Abstract
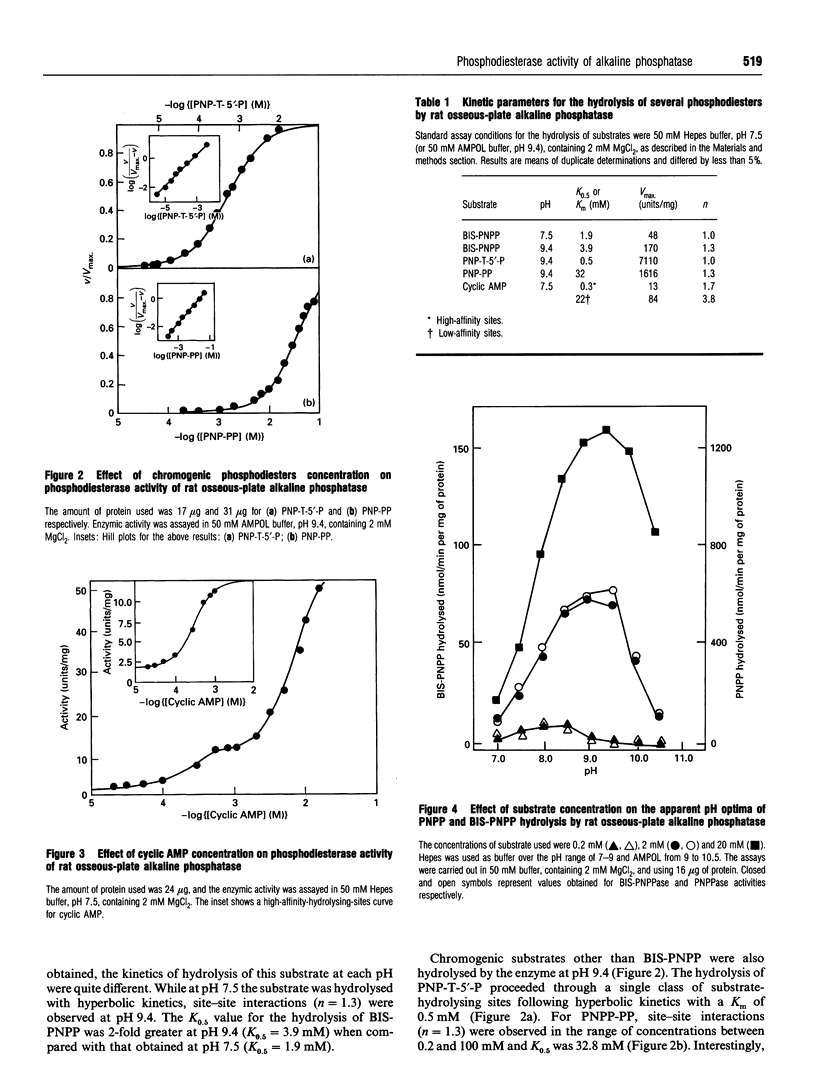
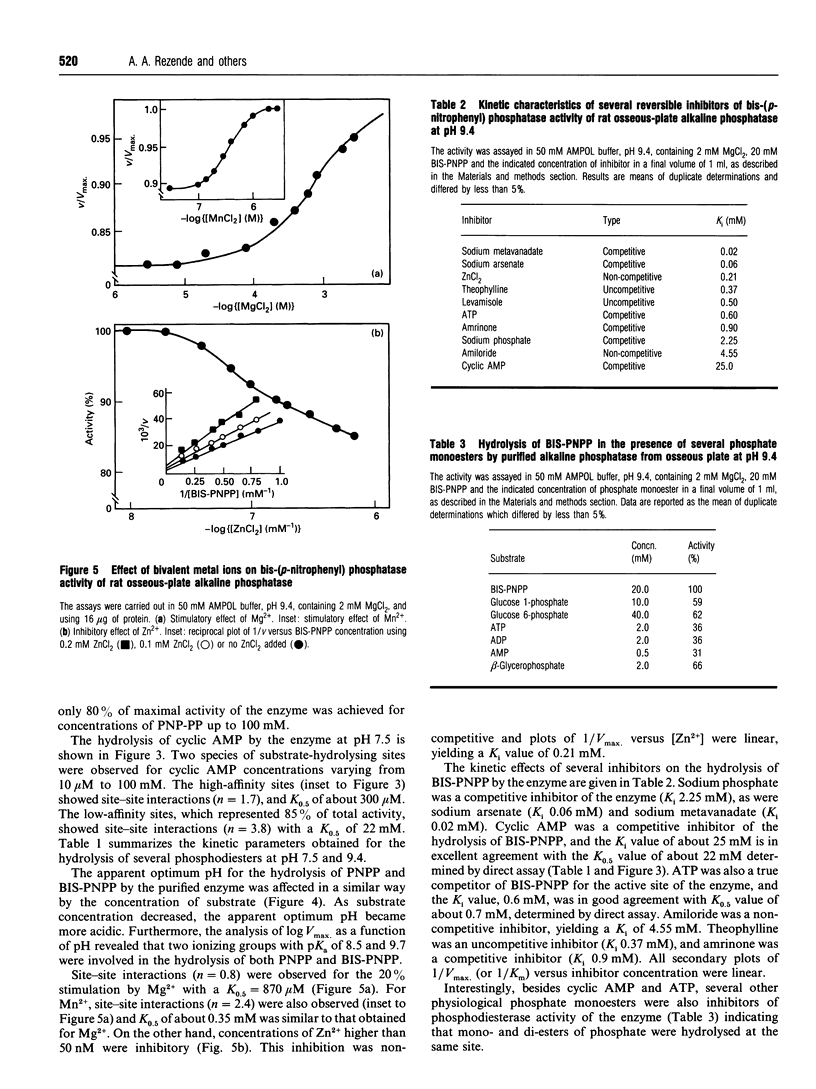
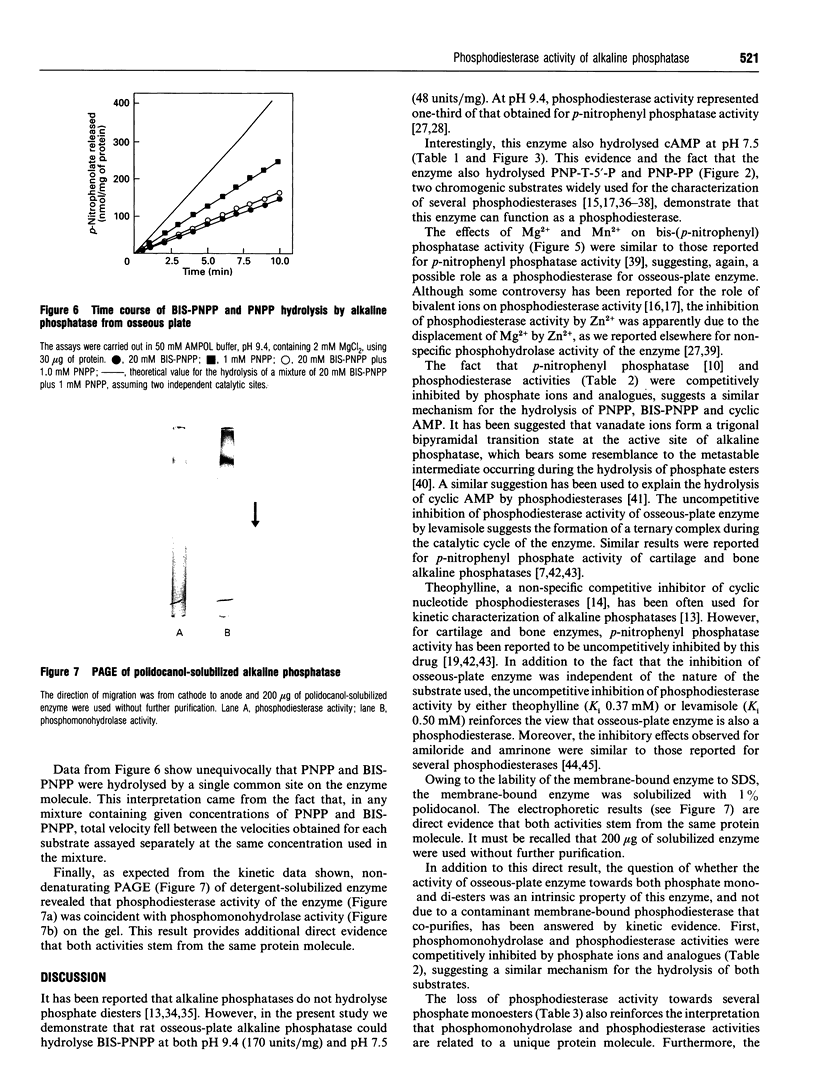
Phosphodiesterase activity is a novel property of the still-enigmatic alkaline phosphatase from osseous plate. Bis-(p-nitrophenyl) phosphate was hydrolysed at both pH 7.5 and 9.4 with an apparent dissociation constant (K0.5) of 1.9 mM and 3.9 mM respectively. The hydrolysis of p-nitrophenyl-5'-thymidine phosphate followed hyberbolic kinetics with a K0.5 of 500 microM. For p-nitrophenyl phenylphosphonate, site-site interactions [Hill coefficient (h) = 1.3] were observed in the range between 0.2 and 100 microM, and K0.5 was 32.8 mM. The hydrolysis of cyclic AMP by the enzyme followed more complex kinetics, showing site-site interactions (h = 1.7) and K0.5 = 300 microM for high-affinity sites. The low-affinity sites, representing 85% of total activity, also showed site-site interactions (h = 3.8) and a K0.5 of about 22 mM. ATP and cyclic AMP were competitive inhibitors of bis-(p-nitrophenyl) phosphatase activity of the enzyme and Ki values (25 mM and 0.6 mM for cyclic AMP and ATP respectively) very close to those of the K0.5 (22 mM and 0.7 mM for cyclic AMP and ATP respectively), determined by direct assay, indicated that a single catalytic site was responsible for the hydrolysis of both substrates. Non-denaturing PAGE of detergent-solubilized enzyme showed coincident bands on the gel for phosphomonohydrolase and phosphodiesterase activities. Additional evidence for a single catalytic site was the similar pKa values (8.5 and 9.7) found for the two ionizing groups participating in the hydrolysis of bis-(p-nitrophenyl) phosphate and p-nitrophenyl phosphate. The alkaline apparent pH optima, the requirement for bivalent metal ions and the inhibition by methylxanthines, amrinone and amiloride demonstrated that rat osseous-plate alkaline phosphatase was a type I phosphodiesterase. Considering that there is still confusion as to which is the physiological substrate for the enzyme, the present results describing a novel property for this enzyme could be of relevance in understanding the mineralization process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Mechanism of mineral formation in bone. Lab Invest. 1989 Mar;60(3):320–330. [PubMed] [Google Scholar]
- Arsenis C., Rudolph J., Hackett M. H. Resolution, purification and characterization of the orthophosphate releasing activities from fracture callus calcifying cartilage. Biochim Biophys Acta. 1975 Jun 24;391(2):301–315. doi: 10.1016/0005-2744(75)90254-5. [DOI] [PubMed] [Google Scholar]
- Blumenthal N. C. Mechanisms of inhibition of calcification. Clin Orthop Relat Res. 1989 Oct;(247):279–289. [PubMed] [Google Scholar]
- Carpenedo F., Debetto P., Floreani M., Guarnieri A., Luciani S. Inhibition of cardiac phosphodiesterases by amiloride and its N-chlorobenzyl analogues. Biochem Pharmacol. 1987 Mar 1;36(5):778–780. doi: 10.1016/0006-2952(87)90736-2. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Ciancaglini P., Pizauro J. M., Leone F. A. Polyoxyethylene 9-lauryl ether-solubilized alkaline phosphatase: synergistic stimulation by zinc and magnesium ions. Int J Biochem. 1992 Apr;24(4):611–615. doi: 10.1016/0020-711x(92)90335-x. [DOI] [PubMed] [Google Scholar]
- Ciancaglini P., Pizauro J. M., Rezende A. A., Rezende L. A., Leone F. A. Solubilization of membrane-bound matrix-induced alkaline phosphatase with polyoxyethylene 9-lauryl ether (polidocanol): purification and metalloenzyme properties. Int J Biochem. 1990;22(4):385–392. doi: 10.1016/0020-711x(90)90141-o. [DOI] [PubMed] [Google Scholar]
- Curti C., Pizauro J. M., Ciancaglini P., Leone F. A. Kinetic characteristics of some inhibitors of matrix-induced alkaline phosphatase. Cell Mol Biol. 1987;33(5):625–635. [PubMed] [Google Scholar]
- Curti C., Pizauro J. M., Rossinholi G., Vugman I., Mello de Oliveira J. A., Leone F. A. Isolation and kinetic properties of an alkaline phosphatase from rat bone matrix-induced cartilage. Cell Mol Biol. 1986;32(1):55–62. [PubMed] [Google Scholar]
- Cyboron G. W., Vejins M. S., Wuthier R. E. Activity of epiphyseal cartilage membrane alkaline phosphatase and the effects of its inhibitors at physiological pH. J Biol Chem. 1982 Apr 25;257(8):4141–4146. [PubMed] [Google Scholar]
- Cyboron G. W., Wuthier R. E. Purification and initial characterization of intrinsic membrane-bound alkaline phosphatase from chicken epiphyseal cartilage. J Biol Chem. 1981 Jul 25;256(14):7262–7268. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Erhardt P. W., Chou Y. L. A topographical model for the c-AMP phosphodiesterase III active site. Life Sci. 1991;49(8):553–568. doi: 10.1016/0024-3205(91)90254-9. [DOI] [PubMed] [Google Scholar]
- Farley J. R., Ivey J. L., Baylink D. J. Human skeletal alkaline phosphatase. Kinetic studies including pH dependence and inhibition by theophylline. J Biol Chem. 1980 May 25;255(10):4680–4686. [PubMed] [Google Scholar]
- Felix R., Fleisch H. Pyrophosphatase and ATPase of isolated cartilage matrix vesicles. Calcif Tissue Res. 1976 Nov 24;22(1):1–7. doi: 10.1007/BF02010340. [DOI] [PubMed] [Google Scholar]
- Fortuna R., Anderson H. C., Carty R. P., Sajdera S. W. Enzymatic characterization of the matrix vesicle alkaline phosphatase isolated from bovine fetal epiphyseal cartilage. Calcif Tissue Int. 1980;30(3):217–225. doi: 10.1007/BF02408631. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Rogers C., Harris H. A search for trace expression of placental-like alkaline phosphatase in non-malignant human tissues: demonstration of its occurrence in lung, cervix, testis and thymus. Clin Chim Acta. 1982 Oct 13;125(1):63–75. doi: 10.1016/0009-8981(82)90046-8. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hawley D. M., Hodes M. Z., Crisp M., Ellis G., Karn R. C., Hodes M. E. Characterization of phosphohydrolase activity in bovine spleen extracts: identification of a bis(p-nitrophenyl)phosphate-hydrolyzing activity (phosphodiesterase IV) which also acts on adenosine triphosphate. Anal Biochem. 1985 Dec;151(2):375–380. doi: 10.1016/0003-2697(85)90191-5. [DOI] [PubMed] [Google Scholar]
- Heinonen J. K., Lahti R. J. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem. 1981 May 15;113(2):313–317. doi: 10.1016/0003-2697(81)90082-8. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Ikeda E., Kashimata M., Minami N., Kodama H., Sudo H., Kumegawa M. Effect of dibutyryl cyclic AMP on collagen synthesis in a clonal osteoblast-like cell line derived from newborn mouse calvaria. J Biochem. 1983 Nov;94(5):1353–1358. doi: 10.1093/oxfordjournals.jbchem.a134481. [DOI] [PubMed] [Google Scholar]
- Hsu H. H., Munoz P. A., Barr J., Oppliger I., Morris D. C., Vaananen H. K., Tarkenton N., Anderson H. C. Purification and partial characterization of alkaline phosphatase of matrix vesicles from fetal bovine epiphyseal cartilage. Purification by monoclonal antibody affinity chromatography. J Biol Chem. 1985 Feb 10;260(3):1826–1831. [PubMed] [Google Scholar]
- Ito K., Yamamoto T., Minamiura N. Phosphodiesterase I in human urine: purification and characterization of the enzyme. J Biochem. 1987 Aug;102(2):359–367. doi: 10.1093/oxfordjournals.jbchem.a122062. [DOI] [PubMed] [Google Scholar]
- Ito M., Tanaka T., Saitoh M., Masuoka H., Nakano T., Hidaka H. Selective inhibition of cyclic AMP phosphodiesterase from various human tissues by milrinone, a potent cardiac bipyridine. Biochem Pharmacol. 1988 May 15;37(10):2041–2044. doi: 10.1016/0006-2952(88)90554-0. [DOI] [PubMed] [Google Scholar]
- Kato Y., Shimazu A., Nakashima K., Suzuki F., Jikko A., Iwamoto M. Effects of parathyroid hormone and calcitonin on alkaline phosphatase activity and matrix calcification in rabbit growth-plate chondrocyte cultures. Endocrinology. 1990 Jul;127(1):114–118. doi: 10.1210/endo-127-1-114. [DOI] [PubMed] [Google Scholar]
- Keleti T., Leoncini R., Pagani R., Marinello E. A kinetic method for distinguishing whether an enzyme has one or two active sites for two different substrates. Rat liver L-threonine dehydratase has a single active site for threonine and serine. Eur J Biochem. 1987 Dec 30;170(1-2):179–183. doi: 10.1111/j.1432-1033.1987.tb13684.x. [DOI] [PubMed] [Google Scholar]
- Kelly S. J., Butler L. G. Enzymic hydrolysis of phosphonate esters. Reaction mechanism of intestinal 5'-nucleotide phosphodiesterase. Biochemistry. 1977 Mar 22;16(6):1102–1104. doi: 10.1021/bi00625a011. [DOI] [PubMed] [Google Scholar]
- Klem K. H., Jablonski G., Saether O., Jarosz G., Gautvik K. M., Gordeladze J. O. 1,25-dihydroxyvitamin D-3 and 24,25-dihydroxyvitamin D-3 affect parathormone (PTH) -sensitive adenylate cyclase activity and alkaline phosphatase secretion of osteoblastic cells through different mechanisms of action. Biochim Biophys Acta. 1990 Sep 24;1054(3):304–310. doi: 10.1016/0167-4889(90)90101-i. [DOI] [PubMed] [Google Scholar]
- Landt M., Everard R. A., Butler L. G. 5'-Nucleotide phosphodiesterase: features of the substrate binding site as deduced from specificity and kinetics of some novel substrates. Biochemistry. 1980 Jan 8;19(1):138–143. doi: 10.1021/bi00542a021. [DOI] [PubMed] [Google Scholar]
- Leone F. A., Pizauro J. M., Ciancaglini P. Effect of pH on the modulation of rat osseous plate alkaline phosphatase by metal ions. Int J Biochem. 1992 Jun;24(6):923–928. doi: 10.1016/0020-711x(92)90098-l. [DOI] [PubMed] [Google Scholar]
- Londesborough J. Evidence that the peripheral cyclic AMP phosphodiesterase of rat liver plasma membranes is a metalloenzyme. Biochem J. 1985 Jan 1;225(1):143–147. doi: 10.1042/bj2250143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez V., Stevens T., Lindquist R. N. Vanadium ion inhibition of alkaline phosphatase-catalyzed phosphate ester hydrolysis. Arch Biochem Biophys. 1976 Jul;175(1):31–38. doi: 10.1016/0003-9861(76)90482-3. [DOI] [PubMed] [Google Scholar]
- Lowe M., Strauss A. W., Alpers R., Seetharam S., Alpers D. H. Molecular cloning and expression of a cDNA encoding the membrane-associated rat intestinal alkaline phosphatase. Biochim Biophys Acta. 1990 Feb 9;1037(2):170–177. doi: 10.1016/0167-4838(90)90164-b. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Wuthier R. E. Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim Biophys Acta. 1975 May 23;391(1):51–60. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- Mori N., Nikai T., Sugihara H. Phosphodiesterase from the venom of Crotalus ruber ruber. Int J Biochem. 1987;19(2):115–119. doi: 10.1016/0020-711x(87)90321-1. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Burger E. H., Feyen J. H. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986 Oct;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Klahr S. Cyclic nucleotides in bone and mineral metabolism. Adv Cyclic Nucleotide Res. 1979;11:89–130. [PubMed] [Google Scholar]
- Pizauro J. M., Ciancaglini P., Leone F. A. Allosteric modulation by ATP, calcium and magnesium ions of rat osseous plate alkaline phosphatase. Biochim Biophys Acta. 1993 Sep 3;1202(1):22–28. doi: 10.1016/0167-4838(93)90058-y. [DOI] [PubMed] [Google Scholar]
- Pizauro J. M., Curti C., Ciancaglini P., Leone F. A. Triton X-100 solubilized bone matrix-induced alkaline phosphatase. Comp Biochem Physiol B. 1987;87(4):921–926. doi: 10.1016/0305-0491(87)90413-5. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Matsui Y., Hinek A., Lee E. R. Cartilage macromolecules and the calcification of cartilage matrix. Anat Rec. 1989 Jun;224(2):167–179. doi: 10.1002/ar.1092240207. [DOI] [PubMed] [Google Scholar]
- Sivanaesan L., Kwan T. K., Perumal R. The activation of chick alkaline phosphatase by calmodulin. Biochem Int. 1991 Oct;25(3):561–570. [PubMed] [Google Scholar]
- Tipton K. F., Dixon H. B. Effects of pH on enzymes. Methods Enzymol. 1979;63:183–234. doi: 10.1016/0076-6879(79)63011-2. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Ray K., Henthorn P. S., Lamb B., Kadesch T., Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988 Aug 25;263(24):12002–12010. [PubMed] [Google Scholar]
- Yamane K., Maruo B. Purification and characterization of extracellular soluble and membrane-bound insoluble alkaline phosphatases possessing phosphodiesterase activities in Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):100–107. doi: 10.1128/jb.134.1.100-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



