Abstract
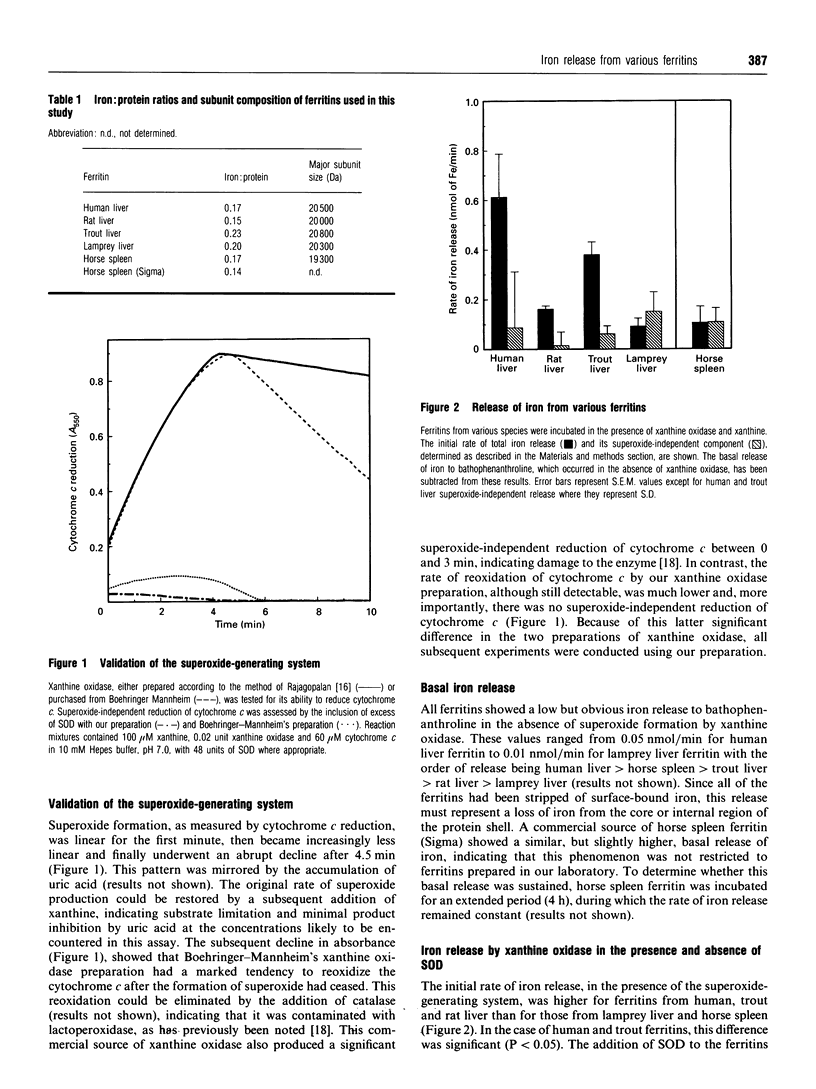
The influence of the superoxide-generating system, xanthine oxidase, on the release of iron from various vertebrate ferritins was determined both in the presence and absence of superoxide dismutase. The initial rate of iron release in the presence of this system was higher for ferritins from human, trout and rat liver than for those from lamprey liver and horse spleen. The proportion of this iron release that was superoxide-dependent in the case of rat, human and trout ferritins was 92, 86 and 84% respectively, whereas no such superoxide-dependent iron release occurred from the ferritins of lamprey liver and horse spleen. On the other hand, the rate of superoxide-independent iron release was of comparable magnitude for all of the species examined. The rate of superoxide-dependent iron release was related neither to the iron: protein ratios nor to the subunit size of the ferritins. However, it is significant that the ferritins with a high rate of superoxide-dependent iron release came from tissues known to be susceptible to iron damage. It is thus proposed that the resistance of lamprey liver ferritin to the mobilization of iron by superoxide ions accounts in part for the tolerance of the lamprey liver to high iron loads.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., Beindorff C. M., Koster J. F. Superoxide-dependent and -independent mechanisms of iron mobilization from ferritin by xanthine oxidase. Implications for oxygen-free-radical-induced tissue destruction during ischaemia and inflammation. Biochem J. 1986 Oct 1;239(1):169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. On the limited ability of superoxide to release iron from ferritin. Eur J Biochem. 1990 Nov 13;193(3):899–904. doi: 10.1111/j.1432-1033.1990.tb19415.x. [DOI] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochem J. 1987 Apr 1;243(1):55–59. doi: 10.1042/bj2430055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell T. H., Charlton R. W. A general approach to the problems of iron deficiency and iron overload in the population at large. Semin Hematol. 1982 Jan;19(1):54–67. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheeseman K. H. Tissue injury by free radicals. Toxicol Ind Health. 1993 Jan-Apr;9(1-2):39–51. doi: 10.1177/0748233793009001-205. [DOI] [PubMed] [Google Scholar]
- Domingo A. Exponential gradient maker using a disposable syringe. Anal Biochem. 1990 Aug 15;189(1):88–90. doi: 10.1016/0003-2697(90)90049-f. [DOI] [PubMed] [Google Scholar]
- Florence A., Ward R. J., Peters T. J., Crichton R. R. Studies of in vivo iron mobilization by chelators in the ferrocene-loaded rat. Biochem Pharmacol. 1992 Sep 25;44(6):1023–1027. doi: 10.1016/0006-2952(92)90363-n. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Funk F., Lenders J. P., Crichton R. R., Schneider W. Reductive mobilisation of ferritin iron. Eur J Biochem. 1985 Oct 1;152(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09177.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992 Jul 27;307(1):108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J., Chambers S., Hoffbrand A. V. Comparative study of iron mobilization from haemosiderin, ferritin and iron(III) precipitates by chelators. Biochem J. 1987 Jan 1;241(1):87–92. doi: 10.1042/bj2410087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey D. J., Cake M. H., Potter I. C. Exceptional iron concentrations in larval lampreys (Geotria australis) and the activities of superoxide radical detoxifying enzymes. Biochem J. 1988 May 15;252(1):167–172. doi: 10.1042/bj2520167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S., Bannister J. V., Williams R. J. Structure and composition of ferritin cores isolated from human spleen, limpet (Patella vulgata) hemolymph and bacterial (Pseudomonas aeruginosa) cells. J Mol Biol. 1986 Mar 20;188(2):225–232. doi: 10.1016/0022-2836(86)90307-4. [DOI] [PubMed] [Google Scholar]
- Miguel J. L., Pablos M. I., Agapito M. T., Recio J. M. Isolation and characterization of ferritin from the liver of the rainbow trout (Salmo gairdneri R.). Biochem Cell Biol. 1991 Oct-Nov;69(10-11):735–741. doi: 10.1139/o91-111. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Ward R. J., Baum H., Peters T. J. Iron release from haemosiderin and ferritin by therapeutic and physiological chelators. Biochem J. 1989 Jun 15;260(3):903–907. doi: 10.1042/bj2600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M., Halliwell B., Moorhouse C. P., Aruoma O. I., Baum H., Peters T. J. Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Is haemosiderin formation a biological protective mechanism? Biochem J. 1986 Mar 15;234(3):727–731. doi: 10.1042/bj2340727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olynyk J., Hall P., Sallie R., Reed W., Shilkin K., Mackinnon M. Computerized measurement of iron in liver biopsies: a comparison with biochemical iron measurement. Hepatology. 1990 Jul;12(1):26–30. doi: 10.1002/hep.1840120106. [DOI] [PubMed] [Google Scholar]
- Smalley S. R., Macey D. J., Potter I. C. Changes in the amount of nonhaem iron in the plasma, whole body, and selected organs during the postlarval life of the lamprey Geotria australis. J Exp Zool. 1986 Feb;237(2):149–157. doi: 10.1002/jez.1402370202. [DOI] [PubMed] [Google Scholar]
- St Pierre T. G., Tran K. C., Webb J., Macey D. J., Heywood B. R., Sparks N. H., Wade V. J., Mann S., Pootrakul P. Organ-specific crystalline structures of ferritin cores in beta-thalassemia/hemoglobin E. Biol Met. 1991;4(3):162–165. doi: 10.1007/BF01141308. [DOI] [PubMed] [Google Scholar]
- Terada L. S., Leff J. A., Repine J. E. Measurement of xanthine oxidase in biological tissues. Methods Enzymol. 1990;186:651–656. doi: 10.1016/0076-6879(90)86161-n. [DOI] [PubMed] [Google Scholar]
- Tran K. C., Webb J., Macey D. J., Pootrakul P. Beta-thalassaemia/haemoglobin E tissue ferritins. II: A comparison of heart and pancreas ferritins with those of liver and spleen. Biol Met. 1990;3(3-4):227–231. doi: 10.1007/BF01140584. [DOI] [PubMed] [Google Scholar]
- Ward R. J., Ramsey M. H., Dickson D. P., Florence A., Crichton R. R., Peters T. J., Mann S. Chemical and structural characterisation of iron cores of haemosiderins isolated from different sources. Eur J Biochem. 1992 Nov 1;209(3):847–850. doi: 10.1111/j.1432-1033.1992.tb17356.x. [DOI] [PubMed] [Google Scholar]
- Yee H. Y., Goodwin J. F. Simultaneous determination of copper and iron in a single aliquot of serum. Clin Chem. 1974 Feb;20(2):188–191. [PubMed] [Google Scholar]



