Abstract
Trametinib, an MEK inhibitor, may offer a new therapeutic option for patients with NF1-related GIST.
Introduction
Neurofibromatosis type 1 (NF1) or von Recklinghausen disease is the most common autosomal dominant inherited disorder in humans, affecting approximately 1 in 3,000 individuals.1 The pathogenesis of this disease is based on genetic alterations in the neurofibromin 1 gene, NF1, located on chromosome 17q11.2, which encodes neurofibromin, a tumor suppressor protein.2 Despite our molecular understanding of this disease, its diagnosis is based on clinical features. National Institutes of Health diagnostic criteria for NF1 require the presence of two or more of the following: (1) six or more café‐au-lait macules >5 mm in greatest diameter in prepubertal individuals and >15 mm in greatest diameter after puberty; (2) two or more neurofibromas of any type or one plexiform neurofibroma; (3) freckling in the axillary or inguinal regions; (4) an optic pathway glioma; (5) two or more Lisch nodules; (6) a distinctive osseus lesion, such as sphenoid dysplasia, anterolateral bowing of the tibia, or pseudarthrosis of long bones; or (7) a heterozygous pathogenic NF1 variant with a variant allele fraction (VAF) of 50% in apparently normal tissue.3 Patients with NF1 are more likely to develop various benign and malignant tumors of neurogenic and non-neurogenic origin than the general population.4,5 Common tumor types in NF1 include low-grade gliomas (LGG, 16.6%), malignant peripheral nerve sheath tumors (MPNST, 15.1%), breast cancer (2.9%), and gastrointestinal stromal tumors (GISTs, 1.2%).4,6
GISTs are mesenchymal tumors that are considered to originate from the intestinal cells of Cajal or their progenitor cells. About 70%-80% of GISTs are caused by mutations in KIT7,8 and approximately 5%-10% are caused by platelet-derived growth factor receptor-α (PDGFRA) mutations.9 Other rare subtypes of GISTs, known as wild-type GISTs, show alterations in BRAF, NF1, or SDHs. Imatinib mesylate, a selective tyrosine kinase inhibitor (TKI) of KIT, has exhibited long-term progression-free survival (PFS) and is well tolerated by patients with advanced GISTs.10,11 The clinical response to imatinib depends on the presence of KIT and PDGFRA mutations, which are predictive markers.9
Wild-type GISTs lack activating mutations in KIT and PDGFRA. Therefore, TKIs such as imatinib are rarely effective.11 There is a report of BRAF-mutated GISTs treated with dabrafenib, a BRAF inhibitor,12 or pazopanib showing long PFS in patients with advanced GISTs resistant to imatinib and sunitinib.12 However, there are no reports on the efficacy of targeted agents in patients with NF1-mutant GISTs. Preclinical data suggest that MEK inhibitors may be therapeutic candidates for tumors caused by NF1 mutations, such as neurofibromas or MPNST.13 BELIEVE trial (jCRTs031190104) is a cross-organ, biomarker-based clinical trial allowing patients to participate in molecular targeted treatments for the off-label use. Here, we report a case of NF1-mutated advanced GIST treated with trametinib, a selective MEK1/MEK2 inhibitor, as part of BELIEVE trial.
Case Presentation
A 78-year-old woman presented at a local hospital with abdominal pain, vomiting, and diarrhea. The patient had more than six café-au-lait macules over 15 mm in greatest diameter and numerous cutaneous neurofibromas at age 40 years and was diagnosed with von Recklinghausen disease in a different hospital. Her second son had numerous cutaneous neurofibromas suggestive of von Recklinghausen's disease, whereas her parents and first son did not have any features or symptoms suggestive of the disease. Computed tomography (CT) revealed a volvulus at the ileum and a mass arising from the small intestine. Operative detorsion was performed as emergency procedure, and three neoplasms were found in the jejunum (5, 10, and 50 cm from the ligament of Treitz). One neoplasm (20 mm in size) was resected for histopathological examination. The tumor is composed of spindle-shaped cells with slightly coarse chromatin and long intertwined oval to elliptical nuclei. The cytoplasm was eosinophilic, and intercellular collagen fibers were not prominent. The cells often had small nuclei, but mitotic figures were not prominent. On immunostaining, the spindle cells showed diffuse cell membrane positivity for c-KIT and CD34, supporting the pathological diagnosis of GIST. The Ki-67 labeling index was low (<1%). One month after surgery, 18F-fluorodeoxyglucose positron emission tomography-CT revealed accumulation in the neoplasms in the duodenum (maximum standardized uptake value [SUVmax], 3.2), jejunum (SUVmax, 3.0), and left chest wall (SUVmax, 3.4). The patient was diagnosed with primary GISTs and was referred to our hospital for chemotherapy.
Comprehensive genomic assay (CGA) was performed using FoundationOne CDx. CGA identified a pathogenic NF1 mutation (R1362*) with a 93.8% VAF and a KIT mutation (D816H) with a 1.1% VAF of therapeutic interest. Since imatinib is not effective in NF1-related GISTs and guidelines do not recommend its use14 and given the previous reports on NF1-associated tumors treated with trametinib,15,16 we enrolled the patient in the BELIEVE trial for the off-label use of trametinib, which was provided by Novartis. Trametinib 2 mg was administered once daily for 2 weeks. The treatment was initially well tolerated; however, the patient developed a grade 3 creatine phosphokinase (CPK) increase after 2 weeks. CPK increase was asymptomatic, but trametinib was temporarily discontinued, and the CPK level gradually recovered to grade 0. Trametinib was restarted with a reduced dose of 1.5 mg daily; however, the patient again developed a grade 3 CPK increase within 1 month. Prompt interruption of trametinib treatment resulted in a smooth decrease in CPK levels. Trametinib was restarted at a dose of 1 mg daily. The patient experienced grade 1 myalgia and grade 2 fatigue at 2 months and 6 months, respectively, after the start of treatment. The fatigue has persisted to this day, but the myalgia improved with about a week of observation and was considered tolerable toxicity. Following 4 months of treatment, CT showed a partial response (Fig 1). The mean density on CT, measured in Hounsfield units (HU), decreased from 96.22 HU to 93.77 HU on the basis of the Choi criteria.17 This response has continued for more than 10 months to date. Chest wall mass revealed no change in size throughout the treatment. All necessary permissions from the patient were obtained from the law and our institution to publish the images in JCO PO.
FIG 1.
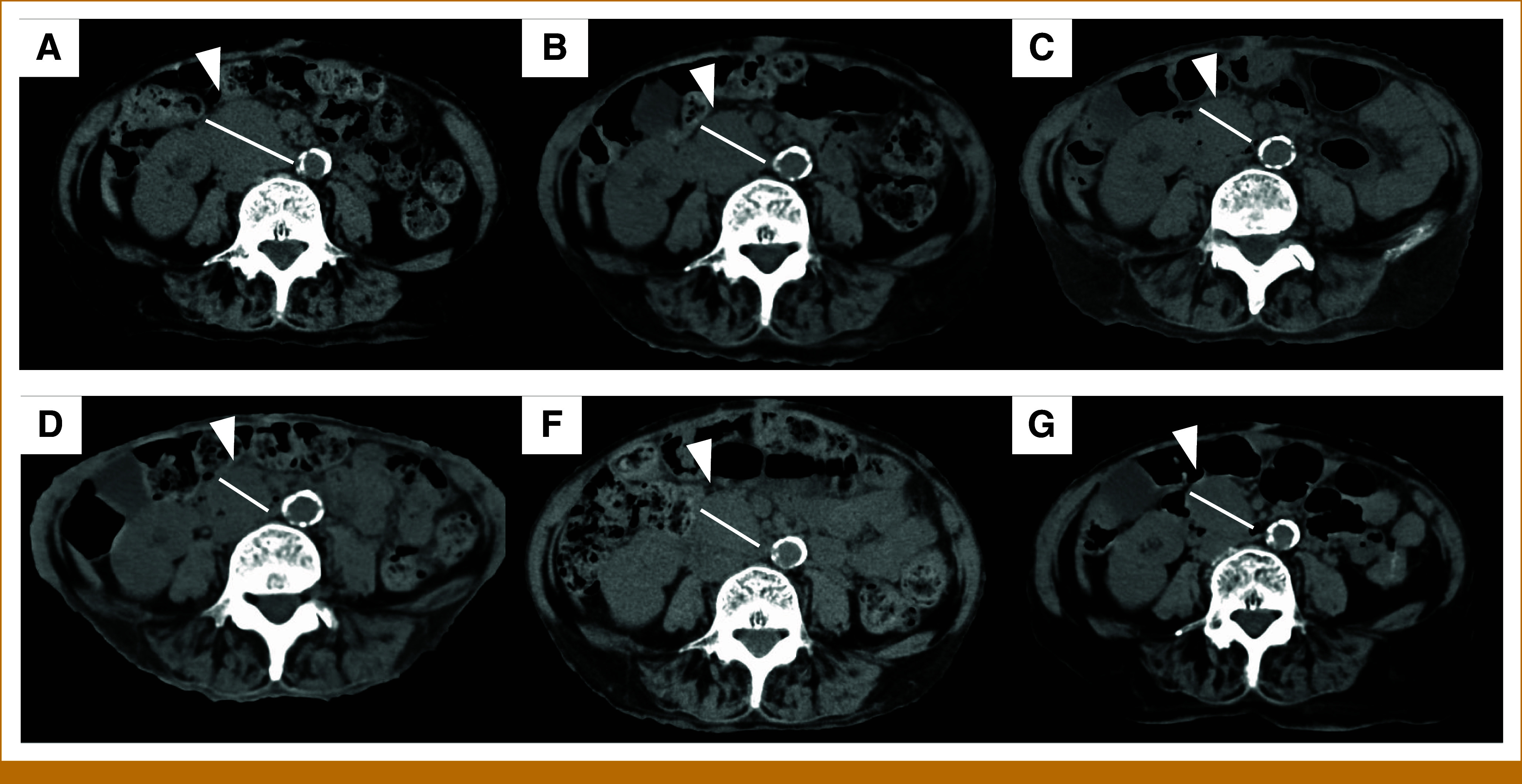
Plane CT performed before (A) and after 4, 6, 10, 18, and 30 months of trametinib therapy (B-F), and tumor (▲) originally 41 mm of size shrank to 27 mm at 4-month CT and has been stable to date. CT, computed tomography.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
A waiver from the requirement to provide written informed consent was granted by the institutional review board of the National Cancer Center.
Discussion
To our knowledge, this is the first report demonstrating the efficacy of an MEK inhibitor in a patient with NF1-mutant GISTs. Although a dose reduction was required, treatment with trametinib resulted in a tumor response with acceptable toxicity.
Neurofibromin is a ubiquitously expressed RAS-GTPase–activating protein that acts as a tumor suppressor by regulating the downstream RAF/MEK/ERK pathway. GTP-RAS is converted to GDP-RAS by increasing the intrinsic GTPase activity, thereby inhibiting RAS signaling.18 When neurofibromin is mutated, GTP-RAS accumulation leads to the upregulation of the downstream RAF/MEK/ERK pathway, resulting in either benign or malignant cell growth. PI3K/AKT, JAK-STAT3, and several other pathways are regulated by neurofibromin, providing therapeutic options for NF1-associated clinical manifestations.
KIT and PDGRA are type III receptor tyrosine kinases, and KIT or PDGFRA-mutant GISTs demonstrate activation of downstream signaling pathways, including the RAF/MEK/ERK, PI3K/AKT, and STAT3 pathways. Wild-type GISTs are a heterogeneous group with various oncogenic mutations with different pathogenesis. NF1-associated GISTs rarely coexpress KIT or PDGFRA mutations, and the PI3K/AKT pathway is rarely activated. Our case uniquely presented with the KIT D816H mutation alongside the NF1 mutation, which is located on exon 17 and is known for imatinib resistance. No mutations were found in exon 9 or 11 in this patient.
There is currently no standard treatment for NF1-associated GISTs. Guidelines suggest treating imatinib-resistant GISTs with sunitinib when druggable mutations (PDGFRA, SDHs, NTRK, and BRAF) are not detected.14 Sunitinib has shown survival benefits in patients with advanced GISTs after imatinib failure.19 However, preclinical studies have suggested that activated forms of KIT mutants, including KIT D816H and wild-type KIT, are resistant to sunitinib and imatinib.20 Regorafenib is another treatment option. It has been shown to improve the PFS in patients with GISTs after progression to imatinib and sunitinib. One case of NF1-associated GISTs treated with regorafenib was reported to show a therapeutic response21 and warrants further investigation.
MEK inhibitors are reported to be effective against NF1-related diseases. Selumetinib causes durable tumor shrinkage in children with NF1 and symptomatic inoperable plexiform neurofibromas (PN) as reported in the SPRINT study.16 Thus, selumetinib is the first FDA-approved targeted therapy for NF1. In the MATCH trial, eight of 21 patients registered in the trial had NF1 mutations. Stable disease was observed in one patient, but there were no reports of tumor shrinkage in the trial cohort. The difference in these two trials may depend on whether the NF1 mutation was a germline mutation or somatic mutation. The MATCH trial included both types of mutations, whereas the SPRINT study recruited patients with germline mutations. We hypothesized that selumetinib is effective when the NF1 mutation is a germline mutation. A preclinical study reported MEK inhibitor showed sensitivity to MPNST-derived cell line, whereas sporadic MPNST cell line did not.22 Another phase II trial of selumetinib in combination with sirolimus in patients with NF1-associated MPNST (ClinicalTrials.gov identifier: NCT03433183) is currently ongoing. These findings suggest that MEK inhibitors can be used as rational treatments for NF1-associated conditions.
Another hypothesis posits that partial inhibition of ERK may be sufficient for more indolent NF1 tumors to elicit response.23 In one phase II trial where selumetinib was administered for pediatric glioma or PN, a response was observed in 40% of patients (ClinicalTrials.gov identifier: NCT03363217). The trial included patients with either NF1-related LGG or NF1-related PN. Furthermore, there is an ongoing trial comparing selumetinib with standard treatment for NF1-related LGG.
One limitation of our study is that it was a single case report. A phase II trial of selumetinib for patients with NF1-mutated GISTs was once underway; however, it was withdrawn because of slow accrual (ClinicalTrials.gov identifier: NCT03109301). Further investigation of the role of MEK inhibitors in NF1-associated GISTs is required. Additionally, any trial targeting NF1-associated GIST should be conducted multicenter and possibly multinational, considering the rarity of this subtype.
In conclusion, in this case report, a patient with NF1-associated GISTs treated with trametinib experienced tumor shrinkage with acceptable toxicity. Considering the scarcity of NF1-associated GISTs,24,25 this case report suggests a potential therapeutic option for targeted therapy.
SUPPORT
Supported by Grant-in-Aid for Rare Cancer Research. This work was conducted as a part of a prospective trial of patient-proposed healthcare services with multiple targeted agents based on the results of gene profiling using a multigene panel test (BELIEVE trial; jCRTs031190104).
AUTHOR CONTRIBUTIONS
Conception and design: Misao Fukuda, Toru Mukohara, Yoichi Naito
Financial support: Yoichi Naito
Administrative support: Yoichi Naito
Provision of study materials or patients: Yoichi Naito
Collection and assembly of data: Misao Fukuda, Yoichi Naito
Data analysis and interpretation: Misao Fukuda, Takeshi Kuwata, Kuniko Sunami, Yoichi Naito
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Toru Mukohara
Honoraria: Eisai, Pfizer, Novartis, Chugai Pharma, Lilly Japan, AstraZeneca, Kyowa Kirin, Taiho Pharmaceutical
Consulting or Advisory Role: Eisai, Micin
Research Funding: Sysmex (Inst), Eisai (Inst), MSD (Inst), Pfizer (Inst), Novartis (Inst), Sanofi (Inst), Chugai Pharma (Inst), Daiichi Sankyo/Astra Zeneca (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst), Gilead Sciences (Inst)
Takeshi Kuwata
Honoraria: MSD, Daiichi Sankyo, Astellas Pharma, Roche, Bayer, FALCO biosystems
Consulting or Advisory Role: Astellas Pharma, Roche, Daiichi Sankyo
Research Funding: Takeda (Inst)
Kuniko Sunami
Honoraria: Sysmex, Chugai Pharma, AstraZeneca, Novartis, Eisai, Riken Genesis, Lilly, Illumina, Pfizer, Konica Minolta Precision Medicine, Guardant Health
Research Funding: Sysmex
Yoichi Naito
Consulting or Advisory Role: Chugai Pharma, Lilly, Pfizer, Taiho Pharmaceutical, Daiichi Sankyo, AstraZeneca, Takeda, Bayer
Speakers' Bureau: Chugai Pharma, Novartis, Lilly, Bayer Yakuhin, Pfizer, AstraZeneca, Ono Yakuhin, Gardant Pharmaceuticals, Takeda, FUJIFILM Toyama Chemistry, Taiho Pharmaceutical, Mundipharma, MSD, Bristol Myers Squibb Japan, Shionogi, Eisai, Hisamitsu Pharmaceutical
Research Funding: AstraZeneca Japan, Lilly, Pfizer, AbbVie, Daiichi Sankyo, Taiho Pharmaceutical, Boehringer Ingelheim, Eisai, Gilead Sciences, Chugai/Roche, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Neurofibromatosis: Conference statement. Arch Neurol 45:575–578, 1988 [PubMed] [Google Scholar]
- 2.Viskochil D, Buchberg AM, Xu G, et al. : Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 62:187-192, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Legius E, Messiaen L, Wolkenstein P, et al. : Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An International Consensus Recommendation. Genet Med 23:1506-1513, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landry JP, Schertz KL, Chiang YJ, et al. : Comparison of cancer prevalence in patients with neurofibromatosis type 1 at An Academic Cancer Center vs in the general population from 1985 to 2020. JAMA Netw Open 4:e210945, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zöller MET, Rembeck B, Odén A, et al. : Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer 79:2125-2131, 1997 [PubMed] [Google Scholar]
- 6.Kiuru M, Busam KJ: The NF1 gene in tumor syndromes and melanoma. Lab Invest 97:146-157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirota S, Nishida T, Isozaki K, et al. : Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol 193:505-510, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Hirota S, Isozaki K, Moriyama Y, et al. : Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577-580, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Heinrich MC, Corless CL, Demetri GD, et al. : Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21:4342-4349, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Blanke CD, et al. : Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472-480, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Blanke CD, Demetri GD, von Mehren M, et al. : Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26:620-625, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Mir O, Cropet C, Toulmonde M, et al. : Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): A randomised, multicentre, open-label phase 2 trial. Lancet Oncol 17:632-641, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Jessen WJ, Miller SJ, Jousma E, et al. : MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest 123:340-347, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casali PG, Blay JY, Abecassis N, et al. : Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 33:20-33, 2022 [DOI] [PubMed] [Google Scholar]
- 15.Galvin R, Watson AL, Largaespada DA, et al. : Neurofibromatosis in the era of precision medicine: Development of MEK inhibitors and recent successes with selumetinib. Curr Oncol Rep 23:45, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross AM, Wolters PL, Dombi E, et al. : Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 382:1430-1442, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi H, Charnsangavej C, Faria SC, et al. : Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol 25:1753-1759, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Mo J, Moye SL, McKay RM, et al. : Neurofibromin and suppression of tumorigenesis: Beyond the GAP. Oncogene 41:1235-1251, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demetri GD, van Oosterom AT, Garrett CR, et al. : Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 368:1329-1338, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gajiwala KS, Wu JC, Christensen J, et al. : KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci USA 106:1542-1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimi A, Nagamachi Y, Yamauchi N, et al. : Gastrointestinal stromal tumor in a patient with neurofibromatosis type 1 that was successfully treated with regorafenib. Intern Med (Tokyo, Japan) 58:1865-1870, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varin J, Poulain L, Hivelin M, et al. : Dual mTORC1/2 inhibition induces anti-proliferative effect in NF1-associated plexiform neurofibroma and malignant peripheral nerve sheath tumor cells. Oncotarget 7:35753-35767, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harder A: MEK inhibitors—Novel targeted therapies of neurofibromatosis associated benign and malignant lesions. Biomark Res 9:26, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corless CL, Barnett CM, Heinrich MC: Gastrointestinal stromal tumours: Origin and molecular oncology. Nat Rev Cancer 11:865-878, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Nishida T, Tsujimoto M, Takahashi T, et al. : Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J Gastroenterol 51:571-578, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]


