Abstract
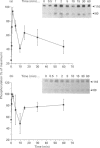
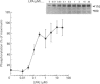
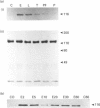
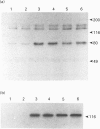
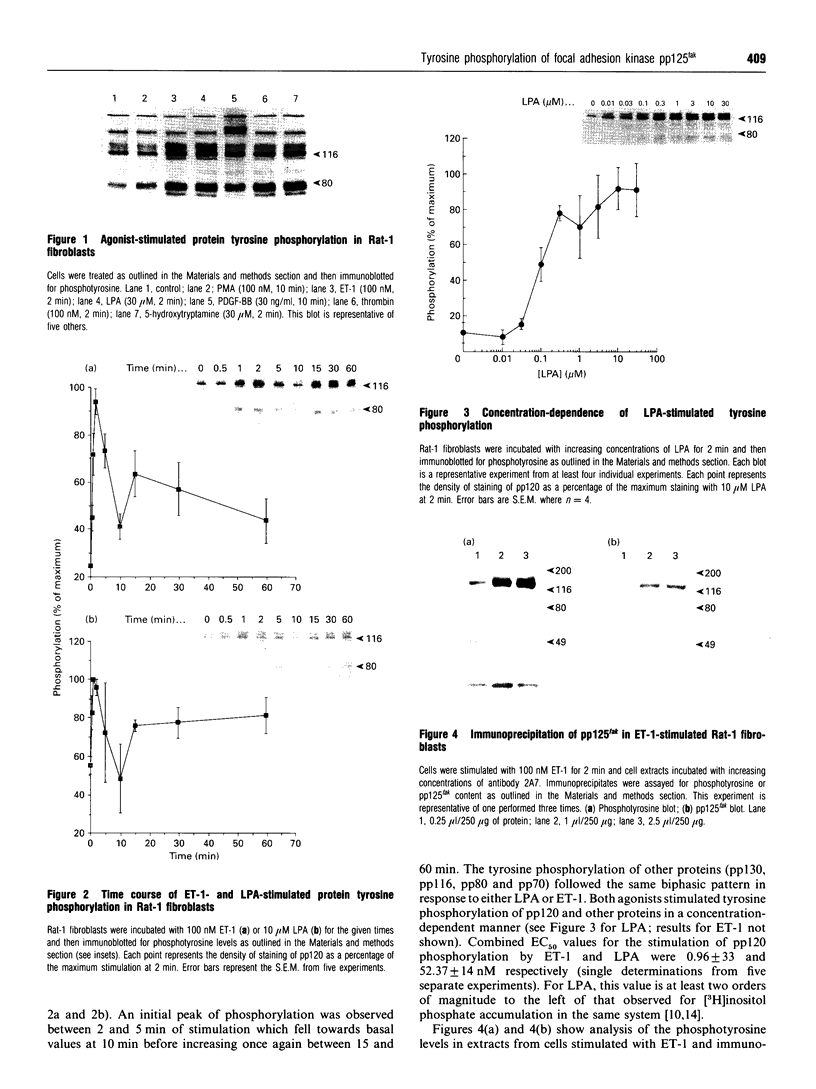
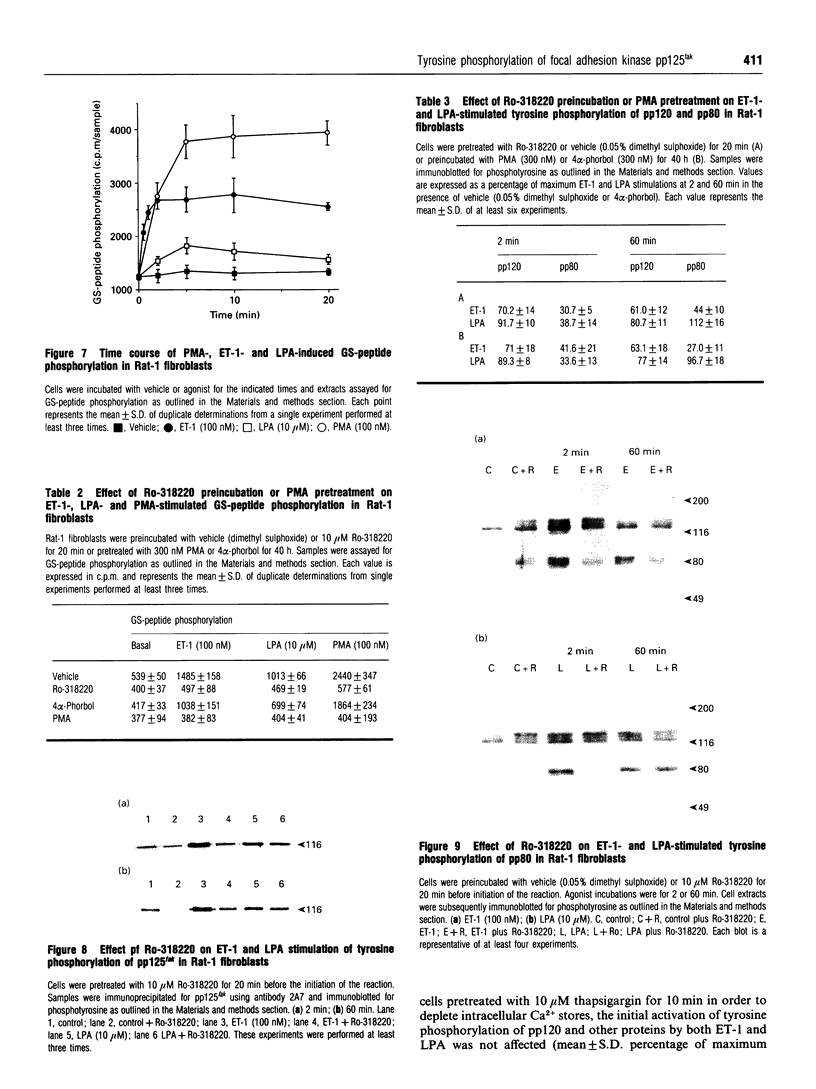
The characteristics of protein tyrosine phosphorylation were examined in Rat-1 fibroblasts in response to endothelin-1 (ET-1) and 1-oleoyl-lysophosphatidic acid (LPA). Both agonists stimulated the biphasic tyrosine phosphorylation of at least three major proteins of approx. 120 kDa (pp116, pp120 and pp130) and two of 80 kDa (pp80 and pp70). Immunoprecipitation experiments indicated that the pp120 protein corresponded to the recently described focal adhesion protein kinase pp125fak. Phorbol 12-myristate 13-acetate, alone or in combination with the calcium ionophore A23187, also stimulated the phosphorylation of pp125fak but to a smaller extent than LPA or ET-1. Removal of both extracellular and intracellular Ca2+ did not significantly reduce LPA- and ET-1-stimulated tyrosine phosphorylation of pp125fak. In cells where protein kinase C activity was down-regulated or inhibited, ET-1-stimulated tyrosine phosphorylation of pp125fak was reduced to a greater extent than phosphorylation in response to LPA. In addition, ET-1-stimulated tyrosine phosphorylation of pp80 was decreased by 50-70% in response to protein kinase C inhibition at both 2 and 60 min whereas LPA-stimulated tyrosine phosphorylation of this protein was only reduced at 2 min. Pretreatment with pertussis toxin reduced the tyrosine phosphorylation of pp42 and pp44 forms of mitogen-activated protein kinase in response to both ET-1 and LPA but reduced the tyrosine phosphorylation of pp125fak only in response to LPA. These results indicate agonist-specific differences in the regulation of pathways mediating the tyrosine phosphorylation of pp125fak and other target proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Graves J. D., Lucas S. C., Cantrell D. A., Crumpton M. J. A method for measuring protein kinase C activity in permeabilized T lymphocytes by using peptide substrates. Evidence for multiple pathways of kinase activation. Biochem J. 1990 Jun 1;268(2):303–308. doi: 10.1042/bj2680303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Force T., Kyriakis J. M., Avruch J., Bonventre J. V. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991 Apr 5;266(10):6650–6656. [PubMed] [Google Scholar]
- Glaser K. B., Asmis R., Dennis E. A. Bacterial lipopolysaccharide priming of P388D1 macrophage-like cells for enhanced arachidonic acid metabolism. Platelet-activating factor receptor activation and regulation of phospholipase A2. J Biol Chem. 1990 May 25;265(15):8658–8664. [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J Biol Chem. 1990 Jul 25;265(21):12232–12239. [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- MacNulty E. E., Plevin R., Wakelam M. J. Stimulation of the hydrolysis of phosphatidylinositol 4,5-bisphosphate and phosphatidylcholine by endothelin, a complete mitogen for Rat-1 fibroblasts. Biochem J. 1990 Dec 15;272(3):761–766. doi: 10.1042/bj2720761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon L. L., Rodland K. D., Forsythe M. L., Magun B. E. Stimulation of phosphatidylinositol hydrolysis, diacylglycerol release, and gene expression in response to endothelin, a potent new agonist for fibroblasts and smooth muscle cells. J Biol Chem. 1989 May 25;264(15):8529–8536. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pang L., Decker S. J., Saltiel A. R. Bombesin and epidermal growth factor stimulate the mitogen-activated protein kinase through different pathways in Swiss 3T3 cells. Biochem J. 1993 Jan 1;289(Pt 1):283–287. doi: 10.1042/bj2890283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Plevin R., MacNulty E. E., Palmer S., Wakelam M. J. Differences in the regulation of endothelin-1- and lysophosphatidic-acid-stimulated Ins(1,4,5)P3 formation in rat-1 fibroblasts. Biochem J. 1991 Dec 15;280(Pt 3):609–615. doi: 10.1042/bj2800609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg S. D., Poder T. C., Lacerda A. E. Endothelin increases single-channel calcium currents in coronary arterial smooth muscle cells. FEBS Lett. 1989 Apr 10;247(1):68–72. doi: 10.1016/0014-5793(89)81242-6. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Herman W. H. Protein kinase C and protein tyrosine kinase activity contribute to mitogenic signaling by endothelin-1. Cross-talk between G protein-coupled receptors and pp60c-src. J Biol Chem. 1993 May 5;268(13):9347–9357. [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Kitagawa H., Yasue S., Yanagi S., Sakai K., Asahi M., Ohta S., Takeuchi F., Nakamura S., Yamamura H. Protein-tyrosine kinase p72syk is activated by thrombin and is negatively regulated through Ca2+ mobilization in platelets. J Biol Chem. 1993 Feb 5;268(4):2277–2279. [PubMed] [Google Scholar]
- Zachary I., Gil J., Lehmann W., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin rapidly stimulate tyrosine phosphorylation in intact Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4577–4581. doi: 10.1073/pnas.88.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992 Dec 11;71(6):891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. Identification of a novel tyrosine kinase as a major substrate. J Biol Chem. 1992 Sep 25;267(27):19031–19034. [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Stimulation of tyrosine kinase activity in anti-phosphotyrosine immune complexes of Swiss 3T3 cell lysates occurs rapidly after addition of bombesin, vasopressin, and endothelin to intact cells. J Biol Chem. 1991 Dec 15;266(35):24126–24133. [PubMed] [Google Scholar]
- van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992 Jul 1;285(Pt 1):235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]