-mediated apoptosis. Cancer Cell. 2021;39(9):1245–61.e6. doi: 10.1016/j.ccell.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Jiang C, Li H, et al. Elevated Aurora B expression contributes to chemoresistance and poor prognosis in breast cancer. Int J Clin Exp Pathol. 2015;8(1):751–757. [PMC free article] [PubMed] [Google Scholar]
- 23.Su CC, Chen NC, Chyau CC, et al. Induction of mitotic catastrophe via inhibition of aurora B by ionizing radiation with additive of mulberry water extract in human bladder cancer cells. Integr Cancer Ther. 2018;18:1534735418808586. doi: 10.1177/1534735418808586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Liu JM, Song HH, et al. Aurora-B knockdown inhibits osteosarcoma metastasis by inducing autophagy via the mTOR/ULK1 pathway. Cancer Cell Int. 2020;20(1):575. doi: 10.1186/s12935-020-01674-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes that Aurora-B was overexpressed in OS compared with normal bone tissues and patients with high Aurora-B expression had poorer clinical outcomes.
- 25.Luo Y, Liu ZL, Shu Y, et al. [Expression of Aurora-B in osteosarcoma and its relationship with metastasis]. China J Modern Med. 2011;21(14):1611–1614; 9. Chinese. [Google Scholar]
- 26.Jiang J, Wang J, Yue M, et al. Direct phosphorylation and stabilization of MYC by aurora B kinase promote T-cell leukemogenesis. Cancer Cell. 2020;37(2):200–15.e5. doi: 10.1016/j.ccell.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Lin X, Wu L, et al. Aurora B induces epithelial-mesenchymal transition by stabilizing Snail1 to promote basal-like breast cancer metastasis. Oncogene. 2020;39(12):2550–2567. doi: 10.1038/s41388-020-1165-z [DOI] [PubMed] [Google Scholar]
- 28.Liu ZL, Yin QS, Shu Y, et al. [Expression of Aurora-B and Ki-67 in osteosarcoma and its correlation with distant metastasis]. Medical J Chinese People's Liberation Army. 2011;36(11):1197–1199. Chinese. [Google Scholar]
- 29.He JY, Xi WH, Zhu LB, et al. Knockdown of Aurora-B alters osteosarcoma cell malignant phenotype via decreasing phosphorylation of VCP and NF-κB signaling. Tumour Biol. 2015;36(5):3895–3902. doi: 10.1007/s13277-014-3032-4 [DOI] [PubMed] [Google Scholar]
- 30.Wu X. [The role and mechanism of Aurora-B knockdown induced autophagy on metastasis in osteosarcoma] [master's thesis]. Nanchang (JX): Nanchang University; 2021. Chinese. [Google Scholar]
- 31.Titova E, Shagieva G, Dugina V, et al. The role of aurora B kinase in normal and cancer cells. Biochemistry (Mosc). 2023;88(12):2054–2062. doi: 10.1134/s0006297923120088 [DOI] [PubMed] [Google Scholar]
- 32.Park J, Kwon MS, Kim EE, et al. USP35 regulates mitotic progression by modulating the stability of Aurora B. Nat Commun. 2018;9(1):688. doi: 10.1038/s41467-018-03107-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassardjian A, Rizkallah R, Riman S, et al. The transcription factor YY1 is a novel substrate for Aurora B kinase at G2/M transition of the cell cycle. PLOS ONE. 2012;7(11):e50645. doi: 10.1371/journal.pone.0050645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang IC, Chen YJ, Hughes D, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell. Biol. 2005;25(24):10875–10894. doi: 10.1128/mcb.25.24.10875-10894.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Listovsky T, Sale JE. Sequestration of CDH1 by MAD2L2 prevents premature APC/C activation prior to anaphase onset. J Cell Biol. 2013;203(1):87–100. doi: 10.1083/jcb.201302060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, et al. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi: 10.1002/jcp.27486 [DOI] [PubMed] [Google Scholar]
- 37.Moghbeli M. MicroRNAs as the pivotal regulators of cisplatin resistance in osteosarcoma. Pathol Res Pract. 2023;249:154743. doi: 10.1016/j.prp.2023.154743 [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Wu KJ, Jia QJ, et al. Roles of miRNA and lncRNA in triple-negative breast cancer. J Zhejiang Univ Sci B. 2020;21(9):673–689. doi: 10.1631/jzus.B1900709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du X, Zhang J, Wang J, et al. Role of miRNA in lung cancer-potential biomarkers and therapies. Curr Pharm Des. 2018;23(39):5997–6010. doi: 10.2174/1381612823666170714150118 [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Shen N, Wicha MS, et al. The roles of the Let-7 Family of MicroRNAs in the regulation of cancer stemness. Cells. 2021;10(9):2415. doi: 10.3390/cells10092415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizuno R, Kawada K, Sakai Y. The molecular basis and therapeutic potential of Let-7 microRNAs against colorectal cancer. Can J Gastroenterol Hepatol. 2018;2018:5769591. doi: 10.1155/2018/5769591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–906. doi: 10.1038/35002607 [DOI] [PubMed] [Google Scholar]
- 43.Zhang GM, Long XH, Liu JM, et al. Let-7i inhibits the malignant phenotype of osteosarcoma cells by targeting Aurora-B. Mol Med Rep. 2015;12(3):3543–3548. doi: 10.3892/mmr.2015.3798 [DOI] [PubMed] [Google Scholar]
- 44.Yu JJ, Pi WS, Cao Y, et al. Let-7a inhibits osteosarcoma cell growth and lung metastasis by targeting Aurora-B. Cancer Manag Res. 2018;10:6305–6315. doi: 10.2147/cmar.S185090 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes that let-7a was underexpressed in OS cell lines and overexpression of let-7a could prevent the growth and metastasis of OS.
- 45.Liu JM, Long XH, Zhang GM, et al. Let-7g reverses malignant phenotype of osteosarcoma cells by targeting Aurora-B. Int J Clin Exp Pathol. 2014;7(8):4596–4606. [PMC free article] [PubMed] [Google Scholar]
- 46.Pi WS. [Let-7a inhibits proliferation, migration and invasion of human osteosarcoma cells by targeting Aurora-B in vitro] [master's thesis]. Nanchang (JX): Nanchang University; 2019. Chinese. [Google Scholar]
- 47.Zhang XX, Fu Z, Zhang Z, et al. Microcystin-LR promotes melanoma cell invasion and enhances matrix metalloproteinase-2/-9 expression mediated by NF-κB activation. Environ Sci Technol. 2012;46(20):11319–11326. doi: 10.1021/es3024989 [DOI] [PubMed] [Google Scholar]
- 48.Song Z, Wang J, Su Q, et al. The role of MMP-2 and MMP-9 in the metastasis and development of hypopharyngeal carcinoma. Braz J Otorhinolaryngol. 2021;87(5):521–528. doi: 10.1016/j.bjorl.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang JF, Wang P, Yan YJ, et al. IL-33 enhances glioma cell migration and invasion by upregulation of MMP2 and MMP9 via the ST2-NF-κB pathway. Oncol Rep. 2017;38(4):2033–2042. doi: 10.3892/or.2017.5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borer RA, Lehner CF, Eppenberger HM, et al. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9 [DOI] [PubMed] [Google Scholar]
- 51.Colombo E, Marine JC, Danovi D, et al. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat Cell Biol. 2002;4(7):529–533. doi: 10.1038/ncb814 [DOI] [PubMed] [Google Scholar]
- 52.Lindström MS. NPM1/B23: a multifunctional chaperone in ribosome biogenesis and chromatin remodeling. Biochem Res Int. 2011;2011:195209. doi: 10.1155/2011/195209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pi WS, Liu JM, Huang SH, et al. Aurora-B-mediated phosphorylation of NPM1 promotes malignant phenotype of OS cells in vitro. Tianjin Med J. 2019;47(1):5–9 + 113. Chinese. doi: 10.11958/20181312 [DOI] [Google Scholar]
- 54.Song H, Zhou Y, Peng A, et al. Aurora-B promotes osteosarcoma cell growth and metastasis through activation of the NPM1/ERK/NF-κβ/MMPs axis. Cancer Manag Res. 2020;12:4817–4827. doi: 10.2147/cmar.S252847 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes that Aurora-B phosphorylats NPM1Ser125 and activates ERK/NF-κβ signaling to promote the malignant phenotype of OS.
- 55.Weng MC, Wang MH, Tsai JJ, et al. Regorafenib inhibits tumor progression through suppression of ERK/NF-κB activation in hepatocellular carcinoma bearing mice. Biosci Rep. 2018;38(3):BSR20171264. doi: 10.1042/bsr20171264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li CH, Ku MC, Lee KC, et al. Magnolol suppresses ERK/NF-κB signaling and triggers apoptosis through extrinsic/intrinsic pathways in osteosarcoma. Anticancer Res. 2022;42(9):4403–4410. doi: 10.21873/anticanres.15940 [DOI] [PubMed] [Google Scholar]
- 57.Zhu LB, Jiang J, Zhu XP, et al. Knockdown of Aurora-B inhibits osteosarcoma cell invasion and migration via modulating PI3K/Akt/NF-κB signaling pathway. Int J Clin Exp Pathol. 2014;7(7):3984–3991. [PMC free article] [PubMed] [Google Scholar]
- 58.Noorolyai S, Shajari N, Baghbani E, et al. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076 [DOI] [PubMed] [Google Scholar]
- 59.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 60.Qu J, Li J, Zhang Y, et al. AKR1B10 promotes breast cancer cell proliferation and migration via the PI3K/AKT/NF-κB signaling pathway. Cell Biosci. 2021;11(1):163. doi: 10.1186/s13578-021-00677-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leng J, Li H, Niu Y, et al. Low-dose mono(2-ethylhexyl) phthalate promotes ovarian cancer development through PPARα-dependent PI3K/Akt/NF-κB pathway. Sci Total Environ. 2021;790:147990. doi: 10.1016/j.scitotenv.2021.147990 [DOI] [PubMed] [Google Scholar]
- 62.Pi WS, Cao ZY, Liu JM, et al. Potential molecular mechanisms of AURKB in the oncogenesis and progression of osteosarcoma cells: a label-free quantitative proteomics analysis. Technol Cancer Res Treat. 2018;18:1533033819853262. doi: 10.1177/1533033819853262 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; •• Speculates that Aurora-B may promote the progression of OS by regulating PTK2/ PI3K/Akt/NF-κβ pathway.
- 63.Xie M, Sun M, Ji X, et al. Overexpression of BACH1 mediated by IGF2 facilitates hepatocellular carcinoma growth and metastasis via IGF1R and PTK2. Theranostics. 2022;12(3):1097–1116. doi: 10.7150/thno.65775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmadi Hedayati M, Khani D, Sheikhesmaeili F, et al. ptk2 and mt2a genes expression in gastritis and gastric cancer patients with Helicobacter pylori Infection. Can J Gastroenterol Hepatol. 2022;2022:8699408. doi: 10.1155/2022/8699408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren K, Lu X, Yao N, et al. Focal adhesion kinase overexpression and its impact on human osteosarcoma. Oncotarget. 2015;6(31):31085–31103. doi: 10.18632/oncotarget.5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mulcahy Levy JM, Thorburn A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27(3):843–857. doi: 10.1038/s41418-019-0474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niu J, Yan T, Guo W, et al. Insight into the role of autophagy in osteosarcoma and its therapeutic implication. Front Oncol. 2019;9:1232. doi: 10.3389/fonc.2019.01232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi KS. Autophagy and cancer. Experimen Mol Med. 2012;44(2):109–120. doi: 10.3858/emm.2012.44.2.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu L, Wang J, Wu Z, et al. AFF4 regulates osteogenic potential of human periodontal ligament stem cells via mTOR-ULK1-autophagy axis. Cell Prolif. 2023;57(2):e13546. doi: 10.1111/cpr.13546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamargo-Gómez I, Mariño G. AMPK: regulation of metabolic dynamics in the context of autophagy. Int J Mol Sci. 2018;19(12):3812. doi: 10.3390/ijms19123812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao C, Huang W, Zhang N, et al. Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis. Cell Prolif. 2018;51(6):e12518. doi: 10.1111/cpr.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu ZL, Wang G, Shu Y, et al. Inhibition effect of AZD1152-HQPA, specific inhibitor of Aurora-B kinase, on human osteosarcoma cell line U2-OS cells. J Nanchang Univ. 2010;50(10):30–32; 6. Chinese. doi: 10.3969/j.issn.1000-2294.2010.10.008 [DOI] [Google Scholar]
- 73.Zhu XP, Liu ZL, Peng AF, et al. Inhibition of Aurora-B suppresses osteosarcoma cell migration and invasion. Exp Ther Med. 2014;7(3):560–564. doi: 10.3892/etm.2014.1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Zhang ZF, Chen J, et al. VX680/MK-0457, a potent and selective Aurora kinase inhibitor, targets both tumor and endothelial cells in clear cell renal cell carcinoma. Am J Transl Res. 2010;2(3):296–308. [PMC free article] [PubMed] [Google Scholar]
- 75.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10(12):825–841. doi: 10.1038/nrc2964 [DOI] [PubMed] [Google Scholar]
- 76.Katayama H, Sen S. Aurora kinase inhibitors as anticancer molecules. Biochim Biophys Acta. 2010;1799(10–12):829–839. doi: 10.1016/j.bbagrm.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tavanti E, Sero V, Vella S, et al. Preclinical validation of Aurora kinases-targeting drugs in osteosarcoma. Br J Cancer. 2013;109(10):2607–2618. doi: 10.1038/bjc.2013.643 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the preclinical validation of drugs targeting Aurora kinases in OS and suggests Aurora-B as a novel therapeutic target for this tumor.
- 78.Tao Y, Leteur C, Calderaro J, et al. The aurora B kinase inhibitor AZD1152 sensitizes cancer cells to fractionated irradiation and induces mitotic catastrophe. Cell Cycle. 2009;8(19):3172–3181. doi: 10.4161/cc.8.19.9729 [DOI] [PubMed] [Google Scholar]
- 79.Krex D, Bartmann P, Lachmann D, et al. Aurora B kinase inhibition by AZD1152 concomitant with tumor treating fields is effective in the treatment of cultures from primary and recurrent glioblastomas. Int J Mol Sci. 2023;24(5):5016. doi: 10.3390/ijms24055016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gully CP, Zhang F, Chen J, et al. Antineoplastic effects of an Aurora B kinase inhibitor in breast cancer. Mol Cancer. 2010;9:42. doi: 10.1186/1476-4598-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aihara A, Tanaka S, Yasen M, et al. The selective Aurora B kinase inhibitor AZD1152 as a novel treatment for hepatocellular carcinoma. J Hepatol. 2010;52(1):63–71. doi: 10.1016/j.jhep.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 82.Zhao Z, Jin G, Yao K, et al. Aurora B kinase as a novel molecular target for inhibition the growth of osteosarcoma. Mol Carcinog. 2019;58(6):1056–1067. doi: 10.1002/mc.22993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bavetsias V, Linardopoulos S. Aurora kinase inhibitors: current status and outlook. Front Oncol. 2015;5:278. doi: 10.3389/fonc.2015.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin G, Li J, Guo Z, et al. Molecular mechanism of APIO-EE-7 suppresses cell metastasis by targeting Aurora B in osteosarcoma. Cancer Res. 2019;79(13). doi: 10.1158/1538-7445.Am2019-4292 [DOI] [Google Scholar]
- 85.Qin Y, Liu Y, Xiang X, et al. Cuproptosis correlates with immunosuppressive tumor microenvironment based on pan-cancer multiomics and single-cell sequencing analysis. Mol Cancer. 2023;22(1):59. doi: 10.1186/s12943-023-01752-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403. doi: 10.1016/j.ccell.2023.02.016 [DOI] [PubMed] [Google Scholar]
- 87.Zhou LD. [Silence Aurora-B inhibit osteosarcoma cell line 143B grown in nude mice] [master's thesis]. Nanchang (JX): Nanchang University; 2015. Chinese. [Google Scholar]


