Abstract
PURPOSE
Thrombocytopenia is a relatively common dose-limiting toxicity during peptide receptor radionuclide therapy (PRRT) in patients with NET. Although uncommon, some patients develop persistent cytopenia and eventually therapy-related myeloid neoplasm (t-MN), which has a dismal prognosis. As the indications for PRRT are expanding, it is important to investigate factors that may predict cytopenias during/after PRRT. We prospectively evaluated the prevalence of clonal hematopoiesis (CH) and cytopenia in patients with NET undergoing PRRT.
MATERIALS AND METHODS
Patients with metastatic NET with plan to receive four cycles of lutetium-177 were enrolled. CH was evaluated before PRRT using a panel of 220 genes with a targeted depth of ≥1,000×. Patients were followed during PRRT and every 3 months thereafter.
RESULTS
Of 37 patients enrolled, the median age was 68 years and 51.4% were male. Previous treatment exposures included alkylating agents in 30%, platinum agents in 8%, and external radiation in 13%. CH was detected in 35.1% using a variant allele frequency (VAF) cutoff of ≥2% and 45.9% with a VAF of ≥1%. The most common mutations were in age-related genes (DNMT3A, TET2). CH was not associated with anemia or neutropenia; however, it was associated with lower platelet count at baseline and more time spent in a thrombocytopenic state during/after PRRT. Five patients had bone marrow biopsies (BMBs) because of sustained hematologic dysfunction post-PRRT, and of those, diagnoses included clonal cytopenia of undetermined significance (CCUS) in three and idiopathic cytopenia of undetermined significance (ICUS) in two.
CONCLUSION
CH is present in 35.1% of patients with NET and is associated with thrombocytopenia risk during PRRT. Future studies with long-term follow-up will delineate whether CH might be a predictor for higher risk of t-MN after PRRT.
INTRODUCTION
The incidence of neuroendocrine tumors (NETs) has been increasing overtime, and prognosis depends on multiple factors including primary tumor location, disease grade, stage, and rate of differentiation and proliferation.1-3 Given that most NETs have high expression of somatostatin receptor (SSTR), somatostatin analogs (SSA) are used as first-line therapy.4 Second-line therapies include peptide receptor radionuclide therapy (PRRT), which allows for the delivery of radiopharmaceutical agents directly to tumor cells by targeting SSTRs. In PRRT, the SSA is linked to a specific radioisotope such as Lu-DOTATATE (177Lu).5 177Lu received FDA approval in 2018 after the NETTER-1 trial showed improved progression-free survival (PFS) with 177Lu + SSA compared with SSA alone in patients with progressive gastroenteropancreatic (GEP) NETs.6,7 More recently, the NETTER-2 trial showed the efficacy of PRRT in the frontline setting in patients with grade 2 or 3 NET.8 Hematotoxicity, and in particular, thrombocytopenia, is a relatively common adverse event of PRRT and is often a limiting factor to receiving a full therapeutic course.6,9-12 Furthermore, therapy-related myeloid neoplasms (t-MNs) have been reported post-PRRT with rates ranging from 2% to 20% and a median time from first PRRT to t-MN of 2.8 years.9,13-19 Our recent retrospective analysis revealed that among 346 patients treated with at least one cycle of PRRT at Mayo Clinic-Rochester, 4% were diagnosed with t-MN or therapy-related clonal cytopenia of undetermined significance (t-CCUS).20 The development of t-MN carries a poor prognosis with a 5-year overall survival (OS) of <10%, and therefore, it is imperative that we elucidate the risks of PRRT.21,22 Previous studies have evaluated risk factors for t-MN, including alkylating agents, previous external radiation (RT) exposure, and others, including clonal hematopoiesis (CH).13,14,19
CONTEXT
Key Objective
In patients with neuroendocrine tumors (NETs) who are receiving peptide receptor radionuclide therapy (PRRT), is underlying clonal hematopoiesis (CH) a risk for hematologic toxicity?
Knowledge Generated
Prevalence of CH was 35% before PRRT, with age-related CH variants being most common (DNMT3A, TET2). Patients with CH had lower baseline platelet counts before PRRT and spent more time thrombocytopenic during PRRT. Post-PRRT, variants in DNA damage response genes were common.
Relevance
In patients with NET with CH, PRRT may lead to increased short- and long-term hematologic dysfunction. Screening before PRRT and hematologic monitoring during and after PRRT should be considered.
CH defines somatic mutations of leukemia-associated driver genes within subpopulations of hematopoietic stem cells (HSCs) and is a precursor to myeloid neoplasms. When CH is associated with unexplained cytopenia, it is termed CCUS.23 T-CCUS describes unexplained cytopenia with clonal abnormality after DNA-damaging therapy and is associated with progression to t-MN.24,25 The progression from CH/CCUS to t-MN depends on mutation, growth rates, context exposures, and the acquisition of additional genetic aberrations.26-32 RT exposure has also been associated with t-MN with a median latency of 6.5 years.33
While the rate of CH/CCUS has been explored in various solid tumors, the prevalence is not well known in patients with NET.26,29 Herein, we hypothesized that PRRT-induced thrombocytopenia may be related to underlying CH in patients with NET. To address this, we prospectively followed 37 patients with NET treated with 177Lu to characterize the incidence of cytopenias and CH/CCUS.
MATERIALS AND METHODS
Study Population
This prospective study was approved by the Mayo Clinic Institutional Review Board (IRB); informed written consent was provided by patients at enrollment. Inclusion criteria included patients with metastatic NET 18 years and younger undergoing PRRT between September 2020 and May 2022. 177Lu was administered intravenously at a dose of 200 mCi once every 8 weeks for four doses.6 Complete blood counts were obtained before each treatment and at 3-month follow-up intervals post-PRRT. Toxicity was recorded with the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. CH and CCUS were defined per 2022 WHO and International Consensus Classification (ICC) criteria.34,35 The primary end point of the study was thrombocytopenia (≥grade 1) during treatment and follow-up. Secondary end points included the prevalence of CH and cytopenia at baseline, incidence of t-MN, and overall survival (OS). We chose thrombocytopenia as the primary end point for the following reasons: (1) it is considered the most common hematologic toxicity post-PRRT, (2) nadir usually occurs 4-6 weeks post-PRRT, and (3) almost all patients who develop t-MN had previous thrombocytopenia.36
Next-Generation Sequencing
DNA was extracted from primary mononuclear cells enriched by density gradient centrifugation. Target capture was performed as described.37-40 Briefly, libraries were prepared by custom capture using a hybrid-target enrichment covering the entire coding regions for 220 genes. Samples were sequenced using Illumina NovaSeq SP (Illumina, San Diego, CA) to accommodate a targeted depth of ≥1,000×. Initial filters were provided by the Genome Analysis Toolkit (GATK) for single nucleotide and small insertion/deletion variant calling. For clinical NGS performed on bone marrow biopsy (BMB), a 42-gene panel with a sensitivity of 5%-10% and a minimum depth of 250× was used as described.40 Variants with minor allele fractions ≥0.6% by GnomAD were excluded. The remaining variants were functionally annotated using ANNOVAR software, and Integrative Genomics Viewer (IGV) was used for manual reviews of alignment for selected variant calls.
Statistical Analysis
Continuous variables were presented as medians with ranges and compared between CH and no CH patients using the Wilcoxon rank-sum test. Categorical variables were expressed as counts with percentages and compared using the chi-square test. OS was estimated using Kaplan-Meier and compared using the log-rank test. A multistate model was used to investigate longitudinal changes in blood counts.41 Illness-death models were used where patients can transition between healthy and illness (eg, thrombocytopenic), with death as the absorbing state. Probability in state and time spent in the state (ie, sojourn time) were estimated using Aalen-Johansen estimator.42 Multistate hazards model was used to estimate the transition rate while adjusting for bone mets. Additional details are given in the Data Supplement. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC) and R (version 4.2.2). Two-sided P values of <.05 were considered statistically significant, and there was no adjustment for multiple comparisons.
RESULTS
Patient Characteristics
A total of 37 patients (51.4% male) with a median age of 68 years (range, 34-85) were enrolled (Table 1). Most patients had small-bowel NET (n = 19, 51.4%), all patients had stage IV well-differentiated NET, and 81.1% (n = 30) had grade 1 or 2 disease. About one third (n = 11, 29.7%) had previous exposure to temozolomide or platinum agents, and 13.5% (n = 5) had a history of RT (4 to bone mets, one for lymphoma 40 years ago to a nonbony site). Among patients with a history of chemotherapy (chemo) or RT, the median time from first chemo to NGS was 2.5 years (range, 0.23-14.7) and the median time from first RT to NGS was 4.8 years (range, 0.3-40.5).
TABLE 1.
Patient Characteristics
| Clinical Value | No CH (n = 24) | CH (≥2%) (n = 13) | Total (n = 37) | P |
|---|---|---|---|---|
| Age, years, median (range) | 68.0 (34.0-81.0) | 69.0 (59.0-85.0) | 68.0 (34.0-85.0) | .60a |
| Male, No. (%) | 14 (58.3) | 5 (38.5) | 19 (51.4) | .25b |
| Primary tumor type, No. (%) | .71b | |||
| Gastric | 1 (4.2) | 0 (0.0) | 1 (2.7) | |
| Head/neck | 0 (0.0) | 1 (7.7) | 1 (2.7) | |
| Other | 2 (8.3) | 1 (7.7) | 3 (8.1) | |
| Pancreatic | 8 (33.3) | 3 (23.1) | 11 (29.7) | |
| Paraganglioma | 1 (4.2) | 1 (7.7) | 2 (5.4) | |
| Small bowel | 12 (50.0) | 7 (53.8) | 19 (51.4) | |
| Grade, No. (%) | .46b | |||
| 1 | 5 (20.8) | 3 (23.1) | 8 (21.6) | |
| 2 | 15 (62.5) | 7 (53.8) | 22 (59.5) | |
| 3 | 2 (8.3) | 0 (0.0) | 2 (5.4) | |
| Unknown | 2 (8.3) | 3 (23.1) | 5 (13.5) | |
| Ki-67%, median (range) | 7.3 (1.0-65) | 8.5 (2.0-20) | 8.5 (1.0-65) | .54a |
| Bone mets, No. (%) | 9 (37.5) | 10 (76.9) | 19 (51.4) | .02b |
| Severity of bone mets, No. (%) | .07b | |||
| None | 15 (62.5) | 3 (23.1) | 18 (48.6) | |
| Mild | 5 (20.8) | 6 (46.2) | 11 (29.7) | |
| Severe | 4 (16.7) | 4 (30.8) | 8 (21.6) | |
| Previous therapy | 6 (25.0) | 5 (38.5) | 11 (29.7) | .39b |
| Alkylating | 2 (8.3) | 1 (7.7) | 3 (8.1) | .95b |
| Platinum mTOR inhibitor | 4 (16.7) | 4 (30.8) | 8 (21.6) | .32b |
| Somatostatin analog | 22 (91.7) | 13 (100) | 35 (94.6) | .28b |
| External radiation | 2 (8.3) | 3 (23.1) | 5 (13.5) | .21b |
| Baseline laboratory tests | ||||
| Hemoglobin (g/dL), median (range) | 13.0 (10.6-16.3) | 13.3 (10.7-15.4) | 13.0 (10.6-16.3) | .57a |
| RDW (%), median (range) | 13.9 (11.9-21.3) | 14.1 (12.4-17.6) | 14.0 (11.9-21.3) | .76a |
| MCV (fL), median (range) | 91.0 (76.5-100.2) | 92.5 (88.7-96.0) | 91.4 (76.5-100.2) | .35a |
| Platelets (×109/L), median (range) | 230.5 (153-473) | 184.5 (113-367) | 220.0 (113-473) | .03a |
| Leukocytes (×109/L), median (range) | 6.4 (4.0-12.7) | 6.0 (2.4-8.8) | 6.1 (2.4-12.7) | .43a |
| Neutrophils (×109/L), median (range) | 4.4 (2.0-10.0) | 3.7 (1.3-7.3) | 3.8 (1.3-10) | .51a |
| Lymphocytes (×109/L), median (range) | 1.3 (0.4-2.3) | 1.5 (0.5-2.0) | 1.4 (0.4-2.3) | .46a |
| Completed four cycles, No. (%) | 17 (70.8) | 8 (61.5) | 25 (67.6) | .56b |
| Total GBq received, median (range) | 789.1 (187-840) | 765.2 (211-841) | 781.2 (187-841) | .17a |
| 12-month laboratory tests | ||||
| Hemoglobin (g/dL), median (range) | 12.1 (7.4-17.0) | 12.6 (9.4-17.2) | 12.5 (7.4-17.2) | .88a |
| RDW (%), median (range) | 14.1 (12.0-21.0) | 13.2 (12.2-16.5) | 14.1 (12.0-21.0) | .29a |
| MCV (fL), median (range) | 97.1 (64.7-111.0) | 97.1 (91.1-101.0) | 97.1 (64.7-111.0) | .77a |
| Platelets (×109/L), median (range) | 169.0 (36.0-264.0) | 199.5 (103.0-427.0) | 172.0 (36.0-427.0) | .43a |
| Leukocytes (×109/L), median (range) | 4.4 (1.8-13.0) | 5.5 (3.6-9.2) | 5.4 (1.8-13.0) | .72a |
| Neutrophils (×/L), median (range) | 2.8 (1.1-5.1) | 4.0 (2.4-4.7) | 2.8 (1.1-5.1) | .16a |
| Lymphocytes (×109/L), median (range) | 0.7 (0.3-2.2) | 0.8 (0.3-2.7) | 0.8 (0.3-2.7) | .60a |
Abbreviations: CH, clonal hematopoiesis; MCV, mean corpuscular volume; mTOR, mammalian target of rapamycin; RDW, red cell distribution width.
Wilcoxon rank-sum P value.
Chi-square P value.
Prevalence of CH Before PRRT
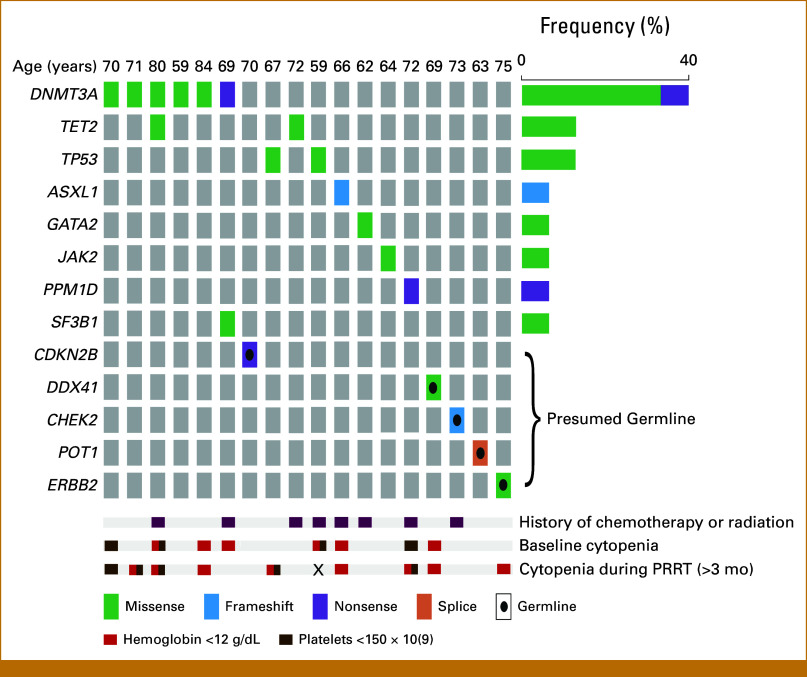
Of the 37 patients, 28 pathogenic variants were identified among 21 patients (Fig 1). Five (18%) of the 28 variants were presumed germline on the basis of VAF (CDKN2B, DDX41, CHEK2, POT1, ERBB2; median VAF 46.5%, range, 44.0%-52.1%; Fig 1).43,44 Eight (28.5%) of the 28 pathogenic variants had a VAF of <2%. In line with the commonly accepted threshold of VAF ≥2%,34 and after excluding the presumed germline variants (n = 5), the prevalence of CH was 35.1% (15 variants among 13 patients, of 37 sequenced). When evaluating patients with VAF ≥1%, the prevalence was 45.9%. Among those with VAF ≥2% (n = 13), the median VAF of all pathogenic variants was 7.3% (range, 2.0%-50.30%). The most common pathogenic variant with VAF ≥2% was in DNMT3A (n = 6), followed by TET2 (n = 2). Patients with bone mets were more likely to have CH (P = .02) although severity of bone metastatic burden was not associated with CH (P = .07, Table 1). Previous history of chemo or RT was not associated with CH (Table 1).
FIG 1.
Oncoplot of pathogenic variants, previous therapies, and cytopenias among patients with variants detected with VAF ≥2%. The overall prevalence of CH was 35%. One patient did not receive PRRT, and therefore, cytopenia could not be evaluated during treatment (indicated by X). Variants with VAF ≥2% were considered CH. PRRT, peptide receptor radionuclide therapy.
Baseline Cytopenias
We first investigated whether patients with CH were more likely to have cytopenias at baseline pre-PRRT. There were no statistically significant differences in baseline hemoglobin (P = .57), red cell distribution width (RDW, P = .76), mean corpuscular volume (MCV, P = .35), leukocytes (P = .43), neutrophils (P = .51), or lymphocytes (P = .46) between those with CH and those without (Table 1, Fig 2A). However, patients with CH (VAF ≥2%) had a lower platelet count at baseline with a median of 185 × 109/L (range, 113-367) compared with 231 × 109/L (range, 153-473) in those without CH (P = .03, Fig 2A).
FIG 2.
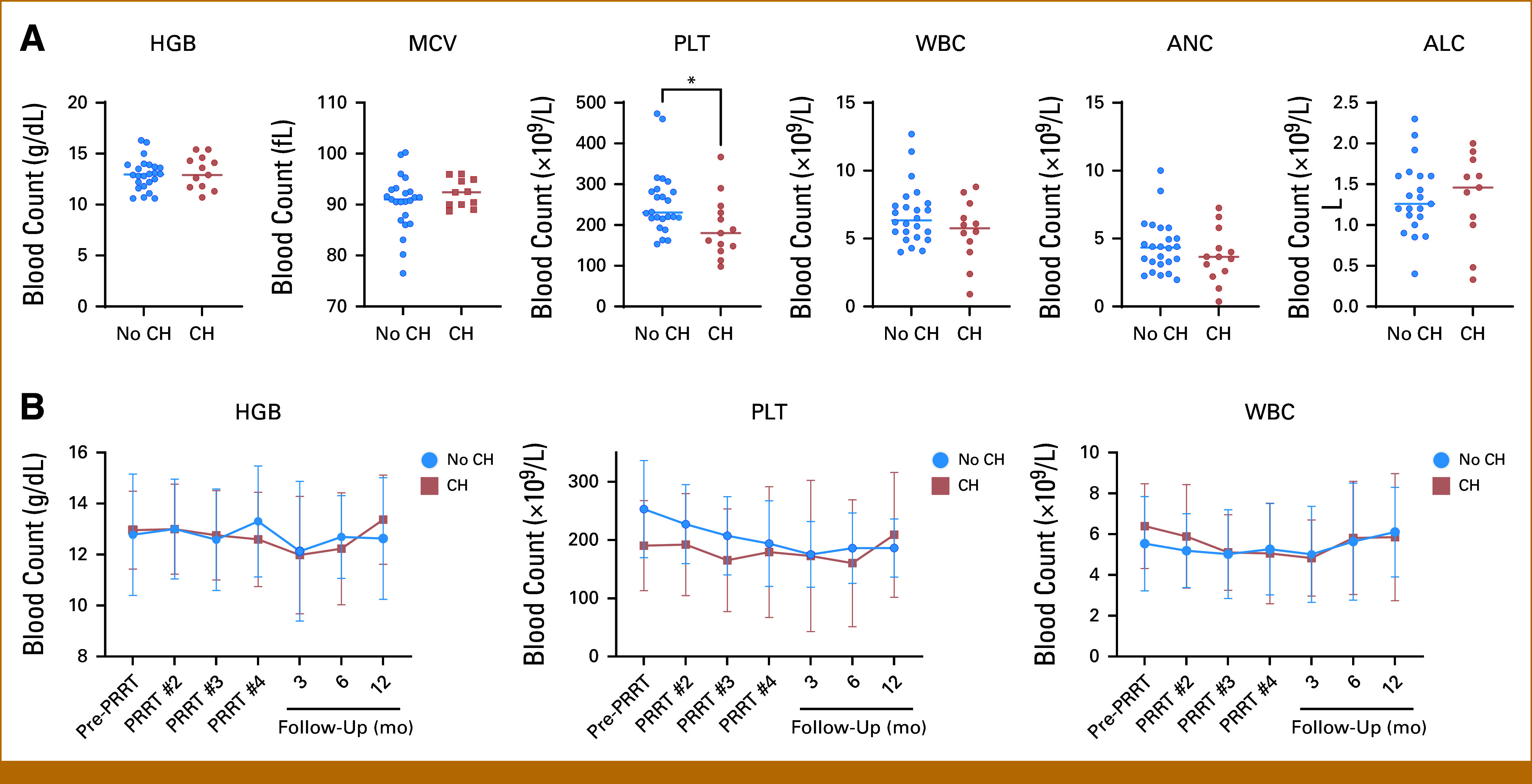
Patients with NET with CH had a lower platelet count at baseline. (A) Baseline blood counts. Patients with CH (VAF ≥2%) had lower platelet counts than those without CH (P = .03). There were no differences in HGB, MCV, WBC, ANC, or ALC. The horizontal line depicts median. (B) During four cycles of PRRT and at 3-month, 6-month and 12-month follow-ups, there was no significant difference in blood counts between groups. Mean with SD at each cycle and follow-up are shown. ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CH, clonal hematopoiesis (defined by VAF ≥2%); Hgb, Hemoglobin; MCV, mean corpuscular volume; NET, neuroendocrine tumor; PLT, platelet; SD, standard deviation.
PRRT and Toxicity
The median follow-up was 26.0 months (IQR, 21.2-29.0). Most patients received four PRRT treatments (n = 25, 67.6%), with a total median dose of 781 mCi (range, 187-841). One patient (ID #4) did not receive any PRRT because of cytopenias, comorbidities, and prolonged hospitalization, which ultimately resulted in death. Overall, two patients discontinued PRRT after three cycles because of cytopenias and three patients required transfusions during PRRT treatment (all without CH). During PRRT and follow-up, there were no statistically significant differences in cytopenias between groups (Fig 2B), including in grade 3 or 4 cytopenias. Thirteen patients transitioned from normal platelet count (health) to thrombocytopenia once, and two patients made the same transition twice (Fig 3A). Notably, there were 10 instances of patients who recovered from thrombocytopenia to health, whereas 10 patients died without experiencing thrombocytopenia. Patients with CH had a higher probability of being thrombocytopenic than patients without CH overtime (Fig 3B). The highest probability of thrombocytopenia, among patients with CH, was 58.3%, which was observed about 300 days after starting PRRT (Fig 3B). CH was not associated with an increased incidence of thrombocytopenia (HR, 0.73 [95% CI, 0.26 to 2.01]), but patients with CH were less likely to recover from thrombocytopenia to normal platelets (HR, 0.17 [95% CI, 0.04 to 0.66]) compared with patients without CH. On average, patients with CH spent more time thrombocytopenic (200 days, [95% CI, 88 to 312]) than patients without CH (99 days [95% CI, 45 to 153]), restricting to 500 days after initiation of PRRT, and this was true for grade ≥3 thrombocytopenia as well (8 days more [95% CI, –16.2 to 32.1]; Figs 3C, Data Supplement, Fig S1). There were no differences across groups for anemia, leukopenia, neutropenia, or lymphopenia (Data Supplement, Fig S2). The 1-year OS rate was 75.0% and 79.2% for those with and without CH, respectively (P = .77), whereas median was not reached (Data Supplement, Fig S3).
FIG 3.
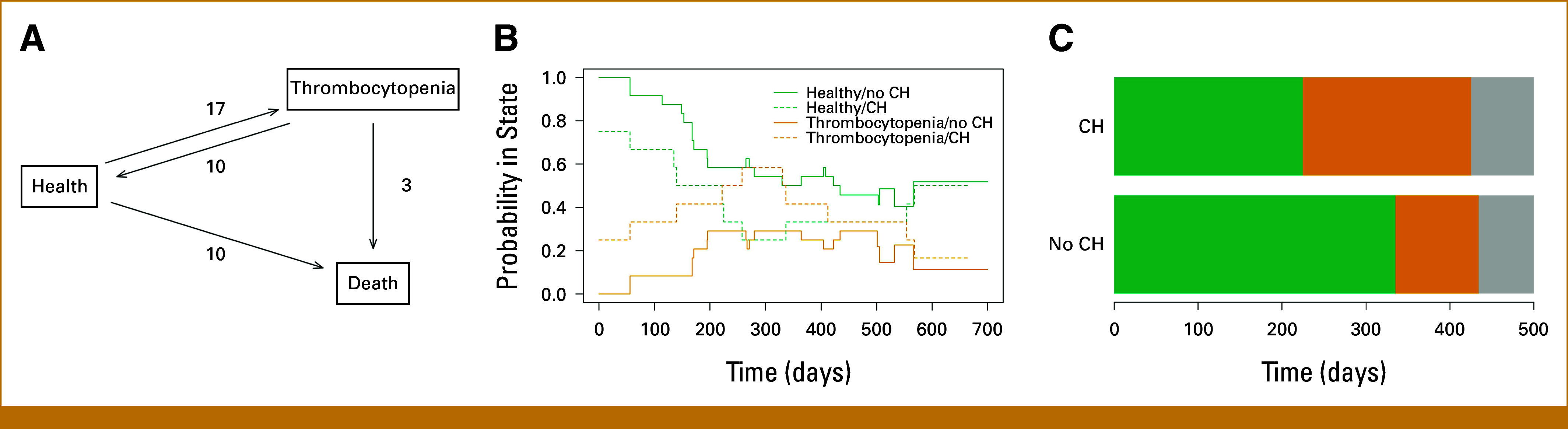
Patients with NET with CH were more likely to be thrombocytopenic during PRRT. (A) State space and transition counts for thrombocytopenia depict patient transitions from normal platelet count (health) to any grade of thrombocytopenia or death. (B) Aalen-Johansen plot showing that patients with CH (VAF ≥2%) were more likely to experience thrombocytopenia and remain thrombocytopenic longer than patients without CH. (C) On average, patients with CH spent about 200 days (95% CI, 88.3 to 312.4) being thrombocytopenic compared with 99 days (95% CI, 45.3 to 153.3) without. Green represents healthy, orange represents thrombocytopenia, and gray represents death. CH, clonal hematopoiesis.
CH Assessment Post-PRRT
In total, five patients (13.5%) enrolled had BMB performed post-PRRT because of persistent cytopenias with a median time from first PRRT to BMB of 1.13 years (range, 0.8-1.3) (Table 2, Data Supplement, Fig S4). Of these 5, one patient (ID #18) had a history of temozolomide and RT to bone mets (femur, hip, and thoracic spine). Pre-PRRT, ID #18 had one TET2 pathogenic variant detected with a VAF of 1.5%, which was not detected on post-PRRT NGS, likely because of differences in sequencing sensitivities. ID #2, who had a history of lymphoma treated with single-agent RT 40 years before PRRT, had a low-level pathogenic PPM1D variant (c.1433dupG) with a VAF of 0.80% detected pre-PRRT; and on post-PRRT BMB, the same variant was detected with a VAF of 18.9%.
TABLE 2.
Characteristics of Patients With NET With Post-PRRT CH Data Available
| ID | Previous Chemotherapy or RT | Pre-PRRT NGS (blood) | PRRT Cycles | Cytopenia During/After PRRT | Time Between Enrollment and BMB | Karyotype | NGS (BM)a | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| #3 | No |
DNMT3A c.2014del (1.5%) DNMT3A c.1906G>T (3.3%) |
4 | Gr1 thrombocytopenia and anemia >6 mo | 13.6 mo | 46,XY[20] | None detected | ICUS v CCUSb | Stable |
| #9 | No | DNMT3A c.2396C>A (3.9%) | 3 | Gr2 anemia Gr3 thrombocytopenia |
10.8 mo | 46,XY[12] |
PPM1D c.1434C>A; p.C478* (3%) PPM1D c.1627dup; p.S543Ffs*9 (2%) DNMT3A c.2396C>A; p.P799H (26%) |
t-CCUS | Deceased |
| #2 | RT 40 years before for lymphoma (nonbony site) | None detected | 3 | Gr2 anemia >6 mo | 10.0 mo | 46,XY[20] | PPM1D c.1433dup; p.C478fs* (19%) | t-CCUS | Deceased |
| #18 | TMZ and RT to bone mets | TET2 c.1454_1457dupTGAA (1.3%) | 2 | None | 13.6 mo | 46,XX[20] | None detected | ICUS v CCUSb | Stable |
| #30 | No | None detected | 4 | Gr1 anemia Gr2 thrombocytopenia |
16.0 mo | 46,XY[20] |
PPM1D c.1608delG; T537Hfs*2 (14%) PPM1D c.1654C>T; R552* (11.7%) |
t-CCUS | Stable |
Abbreviations: BMB, bone marrow biopsy; ICUS, idiopathic cytopenia of undetermined significance; ID, patient identifier; NGS, next generation sequencing; RT, radiotherapy; t-CCUS, therapy related clonal cytopenia of undetermined significance; TMZ, temozolomide.
NGS assay used includes 42 genes; sensitivity is reported as 5%-10% VAF with a minimum depth of 250×.
ID #3 and #18 were classified as ICUS, as on the basis of the time of BMB, no variants were detected. Notably, ID #3 had two DNMT3A variants detected with VAFs of 1.5% and 3.3% and ID #18 had one TET2 variant detected with a VAF of 1.5%. This discrepancy is likely due to variations in sequencing sensitivity.
BMB evaluations were consistent with diagnoses of idiopathic cytopenia of undetermined significance (ICUS) in two patients (ID #3 and #18) as no variants were detected on NGS at the time of BMB. Importantly, both these patients had low-level variants detected pre-PRRT. ID #3 had two pathogenic DNMT3A variants (c.1906G>T, c.2014del) detected pre-PRRT with VAFs of 3.3% and 1.5%; however, post-PRRT, these variants were not detected. ID #18 had one pathogenic variant in TET2 (c.1454_1457dupTGAA) detected pre-PRRT, which was not detected on post-PRRT BMB sequencing. In both cases (ID #3, #18), it is likely that these variants were not detected because of differences in sequencing sensitivities. Three other patients (ID #9, #2, #30) were diagnosed with CCUS (Table 2, Data Supplement, Fig S4), and of those three, NGS revealed five PPM1D pathogenic variants, one of which was detected pre-PRRT (ID #2, c.1433dupG) at a low VAF of 0.80%, whereas the other four variants were not detected pre-PRRT. ID #9 had a DNMT3A variant pre-PRRT (c.2396C>T) with a VAF of 3.9%, and this variant was detected on post-PRRT NGS with a VAF of 26%.
DISCUSSION
In patients with NET treated with 177Lu, reports of t-MN have indicated variable incidence, with rates as high as 20%.9,13-15,19,20,45-47 Previous studies have assessed potential risk factors for t-MN after PRRT; however, there have been no significant associations across different studies.13,14,19 Here, we evaluated CH in patients with NET before receiving 177Lu-DOTATATE and prospectively followed them over time. CH was present in 35% of patients when a VAF cutoff of 2% was used and 46% with a cutoff of 1%. This is consistent with a French study where 46.5% (27 of 58) of patients with NET were found to have CH.45 In addition, a smaller study of 13 patients with NET recently reported a CH prevalence of 62% (8 of 13) with a VAF cutoff of ≥1%.48 This is in comparison with other solid tumors where the prevalence of CH has been reported at 30%.26 It is important to note, however, that in addition to VAF cutoff, the prevalence of these mutations depends on age and method of sequencing, as more sensitive methods can detect mutations at lower frequencies.49
While somatic mutations in the epigenetic modifier genes DNMT3A, TET2, ASXL1 (termed DTA) are most common in the general aging population, patients with a history of cytotoxic chemo and/or RT exposure have an increased incidence of CH in DNA damage response (DDR) genes (PPM1D, ATM2, TP53, CHEK2).26,29,50 Pathogenic mutations in DDR genes are significantly more common in t-MN when compared with primary de novo myeloid neoplasms,50 and previous reports in patients with NET have shown that PRRT exposure led to clonal expansion of these DDR genes.26,45,48 In our study, DTA mutations were most common, in line with general population data. Interestingly, among the patients included in our cohort with post-PRRT CH data available, 60% (3 of 5) developed new PPM1D mutations on bone marrow NGS, consistent with the abovementioned reports of clonal expansion of DDR genes post-PRRT. It remains to be seen whether DDR gene variants are present before PRRT, perhaps at low levels, and are selected for by PRRT leading to clonal expansion during PRRT, or whether PRRTs are inducing new DNA damage leading to these variants. While our data would suggest that these PPM1D mutations are new, given that they were not present in the three patients who seemed to acquire them post-PRRT, differences in sequencing technique and sensitivity are likely contributory. With a median follow-up of 26.0 months, no patients have been diagnosed with t-MN thus far in our study although of the five patients, 3 (60%) were diagnosed with t-CCUS. Prospective evaluation is ongoing in these patients.
Our study highlights the prevalence of CH in patients with NET. Patients with CH do not have a higher risk of developing thrombocytopenia compared with those without CH; however, once thrombocytopenic, patients with CH are less likely to recover and therefore spend more time in a thrombocytopenic state. Such patients are potentially at risk of developing t-MN when they acquire further genetic mutations.26 However, it is important to note that these patients receive multiple chemotherapeutic agents and RT, which have been associated with CH.26 Our data, and the work of others, support the hypothesis of CH as an important risk factor for t-MN in patients with NET planned for PRRT. Further follow-up with longitudinal CH assessment post-PRRT is important to fully elucidate this relationship between CH and t-MN post-PRRT.
Identifying patients at risk of t-MN before PRRT is essential as the prognosis of NET patients is excellent and estimated to be in years. The 5-year follow-up data from the original NETTER-1 trial revealed that 2% of the patients developed refractory cytopenias leading to the diagnosis of t-MN. Importantly, the NETTER-1 study included patients with no previous exposure to other systemic therapies except somatostatin analogs. In addition, primary analysis of the NETTER-2 study has already reported one patient with t-MN (0.7%) compared with none in the SSA arm.8 Recent studies combining PRRT with temozolomide reported ≥ grade 3 hematologic toxicity in up to 31% of patients, and a phase III study is ongoing using this regimen.51-53 Retrospective studies of patients who received both PRRT and capecitabine with temozolomide revealed that 8% developed MDS/AML with a median time to event of 2.8 years.19,54 In addition to combination therapy, others have even recommended the use of poly(ADP-ribose) polymerase (PARP) inhibitors to act as a potentiator of PRRT.55 However, PARP inhibitors have been linked to CH and t-MN56 with a mechanism potentially related to clonal expansion of DDR CH.26 Consequently, the combination of PRRT and PARP inhibitors poses a significant potential risk of t-MN in these patients.
The indications for PRRT are expanding. Recently, the VISION trial revealed the clinical efficacy of PRRT in patients with prostate cancer, leading to its approval.57 To our knowledge, there are no reports of t-MN after PRRT for prostate cancer; however, this may be due to the relatively recent approved indication. The high rates of hematologic toxicity are concerning that long-term follow-up may reveal increased incidence of t-MN especially in the relatively older population with prostate cancer where PARP inhibitors may be indicated.
Given that reports have shown patients who develop t-MN post-PRRT have a dismal prognosis with a reported median OS of only 13 months,58,59 it is imperative that patients who are at risk of t-MN are identified early and counseled on this risk. In this study, we obtained NGS using a comprehensive gene panel with high sensitivity in an unselected group of patients with NET (to our knowledge, the largest prospective sample) with no known previous hematologic malignancies and prospectively followed them during PRRT. Our results indicate that CH may indeed be a risk for hematologic dysfunction during PRRT. However, further studies are needed with larger sample sizes to fully characterize these variants and their impact on hematologic toxicities. Identifying patients who are more likely to experience cytopenias before PRRT may allow for proactive management to avoid treatment delays. In addition, whether bone mets increase the hematologic toxicity risk of PRRT should be explored further. Novel molecular predictors of PRRT activity such as the PRRT predictive quotient (PPQ) have the potential to select the patients most likely to derive benefit from PRRT, and in combinations with predictors of risk, we may ultimately be able to select patients who are likely to benefit from PRRT and have a low risk of severe hematologic complications such as t-MN.60,61
One limitation of our study lies in the relatively short follow-up duration, which may constrain the comprehensive assessment of longer-term outcomes such as t-MN. The median follow-up of 26.0 months provides valuable insights into the immediate effects of PRRT on CH and cytopenias. As such, our study's shorter follow-up duration limits our ability to capture the full spectrum of potential hematologic consequences, particularly those with prolonged latency periods. In addition, the prevalence of CH largely depends on the sequencing method, and therefore, it is possible that low-level variants exist and were not detected in our study. The small sample size limits our analysis to mostly descriptive, and all conclusions should be interpreted as exploratory and discovery in nature. Large-scale prospective studies with longer follow-up duration are warranted.
In conclusion, we have shown that among patients with NET, the prevalence of CH (VAF ≥2%) is 35%, with DTA gene variants being most common. Patients with CH had lower platelet counts at baseline compared with patients without CH and remained thrombocytopenic longer on average. In addition, we identified DDR genes in 3 of 5 patients with post-PRRT samples available. With rates of t-MN post-PRRT being reported as high as 20%, long-term follow-up is essential.
PRIOR PRESENTATION
Presented at the AACR, Orlando, FL, April 14-19, 2023; the NANETS, Montreal, Canada, October 4-6, 2023.
SUPPORT
Supported in part by the Kemper and Ethel Marley Foundation, the Daniel J. Sargent, PhD, Career Development Award in Cancer Research (FSO).
F.-S.O. and M.B.S. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Yael Kusne, Christy Finke, Tanios Bekaii-Saab, Thorvardur R. Halfdanarson, Mrinal M. Patnaik, Mohamad Bassam Sonbol
Provision of study materials or patients: Thorvardur R. Halfdanarson
Collection and assembly of data: Yael Kusne, Terra Lasho, Christy Finke, Zaid Elsabbagh, Thorvardur R. Halfdanarson, Mrinal M. Patnaik, Mohamad Bassam Sonbol
Data analysis and interpretation: Yael Kusne, Terra Lasho, Christy Finke, Shaylene McCue, Timothy Hobday, Jason Starr, Tanios Bekaii-Saab, Thorvardur R. Halfdanarson, Mrinal M. Patnaik, Fang-Shu Ou, Mohamad Bassam Sonbol
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christy Finke
Employment: Mayo Clinic
Timothy Hobday
Research Funding: Novartis (Inst)
Jason Starr
Consulting or Advisory Role: Natera, Ipsen, Pfizer, Taiho Oncology, Tersera, Tempus, Helsinn Therapeutics, Cancer Expert Now
Research Funding: Amgen (Inst), Camurus (Inst), Arcus Biosciences (Inst), RayzeBio (Inst), Aminex (Inst), Cardiff Oncology (Inst), Aprea Therapeutics (Inst)
Travel, Accommodations, Expenses: Camurus
Tanios Bekaii-Saab
Consulting or Advisory Role: Amgen (Inst), Ipsen (Inst), Lilly (Inst), Bayer (Inst), Roche/Genentech (Inst), Abbvie, Incyte (Inst), Immuneering, Seagen (Inst), Pfizer (Inst), Boehringer Ingelheim, Janssen, Eisai, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, SOBI, BeiGene, Foundation Medicine, Arcus Biosciences (Inst), Stemline Therapeutics, Kanaph Therapeutics, Deciphera, Illumina, Foundation Medicine, Caladrius Biosciences, Caladrius Biosciences, Zai Lab
Patents, Royalties, Other Intellectual Property: Patent WO/201083488, Patent WO/2019/055687
Other Relationship: Exelixis, Merck (Inst), AstraZeneca, Lilly, Pancreatic Cancer Action Network, FibroGen, Suzhou Kintor Pharmaceuticals, 1Globe Health Institute, Imugene, Xilis, Replimune, Sun Biopharma, UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/636276
Thorvardur R. Halfdanarson
Consulting or Advisory Role: Ipsen (Inst), Advanced Accelerator Applications (Inst), Tersera, Crinetics Pharmaceuticals (Inst), ITM Isotope Technologies Munich (Inst), Viewpoint Molecular Targeting (Inst), Camurus (Inst)
Research Funding: Thermo Fisher Scientific (Inst), Turnstone Bio (Inst), Advanced Accelerator Applications (Inst), Novartis (Inst), ITM Isotope Technologies Munich (Inst), Camurus (Inst), Crinetics Pharmaceuticals (Inst), Perspective Therapeutics (Inst)
Uncompensated Relationships: North American Neuroendocrine Tumor Society
Mrinal M. Patnaik
Consulting or Advisory Role: Kura Oncology (Inst)
Research Funding: Stemline Therapeutics (Inst), Epigenetix (Inst), Solutherapeutics (Inst), Kura Oncology (Inst), Polaris (Inst)
Mohamad Bassam Sonbol
Honoraria: Novartis
Research Funding: Lilly (Inst), Taiho Oncology (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dasari A, Shen C, Halperin D, et al. : Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3:1335-1342, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panzuto F, Nasoni S, Falconi M, et al. : Prognostic factors and survival in endocrine tumor patients: Comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 12:1083-1092, 2005 [DOI] [PubMed] [Google Scholar]
- 3.White BE, Rous B, Chandrakumaran K, et al. : Incidence and survival of neuroendocrine neoplasia in England 1995-2018: A retrospective, population-based study. Lancet Reg Health Eur 23:100510, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplin ME, Pavel M, Ruszniewski P: Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:1556-1557, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Hennrich U, Kopka K: Lutathera®: The first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals (Basel) 12:114, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strosberg J, El-Haddad G, Wolin E, et al. : Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 376:125-135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strosberg JR, Caplin ME, Kunz PL, et al. : (177)Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol 22:1752-1763, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Ferone D, Halperin D, Myrehaug S, et al. : [177Lu]Lu-DOTA-TATE in newly diagnosed patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors: Primary analysis of the phase 3 randomized NETTER-2 study. J Clin Oncol 42, 2024. (suppl 3; abstr LBA588) [DOI] [PubMed] [Google Scholar]
- 9.Sabet A, Ezziddin K, Pape UF, et al. : Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 54:1857-1861, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Bergsma H, van Lom K, Raaijmakers M, et al. : Persistent hematologic dysfunction after peptide receptor radionuclide therapy with (177)Lu-DOTATATE: Incidence, course, and predicting factors in patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med 59:452-458, 2018 [DOI] [PubMed] [Google Scholar]
- 11.van der Zwan WA, Brabander T, Kam BLR, et al. : Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 46:704-717, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yordanova A, Mayer K, Brossart P, et al. : Safety of multiple repeated cycles of (177)Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur J Nucl Med Mol Imaging 44:1207-1214, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Brieau B, Hentic O, Lebtahi R, et al. : High risk of myelodysplastic syndrome and acute myeloid leukemia after 177Lu-octreotate PRRT in NET patients heavily pretreated with alkylating chemotherapy. Endocr Relat Cancer 23:L17-L23, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Sonbol MB, Halfdanarson TR, Hilal T: Assessment of therapy-related myeloid neoplasms in patients with neuroendocrine tumors after peptide receptor radionuclide therapy: A systematic review. JAMA Oncol 6:1086-1092, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Bodei L, Kidd M, Paganelli G, et al. : Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 42:5-19, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Imhof A, Brunner P, Marincek N, et al. : Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 29:2416-2423, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kwekkeboom DJ, de Herder WW, Kam BL, et al. : Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J Clin Oncol 26:2124-2130, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kesavan M, Claringbold PG, Turner JH: Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology 99:108-117, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Al-Toubah TE, Pelle E, Strosberg JR: Risk of myelodysplastic syndrome/acute leukemia with sequential capecitabine/temozolomide and 177Lu-dotatate. J Clin Oncol 40:513, 2022 [Google Scholar]
- 20.Pritzl SL, KusneHalfdanarson YTR, Hobday T, et al. : Spectrum of therapy-related clonal cytopenias and neoplasms after exposure to Lutetium-177-Spectrum of therapy-related clonal cytopenias and neoplasms after exposure to Lutetium-177-Dotatate. Leuk Res 136:107434, 2024 [DOI] [PubMed] [Google Scholar]
- 21.Fianchi L, Criscuolo M, Fabiani E, et al. : Therapy-related myeloid neoplasms: Clinical perspectives. Onco Targets Ther 11:5909-5915, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuendgen A, Nomdedeu M, Tuechler H, et al. : Therapy-related myelodysplastic syndromes deserve specific diagnostic sub-classification and risk-stratification-an approach to classification of patients with t-MDS. Leukemia 35:835-849, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeZern AE, Malcovati L, Ebert BL: CHIP, CCUS, and other acronyms: Definition, implications, and impact on practice. Am Soc Clin Oncol Educ Book 39:400-410, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Shah MV, Mangaonkar AA, Begna KH, et al. : Therapy-related clonal cytopenia as a precursor to therapy-related myeloid neoplasms. Blood Cancer J 12:106, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MM, Baranwal A, Gurney M, et al. : The impact of cytotoxic therapy on the risk of progression and death in clonal cytopenia(s) of undetermined significance. Blood Adv:bloodadvances.2023012357, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolton KL, Ptashkin RN, Gao T, et al. : Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 52:1219-1226, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabre MA, de Almeida JG, Fiorillo E, et al. : The longitudinal dynamics and natural history of clonal haematopoiesis. Nature 606:335-342, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren JT, Link DC: Clonal hematopoiesis and risk for hematologic malignancy. Blood 136:1599-1605, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coombs CC, Zehir A, Devlin SM, et al. : Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 21:374-382.e4, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamond B, Ziccheddu B, Maclachlan K, et al. : Tracking the evolution of therapy-related myeloid neoplasms using chemotherapy signatures. Blood 141:2359-2371, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soerensen JF, Aggerholm A, Rosenberg CA, et al. : Clonal evolution in patients developing therapy-related myeloid neoplasms following autologous stem cell transplantation. Bone Marrow Transpl 57:460-465, 2022 [DOI] [PubMed] [Google Scholar]
- 32.Weeks LD, Niroula A, Neuberg D, et al. : Prediction of risk for myeloid malignancy in clonal hematopoiesis. NEJM Evid 2, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel AA, Rojek AE, Drazer MW, et al. : Therapy-related myeloid neoplasms in 109 patients after radiation monotherapy. Blood Adv 5:4140-4148, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arber DA, Orazi A, Hasserjian RP, et al. : International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 140:1200-1228, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury JD, Solary E, Abla O, et al. : The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 36:1703-1719, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goncalves I, Burbury K, Westerman D, et al. : Characteristics and outcomes of therapy-related myeloid neoplasms after peptide receptor radionuclide therapy (PRRT) for metastatic neuroendocrine neoplasm (NEN): A single centre series. Neuroendocrinology 108:212, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Carr RM, Vorobyev D, Lasho T, et al. : RAS mutations drive proliferative chronic myelomonocytic leukemia via a KMT2A-PLK1 axis. Nat Commun 12:2901, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Z, Lasho T, Khurana A, et al. : Prognostic relevance of clonal hematopoiesis in myeloid neoplastic transformation in patients with follicular lymphoma treated with radioimmunotherapy. Haematologica 109:509-520, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, Binder M, Lasho T, et al. : Clinical, molecular, and prognostic comparisons between CCUS and lower-risk MDS: A study of 187 molecularly annotated patients. Blood Adv 5:2272-2278, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrer A, Lasho T, Fernandez JA, et al. : Patients with telomere biology disorders show context specific somatic mosaic states with high frequency of U2AF1 variants. Am J Hematol 98:E357-E359, 2023 [DOI] [PubMed] [Google Scholar]
- 41.Putter H, Fiocco M, Geskus RB: Tutorial in biostatistics: Competing risks and multi-state models. Stat Med 26:2389-2430, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Aalen OO, Johansen S: An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat 5:141-150, 1978 [Google Scholar]
- 43.Kraft IL, Godley LA: Identifying potential germline variants from sequencing hematopoietic malignancies. Hematol Am Soc Hematol Educ Program 2020:219-227, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li MM, Datto M, Duncavage EJ, et al. : Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 19:4-23, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Ferkh R, Hadoux J, Lamartina L, et al. : 888MO Emergence of clonal hematopoiesis after peptide receptor radionuclide therapy for neuroendocrine tumors. Ann Oncol 33:S954-S955, 2022 [Google Scholar]
- 46.Brabander T, van der Zwan WA, Teunissen JJM, et al. : Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 23:4617-4624, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Baum RP, Kulkarni HR, Singh A, et al. : Results and adverse events of personalized peptide receptor radionuclide therapy with (90)Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 9:16932-16950, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh A, Mencia-Trinchant N, Griffiths EA, et al. : Mutant PPM1D- and TP53-driven hematopoiesis populates the hematopoietic compartment in response to peptide receptor radionuclide therapy. JCO Precis Oncol 6:e2100309, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal S, Fontanillas P, Flannick J, et al. : Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371:2488-2498, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu JI, Dayaram T, Tovy A, et al. : PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell 23:700-713.e6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puranik AD, Rangarajan V, Ramaswamy A, et al. : PReCedeNT Trial: Phase III Randomised Controlled Trial of PRRT with Lutetium – 177 DOTATATE Plus Chemotherapy vs PRRT Alone in FDG-Avid, Well-Differentiated Gastro-Entero-Pancreatic Neuroendocrine Tumors. Endocrine Abstracts 89, 2022 (abstr 21441) [Google Scholar]
- 52.Pavlakis N, Ransom DT, Wyld D, et al. : Australasian Gastrointestinal Trials Group (AGITG) CONTROL NET study: 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) and capecitabine plus temozolomide (CAPTEM) for pancreas and midgut neuroendocrine tumours (pNETS, mNETS)—Final results. J Clin Oncol 40:4122, 2022 [Google Scholar]
- 53.Nicolini S, Bodei L, Bongiovanni A, et al. : Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging 48:3260-3267, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kesavan M, Grover P, Lam WS, et al. : Long-term hematologic toxicity of 177Lu-octreotate-capecitabine-temozolomide therapy of GEPNET. Endocr Relat Cancer 28:521-527, 2021 [DOI] [PubMed] [Google Scholar]
- 55.Purohit NK, Shah RG, Adant S, et al. : Potentiation of (177)Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget 9:24693-24706, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marmouset D, Haseny B, Dukan R, et al. : Characteristics, survivals and risk factors of surgical site infections after en bloc sacrectomy for primary malignant sacral tumors at a single center. Orthop Traumatol Surg Res 108:103197, 2022 [DOI] [PubMed] [Google Scholar]
- 57.Sartor O, de Bono J, Chi KN, et al. : Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 385:1091-1103, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goncalves I, Burbury K, Michael M, et al. : Characteristics and outcomes of therapy-related myeloid neoplasms after peptide receptor radionuclide/chemoradionuclide therapy (PRRT/PRCRT) for metastatic neuroendocrine neoplasia: A single-institution series. Eur J Nucl Med Mol Imaging 46:1902-1910, 2019 [DOI] [PubMed] [Google Scholar]
- 59.Chantadisai M, Kulkarni HR, Baum RP: Therapy-related myeloid neoplasm after peptide receptor radionuclide therapy (PRRT) in 1631 patients from our 20 years of experiences: Prognostic parameters and overall survival. Eur J Nucl Med Mol Imaging 48:1390-1398, 2021 [DOI] [PubMed] [Google Scholar]
- 60.Das S, Chauhan A, Du L, et al. : External validation of a clinical score for patients with neuroendocrine tumors under consideration for peptide receptor radionuclide therapy. JAMA Netw Open 5:e2144170, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bodei L, Raj N, Do RK, et al. : Interim analysis of a prospective validation of 2 blood-based genomic assessments (PPQ and NETest) to determine the clinical efficacy of (177)Lu-DOTATATE in neuroendocrine tumors. J Nucl Med 64:567-573, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]