Abstract
Most tongue squamous cell carcinoma (TSCC) classified as T3 require reconstructive surgery, which inevitably causes problems with oral functions. We propose new induction chemotherapy using intra-arterial infusion for TSCC classified as T3 to avoid reconstructive surgery. This chemotherapy regimen consists of intra-arterial infusion of docetaxel and cisplatin and systemic administration of 5-fluorouracil. As a result of this treatment, the therapeutic effect was a complete response in five patients and a partial response in one patient, and the overall response rate was 100%. All six patients underwent partial resection because their tumors shrank with this induction chemotherapy. In addition, adverse events of grade 3 or more did not occur in all six patients. The median follow-up duration for all patients was 34 months, and they are alive. This intra-arterial chemotherapy regimen was shown to be highly efficacious and safe.
Keywords: Induction chemotherapy, intra-arterial infusion, squamous cell carcinoma, tongue
INTRODUCTION
The tongue is an extremely important tissue for swallowing, mastication, and articulation, but its function is impaired by reconstructive surgery in many cases of tongue squamous cell carcinoma (TSCC) classified as T3. We believe that achieving both functional preservation and prognosis improvement will become an important challenge for oral surgeons in the future. Therefore, induction chemotherapy should be considered as one of the treatment options to preserve function and improve prognosis.
We focused on the route of drug administration and proposed more effective induction chemotherapy for TSCC. The treatment regimen included conventional anticancer drugs. Docetaxel (DOC) and cisplatin (CDDP) were administered continuously via the artery, and 5-fluorouracil (5-FU) was administered systemically (intra-arterial DCF therapy). Then, the surgical excision range was reduced as much as possible according to the therapeutic effect to preserve the tongue functions. Here, we report six cases with TSCC classified as T3 who received intra-arterial DCF therapy, avoided reconstructive surgery, and achieved a favorable prognosis.
CASE REPORT
We have performed intra-arterial DCF therapy for six cases of TSCC classified as T3 (five males and one female) with a median age of 71 years. As described in our previous report,[1] an intra-arterial catheter was inserted under local anesthesia via the superficial temporal artery. We performed intra-arterial DCF therapy as follows; on days one and two, 20 mg/body of DOC was continuously administered for 24 h via the artery, and on days three and four, 20 mg/body CDDP was also administered for three hours via the artery. In addition, from days one to five, 500 mg/day of 5-FU was continuously administered intravenously [Figure 1]. The second course of treatment was administered four weeks after the first course and was essentially completed in two cycles. Table 1 shows the clinical features of the six patients. The overall response rate (ORR) was 100%, and all six patients underwent partial glossectomy with functional preservation. The median follow-up period for six patients was 34 months (range: 19–47 months). All six patients are alive. Adverse events of grade 3 or more did not occur in all six patients [Table 2].
Figure 1.
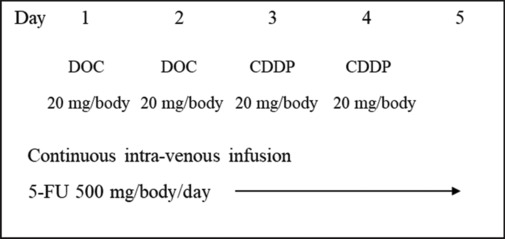
The regimen of intra-arterial infusion of docetaxel and cisplatin and intravenous 5-fluorouracil
Table 1.
Demographics and clinical features of the patients
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex | M | F | M | M | M | M |
| Age | 70 | 84 | 83 | 69 | 63 | 72 |
| TNM | T3N0M0 | T3N1M0 | T3N0M0 | T3N0M0 | T3N0M0 | T3N0M0 |
| Stage | III | IVA | III | III | III | III |
| Treatment effects | CR | CR | CR | CR | CR | PR |
| Histopathological effects | Grade 2 | Grade 3 | Grade 2 | Grade 3 | Grade 3 | Grade 1 |
| Residual tumor diameter | 2 mm | – | 1 mm | – | – | 23 mm |
| Surgery | Partial glossectomy | Partial glossectomy and neck dissection | Partial glossectomy | Partial glossectomy | Partial glossectomy | Partial glossectomy |
| Recurrence | Neck | – | – | – | – | – |
| Prognosis | Alive | Alive | Alive | Alive | Alive | Alive |
| Survival period | 42 months | 27 months | 46 months | 47 months | 21 months | 19 months |
Table 2.
Toxicity (n=6)
| Number of patients |
||
|---|---|---|
| Grade 1 | Grade 2 | |
| Anemia | 5 | 0 |
| Neutropenia | 0 | 0 |
| Thrombocytopenia | 5 | 0 |
| Renal dysfunction | 0 | 0 |
| Hyponatremia | 3 | 0 |
| Hyperkalemia | 3 | 0 |
| Hepatic dysfunction | 2 | 1 |
| Anorexia | 3 | 1 |
| Nausea | 0 | 0 |
| Mucositis | 4 | 1 |
| Pain | 4 | 1 |
CASE 1
A 70-year-old man presented with a lesion measuring 27 × 22 mm on the right tongue margin. Based on the MR images, the depth of invasion was diagnosed as 13 mm [Figure 2a]. Before intra-arterial DCF therapy, the markings were performed approximately 5 mm from the indurated tumor border [Figure 2b]. After two cycles of intra-arterial DCF therapy, the treatment effect achieved CR [Figure 3a]. The excisional margin was inside the marking points and included the muscle layer [Figure 3b]. It has been 42 months since the surgery, but no recurrence has been observed at the primary site.
Figure 2.
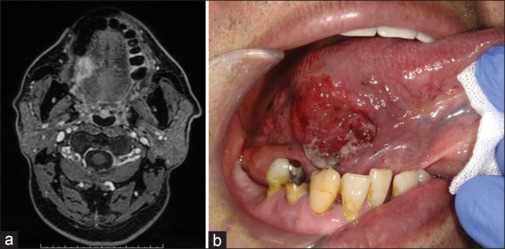
(a) MR image at the initial examination in case 1. (b) The markings before intra-arterial DCF therapy
Figure 3.
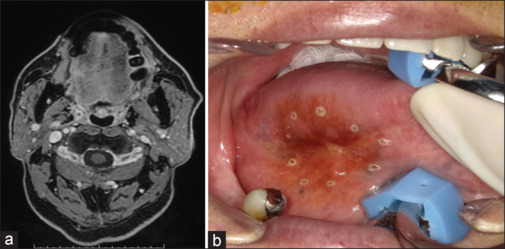
(a) MR image after completion of intra-arterial DCF therapy in case 1. (b) Intraoral photograph at the time of surgery. The excisional margin was inside the marking points
DISCUSSION
The standard treatment for resectable locally advanced TSCC is radical resection. The five-year overall survival (OS) rates according to the cancer stage of patients who underwent this standard treatment were reported to be 61–77% for stage III and 47–60% for stage IV disease.[2,3,4,5] Most of these patients also require reconstructive surgery due to extensive tissue defects,[6] resulting in functional defects and aesthetic concerns. As such, the current challenge in the treatment of locally advanced TSCC is how to concurrently improve OS and preserve oral functions. To solve this problem, we believe that improvement of induction chemotherapy is important, but no chemotherapy has been effective so far. In two randomized studies of chemotherapy for resectable oral SCC, one with CDDP and 5-FU and the other with DOC, CDDP, and 5-FU, CR rates were 33% for T2–T4 and 8.1% for T1–T4, respectively. Although both studies failed to show statistically significant improvement in survival, their results demonstrated that clinical and pathological responses are significant prognostic factors, suggesting that the downstaging of a tumor by induction chemotherapy may lead to less demolition surgery.[7,8]
We focused on the route of administration to enhance the synergistic effect of drugs and proposed intra-arterial chemotherapy using DOC, CDDP, and PEP combined with systemic chemotherapy using 5-FU for locally advanced oral SCC.[1] The results showed a high therapeutic effect with an ORR of 99.3%. Although this regimen has very little hematotoxicity, Grade 3 mucositis occurred frequently (46.8%), and it was necessary to manage the accompanying pain and anorexia. We removed PEP from the regimen in this study, five out of six patients achieved clinical CR (83.3%), and the ORR for all patients was 100%. This indicates that a sufficient effect can be obtained even without PEP. Under standard treatment, all six cases shown in this report would have required reconstructive surgery. However, with effective intra-arterial DCF therapy, all patients were spared demolition surgery. Moreover, no grade 3 or higher adverse events occurred. There was no mucositis nor difficulty in controlling pain or diet during treatment. The intra-arterial DCF therapy has other benefits besides the combination of drugs. That is, the catheter was placed retrogradely. By indwelling the catheter retrograde via the superficial temporal artery, the catheter can be left in place for a long period, and multiple anticancer drugs can be administered continuously or frequently.[9] This is an advantage not seen when using the Seldinger technique via the femoral artery. We believe that intra-arterial DCF therapy is a promising treatment for the functional preservation of TSCC.
CONCLUSION
We showed higher therapeutic effects by devising the drug combination of three conventional anticancer drugs that have been used for oral cancer. The treatment strategy described here can downgrade the stage of T3 TSCC and could be a promising therapy to achieve both functional preservation and good prognosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hasegawa H, Kaneko T, Kanno C, Endo M, Yamazaki M, Kitabatake T, et al. Preoperative intra-arterial chemotherapy with docetaxel, cisplatin, and peplomycin combined with intravenous chemotherapy using 5-fluorouracil for oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2020;49:984–92. doi: 10.1016/j.ijom.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Noble AR, Greskovich JF, Han J, Reddy CA, Nwizu TI, Khan MF, et al. Risk factors associated with disease recurrence in patients with stage III/IV squamous cell carcinoma of the oral cavity treated with surgery and postoperative radiotherapy. Anticancer Res. 2016;36:785–92. [PubMed] [Google Scholar]
- 3.Seo BY, Lee CO, Kim JW. Changes in the management and survival rates of patients with oral cancer: A 30-year single-institution study. J Korean Assoc Oral Maxillofac Surg. 2016;42:31–7. doi: 10.5125/jkaoms.2016.42.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, et al. Surgical outcome of T4a and resected T4b oral cavity cancer. Cancer. 2006;107:337–44. doi: 10.1002/cncr.21984. [DOI] [PubMed] [Google Scholar]
- 5.Stenson KM, Kunnavakkam R, Cohen EE, Portugal LD, Blair E, Haraf DJ, et al. Chemoradiation for patients with advanced oral cavity cancer. Laryngoscope. 2010;120:93–9. doi: 10.1002/lary.20716. [DOI] [PubMed] [Google Scholar]
- 6.Hanasono MM, Friel MT, Klem C, Hsu PW, Robb GL, Weber RS, et al. Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck. 2009;31:1289–96. doi: 10.1002/hed.21100. [DOI] [PubMed] [Google Scholar]
- 7.Licitra L, Grandi C, Guzzo M, Mariani L, Lo Vullo S, Valvo F, et al. Primary chemotherapy in resectable oral cavity squamous cell cancer: A randomized controlled trial. J Clin Oncol. 2003;21:327–33. doi: 10.1200/JCO.2003.06.146. [DOI] [PubMed] [Google Scholar]
- 8.Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Jr, Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744–51. doi: 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsudo K, Koizumi T, Iida M, Iwai T, Nakashima H, Oguri S. Retrograde superselective intra-arterial chemotherapy and daily concurrent radiotherapy for stage III and IV oral cancer: Analysis of therapeutic results in 112 cases. Radiother Oncol. 2014;111:306–10. doi: 10.1016/j.radonc.2014.03.005. [DOI] [PubMed] [Google Scholar]


