Abstract
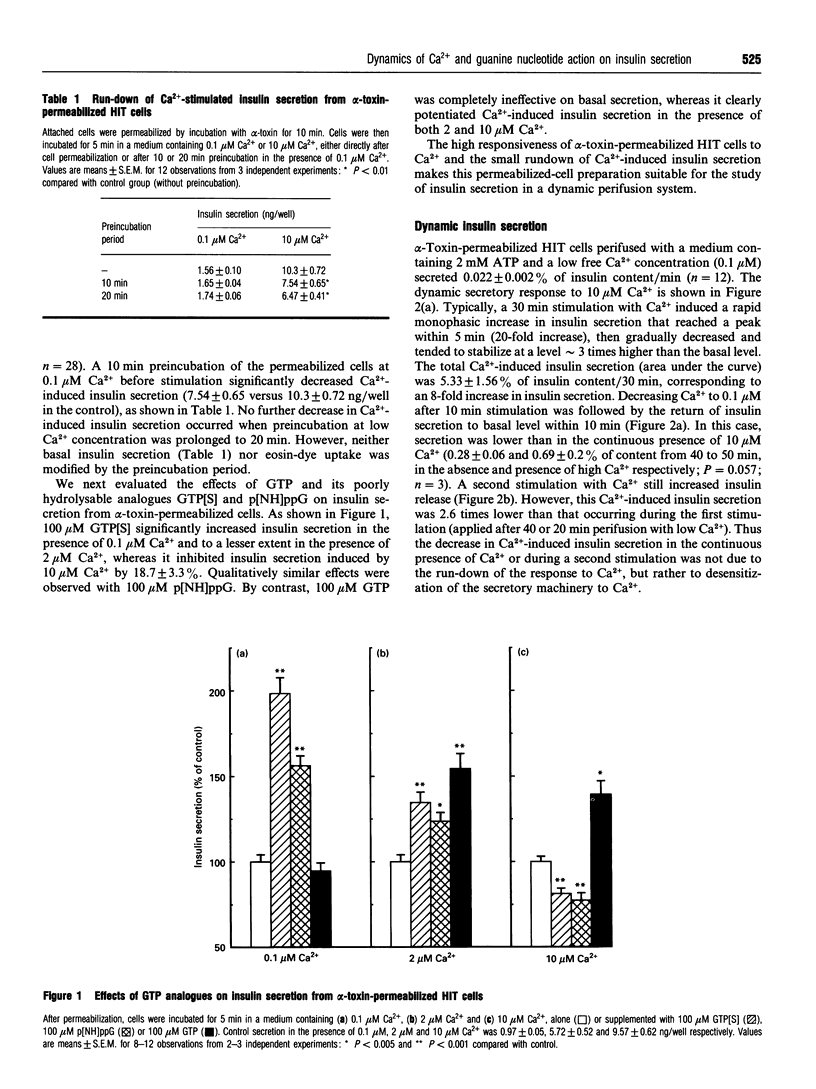
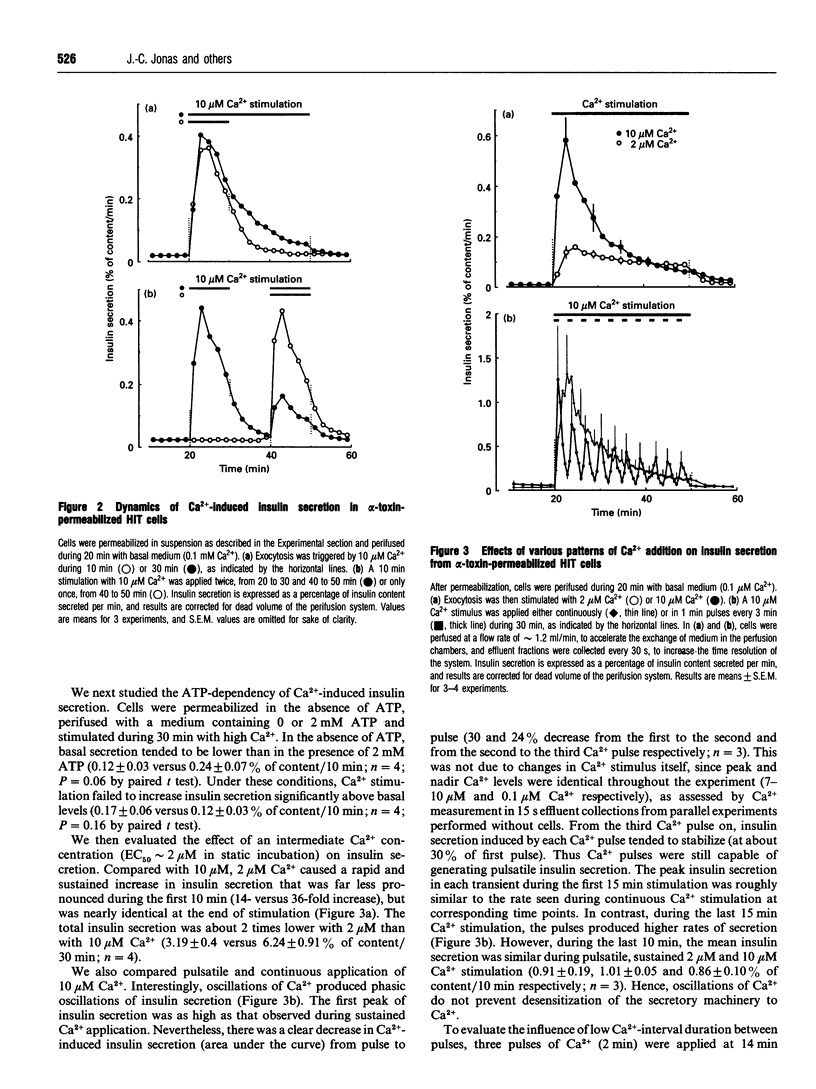
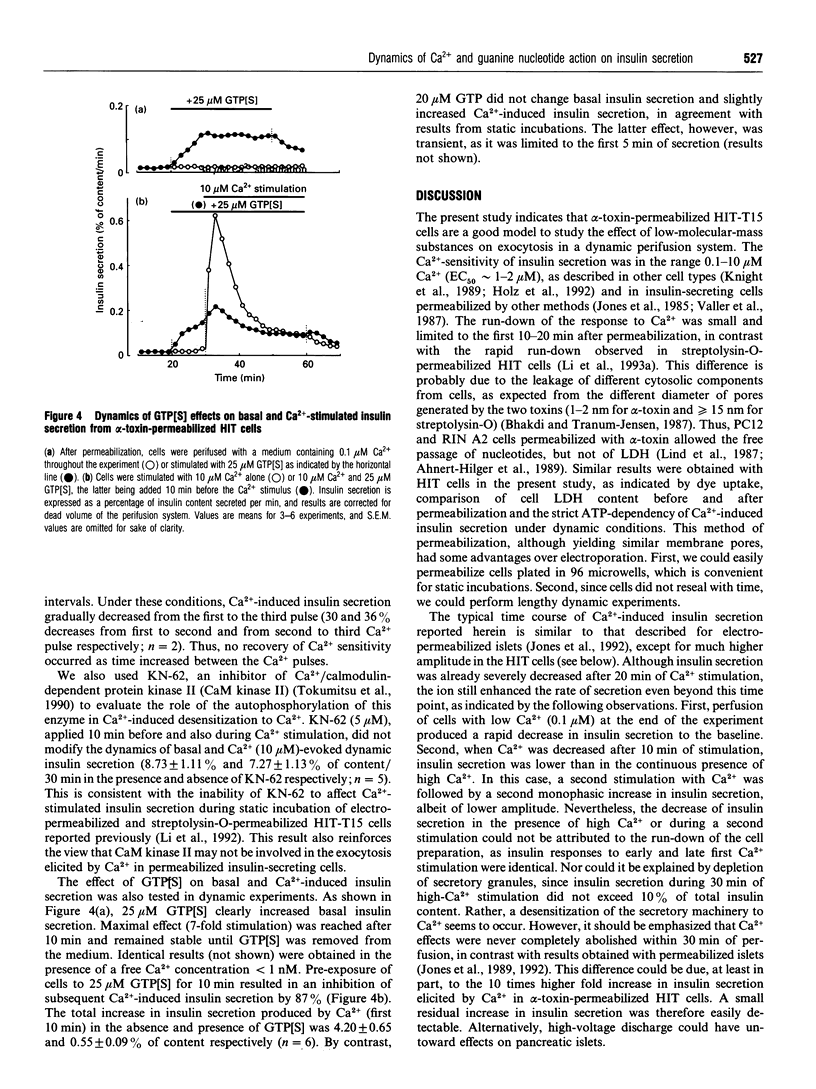
The time course of Ca2+ and GTP-analogue effects on insulin secretion was investigated in HIT-T15 cells permeabilized with Staphylococcus alpha-toxin. These cells responded to Ca2+ in the range 0.1-10 microM and could be used in a dynamic perifusion system because of the minimal run-down of the secretory response. High Ca2+ (10 microM) elicited a monophasic ATP-dependent stimulation of insulin secretion that reached a peak within 5 min (approximately 20-fold increase) and rapidly decreased during the subsequent 15 min to a plateau remaining above basal rates (0.1 microM Ca2+). The decrease in Ca(2+)-induced insulin secretion with time could not be attributed to decreased capacity to respond to Ca2+ after prolonged perfusion at low Ca2+ (run-down), nor to depletion of a particular secretory-granule pool. It was rather due to desensitization of the secretory machinery to Ca2+ that was not reversed by selective inhibition of the Ca2+/calmodulin-dependent kinase II with KN-62. However, an intermediate Ca2+ concentration (2 microM) increased insulin secretion to stable level without causing any desensitization. Imposed oscillations of Ca2+ (0.1-10 microM) produced phasic oscillations of insulin secretion, but did not prevent desensitization to Ca2+. Poorly hydrolysable GTP analogues increased insulin secretion at low Ca2+, whereas they strongly inhibited Ca(2+)-induced insulin secretion. By contrast, GTP did not affect basal secretion, and slightly increased Ca(2+)-evoked secretion. These results indicate the following. (1) Oscillations of insulin secretion are tightly coupled to cytosolic Ca2+ oscillations. (2) Oscillations of Ca2+ do not prevent high-Ca(2+)-induced desensitization to Ca2+; this result does not support the idea of a greater efficiency of oscillations compared with sustained Ca2+ rises in triggering exocytosis. (3) Activation of G-proteins modulates exocytosis in a bimodal manner.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bhakdi S., Gratzl M. Minimal requirements for exocytosis. A study using PC 12 cells permeabilized with staphylococcal alpha-toxin. J Biol Chem. 1985 Oct 15;260(23):12730–12734. [PubMed] [Google Scholar]
- Ahnert-Hilger G., Dayanithi G., Spicher K., Nordmann J. J. G-proteins mediate inhibition and activation of Ca(2+)-induced exocytosis from SLO-permeabilized peptidergic nerve endings. Biosci Rep. 1992 Dec;12(6):463–469. doi: 10.1007/BF01122034. [DOI] [PubMed] [Google Scholar]
- Ahnert-Hilger G., Mach W., Föhr K. J., Gratzl M. Poration by alpha-toxin and streptolysin O: an approach to analyze intracellular processes. Methods Cell Biol. 1989;31:63–90. doi: 10.1016/s0091-679x(08)61602-7. [DOI] [PubMed] [Google Scholar]
- Ahnert-Hilger G., Wegenhorst U., Stecher B., Spicher K., Rosenthal W., Gratz M. Exocytosis from permeabilized bovine adrenal chromaffin cells is differently modulated by guanosine 5'-[gamma-thio]triphosphate and guanosine 5'-[beta gamma-imido]triphosphate. Evidence for the involvement of various guanine nucleotide-binding proteins. Biochem J. 1992 Jun 1;284(Pt 2):321–326. doi: 10.1042/bj2840321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F. M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993 Dec;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Bergsten P., Hellman B. Glucose-induced amplitude regulation of pulsatile insulin secretion from individual pancreatic islets. Diabetes. 1993 May;42(5):670–674. doi: 10.2337/diab.42.5.670. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991 Dec;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembal M., Detimary P., Gilon P., Gao Z. Y., Henquin J. C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. J Clin Invest. 1993 Mar;91(3):871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gilon P., Henquin J. C. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem. 1992 Oct 15;267(29):20713–20720. [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- Herchuelz A., Pochet R., Pastiels C., Van Praet A. Heterogeneous changes in [Ca2+]i induced by glucose, tolbutamide and K+ in single rat pancreatic B cells. Cell Calcium. 1991 Sep;12(8):577–586. doi: 10.1016/0143-4160(91)90076-q. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Bittner M. A., Senter R. A. Regulated exocytotic fusion I: Chromaffin cells and PC12 cells. Methods Enzymol. 1992;219:165–178. doi: 10.1016/0076-6879(92)19019-3. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Fyles J. M., Persaud S. J., Howell S. L. Catecholamine inhibition of Ca2+-induced insulin secretion from electrically permeabilised islets of Langerhans. FEBS Lett. 1987 Jul 13;219(1):139–144. doi: 10.1016/0014-5793(87)81206-1. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Howell S. L. Ca2(+)-induced insulin secretion from electrically permeabilized islets. Loss of the Ca2(+)-induced secretory response is accompanied by loss of Ca2(+)-induced protein phosphorylation. Biochem J. 1992 Aug 1;285(Pt 3):973–978. doi: 10.1042/bj2850973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Howell S. L. Time-course of Ca2+-induced insulin secretion from perifused, electrically permeabilised islets of Langerhans: effects of cAMP and a phorbol ester. Biochem Biophys Res Commun. 1989 Aug 15;162(3):998–1003. doi: 10.1016/0006-291x(89)90772-9. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Stutchfield J., Howell S. L. Effects of Ca2+ and a phorbol ester on insulin secretion from islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1985 Oct 21;191(1):102–106. doi: 10.1016/0014-5793(85)81002-4. [DOI] [PubMed] [Google Scholar]
- Knight D. E., von Grafenstein H., Athayde C. M. Calcium-dependent and calcium-independent exocytosis. Trends Neurosci. 1989 Nov;12(11):451–458. doi: 10.1016/0166-2236(89)90095-7. [DOI] [PubMed] [Google Scholar]
- Li G., Hidaka H., Wollheim C. B. Inhibition of voltage-gated Ca2+ channels and insulin secretion in HIT cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-62: comparison with antagonists of calmodulin and L-type Ca2+ channels. Mol Pharmacol. 1992 Sep;42(3):489–488. [PubMed] [Google Scholar]
- Li G., Regazzi R., Balch W. E., Wollheim C. B. Stimulation of insulin release from permeabilized HIT-T15 cells by a synthetic peptide corresponding to the effector domain of the small GTP-binding protein rab3. FEBS Lett. 1993 Jul 26;327(2):145–149. doi: 10.1016/0014-5793(93)80159-r. [DOI] [PubMed] [Google Scholar]
- Li G., Regazzi R., Roche E., Wollheim C. B. Blockade of mevalonate production by lovastatin attenuates bombesin and vasopressin potentiation of nutrient-induced insulin secretion in HIT-T15 cells. Probable involvement of small GTP-binding proteins. Biochem J. 1993 Jan 15;289(Pt 2):379–385. doi: 10.1042/bj2890379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind I., Ahnert-Hilger G., Fuchs G., Gratzl M. Purification of alpha-toxin from Staphylococcus aureus and application to cell permeabilization. Anal Biochem. 1987 Jul;164(1):84–89. doi: 10.1016/0003-2697(87)90371-x. [DOI] [PubMed] [Google Scholar]
- Longo E. A., Tornheim K., Deeney J. T., Varnum B. A., Tillotson D., Prentki M., Corkey B. E. Oscillations in cytosolic free Ca2+, oxygen consumption, and insulin secretion in glucose-stimulated rat pancreatic islets. J Biol Chem. 1991 May 15;266(14):9314–9319. [PubMed] [Google Scholar]
- Opara E. C., Atwater I., Go V. L. Characterization and control of pulsatile secretion of insulin and glucagon. Pancreas. 1988;3(4):484–487. doi: 10.1097/00006676-198808000-00019. [DOI] [PubMed] [Google Scholar]
- Palmer M., Jursch R., Weller U., Valeva A., Hilgert K., Kehoe M., Bhakdi S. Staphylococcus aureus alpha-toxin. Production of functionally intact, site-specifically modifiable protein by introduction of cysteine at positions 69, 130, and 186. J Biol Chem. 1993 Jun 5;268(16):11959–11962. [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praz G. A., Halban P. A., Wollheim C. B., Blondel B., Strauss A. J., Renold A. E. Regulation of immunoreactive-insulin release from a rat cell line (RINm5F). Biochem J. 1983 Feb 15;210(2):345–352. doi: 10.1042/bj2100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Janjic D., Wollheim C. B. The regulation of extramitochondrial steady state free Ca2+ concentration by rat insulinoma mitochondria. J Biol Chem. 1983 Jun 25;258(12):7597–7602. [PubMed] [Google Scholar]
- Regazzi R., Li G. D., Deshusses J., Wollheim C. B. Stimulus-response coupling in insulin-secreting HIT cells. Effects of secretagogues on cytosolic Ca2+, diacylglycerol, and protein kinase C activity. J Biol Chem. 1990 Sep 5;265(25):15003–15009. [PubMed] [Google Scholar]
- Regazzi R., Li G., Ullrich S., Jaggi C., Wollheim C. B. Different requirements for protein kinase C activation and Ca2+-independent insulin secretion in response to guanine nucleotides. Endogenously generated diacylglycerol requires elevated Ca2+ for kinase C insertion into membranes. J Biol Chem. 1989 Jun 15;264(17):9939–9944. [PubMed] [Google Scholar]
- Regazzi R., Vallar L., Ullrich S., Ravazzola M., Kikuchi A., Takai Y., Wollheim C. B. Characterization of small-molecular-mass guanine-nucleotide-binding regulatory proteins in insulin-secreting cells and PC12 cells. Eur J Biochem. 1992 Sep 15;208(3):729–737. doi: 10.1111/j.1432-1033.1992.tb17241.x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Santos R. M., Rosario L. M., Nadal A., Garcia-Sancho J., Soria B., Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991 May;418(4):417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Theler J. M., Mollard P., Guérineau N., Vacher P., Pralong W. F., Schlegel W., Wollheim C. B. Video imaging of cytosolic Ca2+ in pancreatic beta-cells stimulated by glucose, carbachol, and ATP. J Biol Chem. 1992 Sep 5;267(25):18110–18117. [PubMed] [Google Scholar]
- Ullrich S., Prentki M., Wollheim C. B. Somatostatin inhibition of Ca2(+)-induced insulin secretion in permeabilized HIT-T15 cells. Biochem J. 1990 Aug 15;270(1):273–276. doi: 10.1042/bj2700273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. GTP-dependent inhibition of insulin secretion by epinephrine in permeabilized RINm5F cells. Lack of correlation between insulin secretion and cyclic AMP levels. J Biol Chem. 1988 Jun 25;263(18):8615–8620. [PubMed] [Google Scholar]
- Valdeolmillos M., Santos R. M., Contreras D., Soria B., Rosario L. M. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Lett. 1989 Dec 18;259(1):19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Wollheim C. B., Pozzan T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J Biol Chem. 1984 Feb 25;259(4):2262–2267. [PubMed] [Google Scholar]


