Abstract
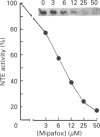
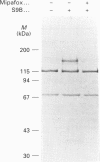
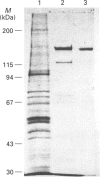
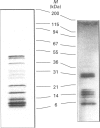
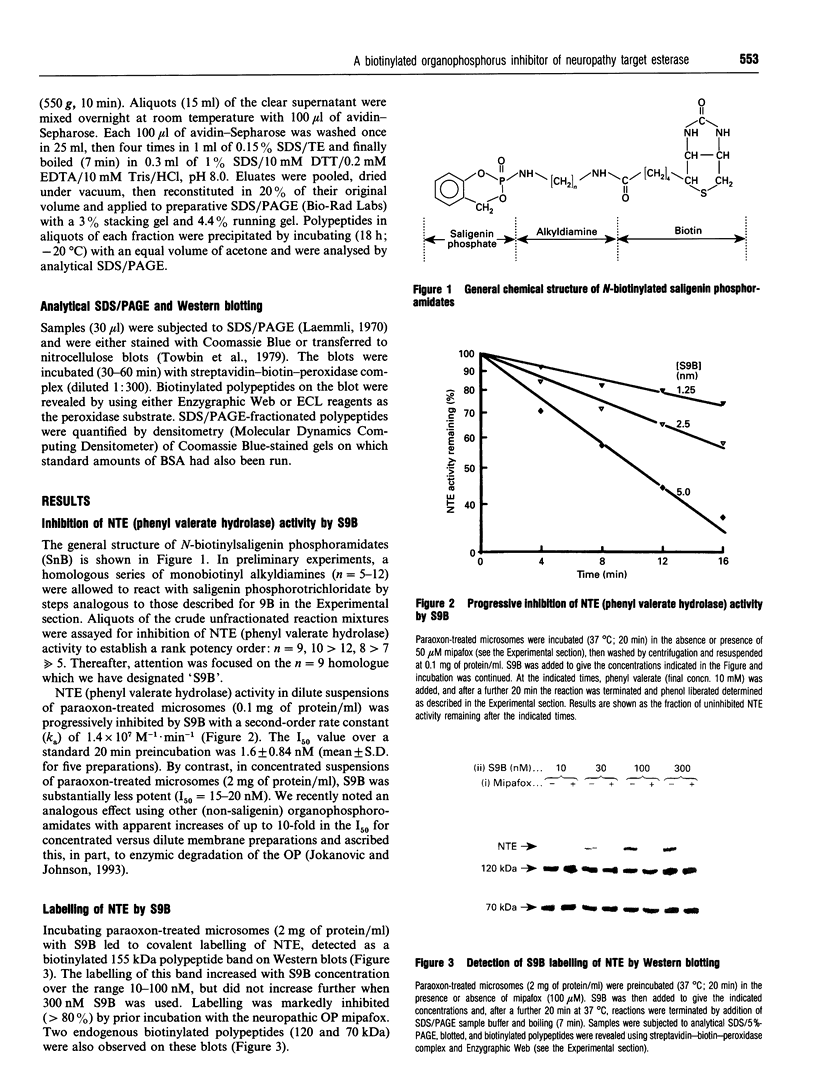
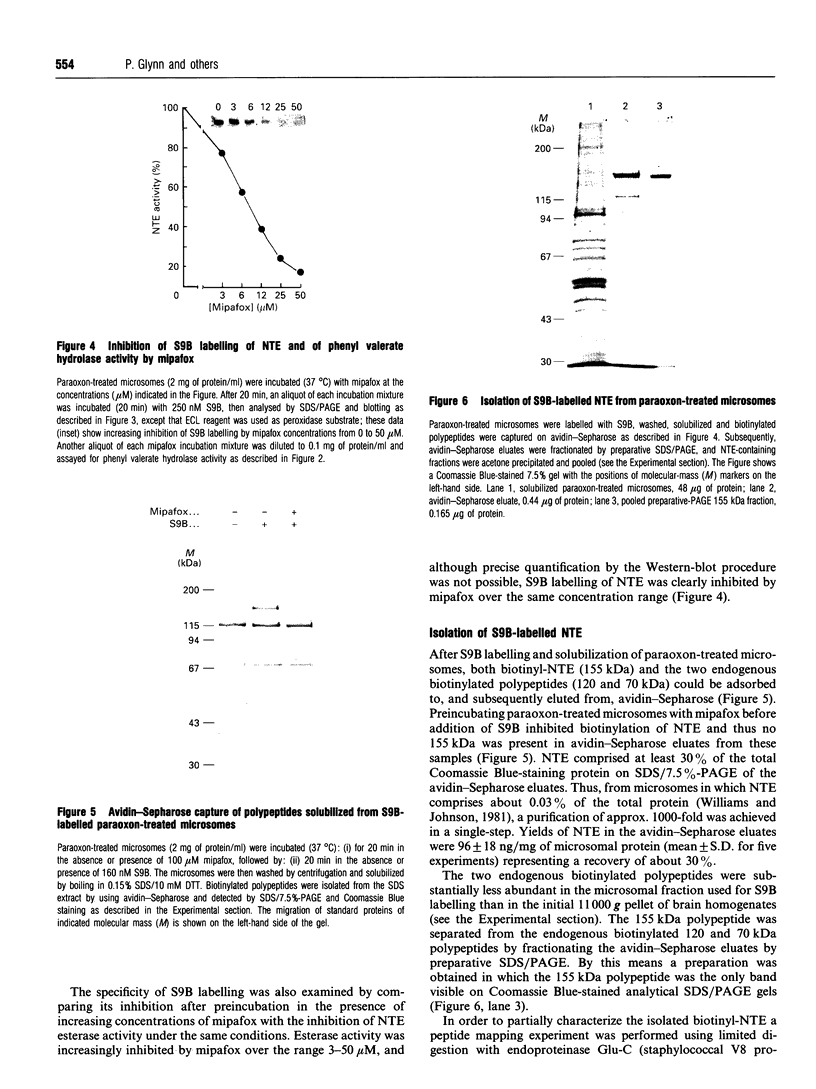
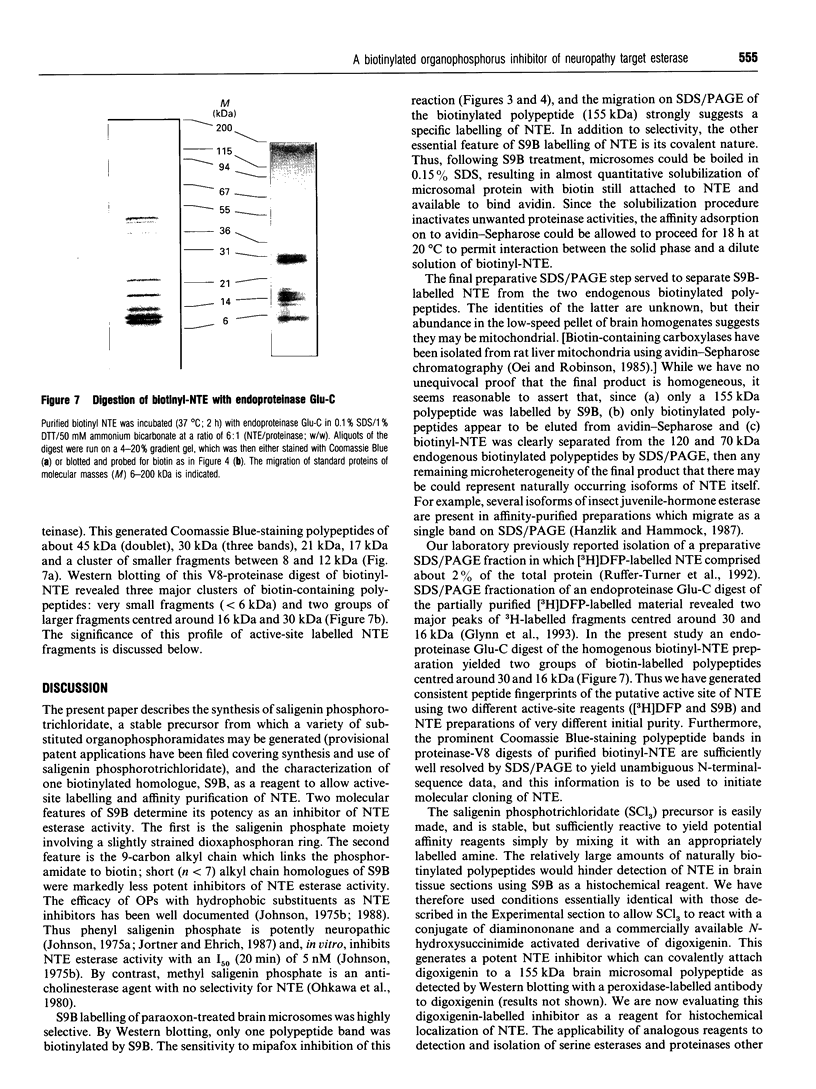
We have synthesized a novel stable precursor, saligenin phosphorotrichloridate, which, on reaction with N-monobiotinyldiamines, generates a series of biotinylated covalent inhibitors of serine esterases. A homologue designated S9B [1-(saligenin cyclic phospho)-9-biotinyldiaminononane] was selected to allow detection and rapid isolation of neuropathy target esterase (NTE). This enzyme is the primary target site for those organophosphorus esters (OPs) which cause delayed neuropathy. NTE comprises about 0.03% of the total protein in brain microsomal fractions and has resisted purification attempts over many years. S9B is a potent progressive inhibitor of NTE esteratic activity (second-order rate constant 1.4 x 10(7) M-1.min-1). Incubation of S9B with brain microsomes led to specific covalent labelling of NTE as determined by detection of a biotinylated 155 kDa polypeptide on Western blots. Specificity of S9B labelling was further demonstrated by inhibition with the neuropathic OP mipafox. Biotinyl-NTE in SDS-solubilized S9B-labelled microsomes was adsorbed on to avidin-Sepharose and subsequently eluted, yielding a fraction enriched approx. 1000-fold in NTE by a single step with recoveries of 30%. Essentially pure NTE was obtained after separation from two endogenous biotinylated polypeptides (120 and 70 kDa) in avidin-Sepharose eluates by preparative SDS/PAGE. Other biotinylated saligenin phosphoramidates derived from the same precursor may be useful for detection and isolation of other serine esterases and proteinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glynn P., Rüffer-Turner M., Read D., Wylie S., Johnson M. K. Molecular characterisation of neuropathy target esterase: proteolysis of the [3H]DFP-labelled polypeptide. Chem Biol Interact. 1993 Jun;87(1-3):361–367. doi: 10.1016/0009-2797(93)90064-6. [DOI] [PubMed] [Google Scholar]
- Hanzlik T. N., Hammock B. D. Characterization of affinity-purified juvenile hormone esterase from Trichoplusia ni. J Biol Chem. 1987 Oct 5;262(28):13584–13591. [PubMed] [Google Scholar]
- Johnson M. K. A phosphorylation site in brain and the delayed neurotoxic effect of some organophosphorus compounds. Biochem J. 1969 Feb;111(4):487–495. doi: 10.1042/bj1110487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Sensitivity and selectivity of compounds interacting with neuropathy target esterase. Further structure-activity studies. Biochem Pharmacol. 1988 Nov 1;37(21):4095–4104. doi: 10.1016/0006-2952(88)90101-3. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. Structure-activity relationships for substrates and inhibitors of hen brain neurotoxic esterase. Biochem Pharmacol. 1975 Apr 1;24(7):797–805. doi: 10.1016/0006-2952(75)90123-9. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. The delayed neuropathy caused by some organophosphorus esters: mechanism and challenge. CRC Crit Rev Toxicol. 1975 Jun;3(3):289–316. doi: 10.3109/10408447509079861. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. The delayed neurotoxic effect of some organophosphorus compounds. Identification of the phosphorylation site as an esterase. Biochem J. 1969 Oct;114(4):711–717. doi: 10.1042/bj1140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokanovic M., Johnson M. K. Interactions in vitro of some organophosphoramidates with neuropathy target esterase and acetylcholinesterase of hen brain. J Biochem Toxicol. 1993 Mar;8(1):19–31. doi: 10.1002/jbt.2570080105. [DOI] [PubMed] [Google Scholar]
- Kay G., Bailie J. R., Halliday I. M., Nelson J., Walker B. The synthesis, kinetic characterization and application of biotinylated aminoacylchloromethanes for the detection of chymotrypsin and trypsin-like serine proteinases. Biochem J. 1992 Apr 15;283(Pt 2):455–459. doi: 10.1042/bj2830455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotti M. The pathogenesis of organophosphate polyneuropathy. Crit Rev Toxicol. 1991;21(6):465–487. doi: 10.3109/10408449209089884. [DOI] [PubMed] [Google Scholar]
- Oei J., Robinson B. H. Simultaneous preparation of the three biotin-containing mitochondrial carboxylases from rat liver. Biochim Biophys Acta. 1985 May 29;840(1):1–5. doi: 10.1016/0304-4165(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Oshita H., Miyamoto J. Comparison of inhibitory activity of various organophosphorus compounds against acetylcholinesterase and neurotoxic esterase of hens with respect to delayed neurotoxicity. Biochem Pharmacol. 1980 Oct 15;29(20):2721–2727. doi: 10.1016/0006-2952(80)90002-7. [DOI] [PubMed] [Google Scholar]
- Pope C. N., Padilla S. Modulation of neurotoxic esterase activity in vitro by phospholipids. Toxicol Appl Pharmacol. 1989 Feb;97(2):272–278. doi: 10.1016/0041-008x(89)90332-3. [DOI] [PubMed] [Google Scholar]
- Rüffer-Turner M. E., Read D. J., Johnson M. K. Purification of neuropathy target esterase from avian brain after prelabelling with [3H]diisopropyl phosphorofluoridate. J Neurochem. 1992 Jan;58(1):135–141. doi: 10.1111/j.1471-4159.1992.tb09288.x. [DOI] [PubMed] [Google Scholar]
- Schoub B. D., Blackburn N. K., Johnson S., McAnerney J. M., Miller B. Low antibody avidity in elderly chickenpox patients. J Med Virol. 1992 Jun;37(2):113–115. doi: 10.1002/jmv.1890370207. [DOI] [PubMed] [Google Scholar]
- Thomas T. C., Ishikawa Y., McNamee M. G., Wilson B. W. Correlation of neuropathy target esterase activity with specific tritiated di-isopropyl phosphorofluoridate-labelled proteins. Biochem J. 1989 Jan 1;257(1):109–116. doi: 10.1042/bj2570109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. G., Johnson M. K. Gel-electrophoretic identification of hen brain neurotoxic esterase, labelled with tritiated di-isopropyl phosphorofluoridate. Biochem J. 1981 Nov 1;199(2):323–333. doi: 10.1042/bj1990323. [DOI] [PMC free article] [PubMed] [Google Scholar]