Abstract
Background
Small cell lung cancer (SCLC) accounts for approximately 20% of all cases of lung cancer. It tends to disseminate early in the course of its natural history and to grow quickly. Approximately 10% to 18% of patients present with brain metastases (BM) at the time of initial diagnosis, and an additional 40% to 50% will develop BM some time during the course of their disease.
Objectives
The aim of this review was to evaluate the effectiveness and toxicity of systemic chemotherapy for the treatment of BM from SCLC.
Search methods
We searched the Cochrane Lung Cancer Review Group Specialised Register (July 2011), CENTRAL (2011, Issue 5), PubMed (1966 to July 2011), EMBASE (2005 to July 2011), LILACS (1982 to July 2011) and the International Clinical Trial Registry Platform (ICTRP).
Selection criteria
Randomized controlled trials (RCTs) comparing systemic chemotherapy (single agent or combination chemotherapy) with another chemotherapy regimen, palliative care, whole brain radiotherapy or any combination of these interventions for the treatment of BM as the only site of progression.
Data collection and analysis
Data extraction and 'Risk of bias' assessment were carried out independently by two review authors. As the included studies evaluated three different treatment modalities meta‐analysis was not possible.
Main results
Three RCTs, involving 192 participants, met inclusion criteria for this review. No significant differences for overall survival (OS) were reported in any of the trials: in the first trial, 33 patients received whole brain radiation therapy and no significant difference was found between patients treated with topotecan and those not treated with topotecan. In a second trial, in which 120 patients were randomized to receive teniposide with or without brain radiation therapy, the authors reported that the median progression‐free survival (brain‐specific progression‐free survival (PFS)) was 3.5 months in the combined modality arm and 3.2 in the teniposide alone arm. In a third trial, comparing sequential and concomitant chemoradiotherapy (teniposide plus cisplatin) in 39 participants, the survival difference between the two groups was not statistically significant. While the first trial reported no significant difference in PFS, the second RCT found a significant difference favoring combined therapy group. The second trial also found that patients receiving chemoradiotherapy (teniposide plus whole brain radiotherapy) had a higher complete response rate than those receiving only the topoisomerase inhibitor.
Authors' conclusions
Given the paucity of robust studies assessing the clinical effects of treatments, available evidence is insufficient to judge the effectiveness and safety of chemotherapy for the treatment of BM from SCLC. Published studies are insufficient to address the objectives of this review. According to the available evidence included in this review, chemotherapy does not improve specific brain PFS and OS in patients with SCLC. The combined treatment of teniposide and brain radiation therapy contributed to outcome in terms of increased complete remission and shorter time to progression (though not OS).
Keywords: Humans, Lung Neoplasms, Antineoplastic Agents, Antineoplastic Agents/therapeutic use, Brain Neoplasms, Brain Neoplasms/drug therapy, Brain Neoplasms/radiotherapy, Brain Neoplasms/secondary, Cisplatin, Cisplatin/therapeutic use, Cranial Irradiation, Cranial Irradiation/methods, Randomized Controlled Trials as Topic, Small Cell Lung Carcinoma, Small Cell Lung Carcinoma/drug therapy, Small Cell Lung Carcinoma/radiotherapy, Small Cell Lung Carcinoma/secondary, Teniposide, Teniposide/therapeutic use, Topotecan, Topotecan/therapeutic use
Plain language summary
Is chemotherapy beneficial to patients with brain metastases from small cell lung cancer?
Lung carcinoma is the single most common source of brain metastases (BM) in adults. Small cell lung cancer (SCLC) accounts for approximately 20% of all cases of lung cancer. It tends to disseminate early in the course of its natural history and to grow quickly. Approximately 10% to 18% of patients present with BM at the time of initial diagnosis, and an additional 40% to 50% will develop BM some time during the course of their disease.
After an extensive review of medical literature we identified three trials assessing different treatment strategies for patients with BM from SCLC. Only one of the studies compared chemotherapy (topotecan) versus no chemotherapy, but in patients treated with whole brain radiotherapy. Another study randomized patients to receive teniposide with or without brain radiation therapy, and the third one, compared sequential and concomitant chemoradiotherapy (teniposide plus cisplatin).
Studies show that people who received chemotherapy did not live longer or have a longer time before the BM grew again compared to those who were treated with brain radiation therapy alone. Hematological toxicities occurred more often in patients exposed to chemoradiotherapy in one study and in patients receiving sequential treatment in another study. A major limitation of this review was the low number of included studies and participants.
Background
Description of the condition
Several studies have described the molecular pathophysiology of brain metastases (BM) and the basis for the differences in 'neurotropism' among various systemic tumors (Nathoo 2005; Palmieri 2007; Gril 2010; Lorger 2010). To form brain metastases successfully, tumor cells must attach to and penetrate the microvessel endothelium, degrade the extracellular matrix, and respond to autocrine and brain‐derived survival and growth factors.
Approximately 150,000 patients in the US develop symptomatic BM each year, making them the most common intracranial malignancies. Any neoplasm is capable of metastasizing to the brain, although two thirds of all adult patients have lung cancer, breast cancer, or melanoma. Lung carcinoma is the single most common source of BM in adults, accounting for 30% to 50% of all cases (Nussbaum 1996; Nayak 2011). Lung carcinomas are also the tumors most likely to spread to the brain in the absence of other systemic metastases.
Small cell lung cancer (SCLC) accounts for approximately 15 to 20% of all cases of lung cancer. It tends to disseminate early in the course of its natural history and grow quickly. Around 10% to 18% of patients present with BM at the time of initial diagnosis, and an additional 40% to 50% will develop BM some time during the course of their disease (Quan 2004; Seute 2004).
The aims of treatment of symptomatic BM from SCLC are to improve survival, reduce symptoms, and to prevent complications, such as neurologic deficits and cognitive impairment. Traditionally, the standard treatment for symptomatic BM is whole brain radiotherapy (WBRT), with an overall response rate (ORR) ranging from 56% to 92% (Kristensen 1992; Seute 2005; Nieder 2006). Factors related to the prognosis of patients with SCLC who develop BM are the performance status, control of extracranial metastases, number of brain lesions, and age (Seute 2004).
Description of the intervention
For a long time systemic chemotherapy was not considered a potential therapy for BM, since it was assumed that the brain was a pharmacologic sanctuary where metastases were protected from cytotoxic drugs by the blood brain barrier (BBB). However, in recent decades it has become clear that the BBB is disrupted by tumor tissue (Stewart 1984; Siegers 1990; Stewart 1993; Stewart 1994). Since then, the effectiveness of first‐line and second‐line systemic chemotherapy for the treatment of BM from SCLC has been the topic of several studies (Kristensen 1992; Postmus 1999; Van den Bent 2003; Schuette 2004; Seute 2004; Seute 2006). In addition, several authors have claimed that synchronous BM from SCLC and other solid tumors have a response rate for systemic chemotherapy that is similar for the primary (Kristensen 1992; Postmus 1999; Van den Bent 2003). It has been suggested that BM from SCLC should initially be treated with systemic chemotherapy (Grossi 2001). The debate about whether WBRT should be part of the initial treatment is still ongoing (Schuette 2004; Soffietti 2005). The aim of this review is to investigate whether there is any evidence that can clarify the role of systemic chemotherapy in the treatment of BM from SCLC.
Objectives
The aim of this review was to evaluate the effectiveness and toxicity of systemic chemotherapy for the treatment of BM from SCLC.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) (phase II (B) or III) of parallel design, published in any language, were eligible for inclusion in the review. We excluded studies presented only in abstract form about which no further or sufficient information could be obtained from the authors.
Types of participants
We included patients with histologically confirmed SCLC in whom BM was found on either computer tomography (CT) scan or magnetic resonance imaging (MRI).
Types of interventions
Systemic chemotherapy (single agent or combination chemotherapy) compared with another chemotherapy regimen, palliative care, WBRT, or any combination of these interventions.
Types of outcome measures
Primary outcomes
Overall survival (OS).
Progression‐free survival (PFS), defined as the time from the start of systemic treatment until progressive brain disease (enlarging BM, the onset of new BM based on CT or MRI, or both).
Radiologic response of BM/local brain response. The radiologic response was determined as the percentage of patients achieving complete remission (CR) or partial remission (PR) on MRI. CR was defined as the complete disappearance of all tumors on MRI. PR was defined as at least a 50% decrease of total tumor size of the lesions measured, without the appearance of any new lesions or progression of any lesions. Stable disease was defined as a less than 50% decrease or less than 25% increase in size of lesions and no new lesions. Progressive disease was defined as a more than 25% increase in the size of lesions or the appearance of new lesions (Macdonald 1990). However, we accepted whatever definitions had been used in individual trials.
Secondary outcomes
Control of the neurologic symptoms and signs.
The response of the primary tumor and systemic metastases.
Quality of life (QoL) measured using validated international scales.
Toxicity (using the National Cancer Institute Common Toxicity Criteria).
Search methods for identification of studies
(1) Electronic databases
We identified relevant trials from:
(a) the Cochrane Lung Cancer Review Group Specialised Register, which contains the results of a comprehensive handsearching of relevant lung cancer journals and conference proceedings (6 July 2011),
(b) the Cochrane Central Register of Controlled Clinical Trials (CENTRAL) (The Cochrane Library, 2011, issue 6), MEDLINE (accessed via Ovid; from 1966 to July 6, 2011), EMBASE (accessed via Ovid, from 1980 to July 6, 2011), LILACS (from 1982 to July 2011) (Manríquez 2008),
(c) ongoing trials in the International clinical trial registry platform (ICTRP) using the following key words: brain AND lung AND metastasis.
We searched MEDLINE (Appendix 1), EMBASE (Appendix 2), and CENTRAL (via Ovid; Appendix 3). We modified and adapted this search strategy for LILACS.
(2) References from published studies
We scanned bibliographies of relevant studies for possible references to additional trials.
(3) Unpublished literature
We searched for electronic addresses of leading researchers or researchers possibly involved in this field in electronic databases, to obtain additional published and unpublished trials.
(4) Adverse effects
We only included data from RCTs and clinical controlled trials. No further search for other types of studies was done (Golder 2005).
Data collection and analysis
Selection of studies
We assessed the eligibility of the retrieved articles from the title and abstracts. Where there was insufficient information for assessment, the authors reviewed the full articles. Two authors (LR and AFC) independently assessed all RCTs. There was no blinding of the author as to the origin or conclusions of the article for eligibility assessment, data extraction or 'Risk of bias' assessment.
Data extraction and management
Two review authors (LR and JRR) independently carried out data extraction using a pre‐designed data extraction form. Data were extracted for all outcomes for all relevant drugs, paying particular attention to the dosage and periodicity of treatment. Data extraction was double‐checked. Disagreements were solved by consensus.
Assessment of risk of bias in included studies
Two review authors (LR and JRR) independently assessed the risk of bias of included studies according to the areas and criteria proposed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and results of those judgments are presented in the 'Risk of bias' tables. Disagreements were solved by consensus.
(1) Sequence generation (checking for possible selection bias)
For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the risk of bias as:
low risk (any truly random process, e.g. random number table; computer random number generator),
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number), or
unclear risk.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the risk of bias as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes),
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth),
unclear risk.
(3) Blinding (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the risk of bias as:
low risk, high risk, or unclear risk for participants;
low risk, high risk, or unclear risk for personnel;
low risk, high risk, or unclear risk for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
For each included study and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We categorized the risk of bias as:
low risk,
high risk,
unclear risk.
(5) Selective reporting bias
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the risk of bias as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
(6) Other sources of bias
Where relevant for each included study any important concern about other possible sources of bias is reported.
We assessed the risk of bias as:
low risk,
high risk,
unclear risk.
Assessment of heterogeneity
No meta‐analysis was conducted in this review. For future updates of this review we will carry out tests for homogeneity using a standard Chi2 test with significance being set at P < 0.1 when possible. The I2 statistic will be used to estimate total variation across studies due to heterogeneity rather than chance. In percentages, less than 25% will be considered as low‐level heterogeneity, 25% to 50% as moderate‐level heterogeneity, and greater than 50% as high‐level heterogeneity (Higgins 2011). We plan that if only significant methodologic heterogeneity is found, we should perform a sensitivity analysis.
Potential sources of heterogeneity
'Risk of bias' assessment (low, intermediate, high).
Methods of symptom assessment.
Type of previous therapy for patients with progressive disease in the brain.
Stratification by Eastern Cooperative Oncology Group performance status.
Potential sources of heterogeneity could not be explored due to the scarcity of trials.
Data synthesis
We planned to estimate differences between treatments by pooling the results of RCTs that evaluated similar interventions and controls (with another chemotherapy regimen, palliative care, or WBRT), and to calculate a weighted treatment effect across RCTs using a random‐effects model, but it was not possible since there was only one study for each comparison of treatments. However, for the individual studies, we expressed the results as risk ratio (RR) with 95% confidence intervals (CI) for dichotomous outcomes (i.e. radiologic response of BM/local brain response) and weighted mean difference (WMD) with 95% CI for continuous outcomes (i.e. survival time); or, when appropriate, we used the standardized mean difference (SMD) with 95% CI. For survival analysis, estimation of the hazard ratio and its variance (Parmar 1998) was used as the summary statistic where the data permit.
We summarized the available information and based our analysis on intention to treat whenever possible. We considered a level of P < 0.05 to be statistically significant. We provided a qualitative description for adverse effects when it was available. We planned to estimate publication bias by a visual inspection of a funnel plot; however there were fewer than nine studies involved in one subgroup (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Even though it was planned to make separate analysis for some subgroups of patients it was not possible because of the lack of necessary data. If information is available, future updates of this review will present separate analysis for:
patients with synchronous BM (diagnosed within four weeks of the diagnosis of SCLC), and
patients with SCLC previously treated with prophylactic cranial radiotherapy or systemic first‐line chemotherapy, who subsequently developed BM as the only site of progression.
Sensitivity analysis
For further updates, we plan to perform sensitivity analysis by systematically excluding studies from the overall analysis based on the potential sources of heterogeneity hypothesized above, and if homogeneous subgroups have not already been identified and analyzed separately (Higgins 2011).
Results
Description of studies
See: Characteristics of included studies.
Results of the search
The search identified 912 references. An initial trawl through this list, done by the three review authors, excluded 895 references that did not comply with the inclusion criteria (Figure 1). Fourteen studies were further excluded after first review because they were not RCTs, did not focus on chemotherapy for the treatment of BM from SCLC, or did not present disaggregated data on SCLC participants. The search identified no RCTs published in abstract form only. We actively contacted authors requesting information about additional trials but had no replies. In addition, no ongoing trials were identified after assessing 62 studies in the ICTRP database.
1.
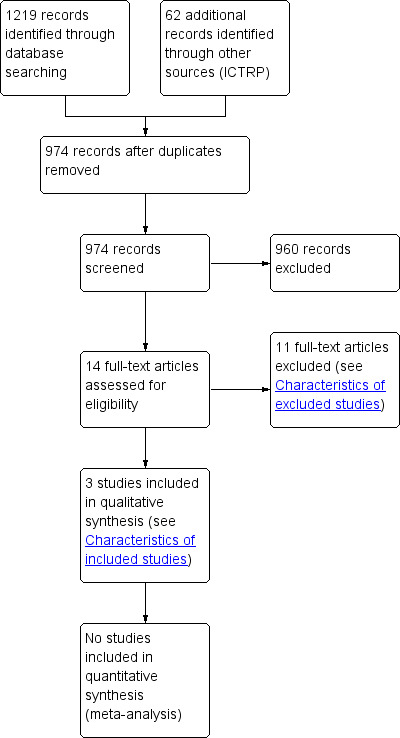
Study flow diagram.
Included studies
We included only three RCTs in the review (see Characteristics of included studies). One study was done in several European countries (Postmus 2000), another one in Germany (Neuhaus 2009) and the third in China (Liu 2010). Chemotherapy regimens were different in all three studies: topotecan, teniposide alone, and teniposide plus cisplatin. All the studies assessed survival time, two reported PFS time and two reported tumor response.
Only one of the RCTs compared chemotherapy with no chemotherapy (Neuhaus 2009) and included 33 patients with SCLC metastases (first line 5; recurrence 28); 17 patients received WBRT alone and 16 patients were treated with WBRT plus topotecan.
In another study that compared two schedules of administration of chemotherapy (teniposide plus cisplatin), a total of 39 patients were randomly allocated to receive either sequential chemoradiotherapy (20 patients) or concomitant chemoradiotherapy (19 patients) (Liu 2010).
In the third study both groups received chemotherapy, but one also received radiotherapy. They randomized 120 patients to receive teniposide (60 patients) or teniposide plus WBRT (60 patients) (Postmus 2000).
Excluded studies
Excluded studies and reasons for exclusion are listed in Characteristics of excluded studies.
Risk of bias in included studies
Allocation
The allocation strategy was considered adequate for one trial (Postmus 2000) and unclear for the other two (Neuhaus 2009; Liu 2010), while allocation concealment was judged as unclear in the three included RCTs (Neuhaus 2009; Postmus 2000; Liu 2010) (Figure 2; Figure 3). Although we actively contacted authors requesting information about their trials, we received no replies.
2.
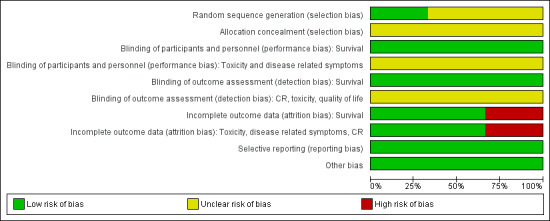
'Risk of bias' graph: review authors' judgments about each 'Risk of bias' item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgments about each 'Risk of bias' item for each included study.
Blinding
Blinding was not used in any of the three trials but main outcome measures, particularly survival, are not likely to be influenced by lack of blinding.
Incomplete outcome data
Losses to follow‐up (Neuhaus 2009; Postmus 2000; Liu 2010) and reasons for stopping protocol therapy were reported (Postmus 2000). In one trial (Postmus 2000) 39 (65.0%) and 50 (83.3%) patients in the combined modality and teniposide‐only arm, respectively, stopped treatment because of tumor progression. None of the patients completed all 12 courses of protocol therapy in the teniposide only arm while six (10%) patients did so in the combined modality arm.
Selective reporting
Relevant outcomes for this review were reported in all the studies.
Other potential sources of bias
The studies seemed to be free of other sources of bias.
Effects of interventions
Overall survival
Neuhaus 2009, in an RCT with 33 patients receiving WBRT, reported that the differences in survival time were not significant between patients treated with topotecan compared to those not receiving topotecan. Data on both SCLC and NSCLC were reported but numerical results for patients with SCLC were not presented in a disaggregated way.
In the RCT in which 120 patients were randomized to receive teniposide with or without WBRT (Postmus 2000), OS rated in the two groups were not statistically significant different, with a median survival of 3.5 months in the combined‐modality arm and of 3.2 months in the teniposide‐alone arm.
In the trial that compared sequential and concomitant chemoradiotherapy (Liu 2010), including 39 patients, the survival difference between the two groups was not statistically significant (Analysis 2.1).
2.1. Analysis.
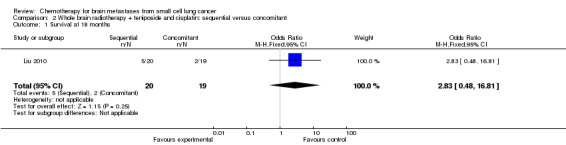
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 1 Survival at 18 months.
Progression‐free survival
Neuhaus 2009 reported that PFS differences in patients receiving WBRT were not significant between the patients treated with topotecan and those not receiving topotecan. However, no numerical data were reported in the article.
In the RCT in which participants were randomized to receive teniposide with or without WBRT (Postmus 2000), time to progression (TTP) in the brain, as assessed by contrast enhanced brain CT scan, was significantly better in the combined therapy group, but data were not provided to calculate the hazard ratio.
Liu 2010 did not publish an analysis of PFS.
Radiologic response of BM/local brain response
Neuhaus 2009 did analyze that outcome in their study but did not provide separate data for patients with SCLC.
Postmus 2000 found that patients receiving the combined treatment of teniposide and brain radiation therapy had higher complete BM response than those receiving only teniposide (RR 3.60 95% CI 1.43 to 9.07 Analysis 1.3).
1.3. Analysis.
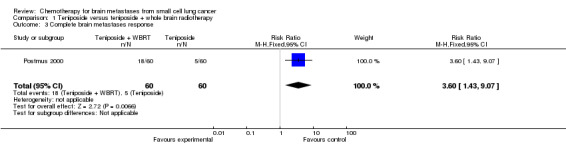
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 3 Complete brain metastases response.
Liu 2010 found that total response rates were similar among groups treated with sequential and concomitant chemoradiotherapy (Analysis 2.2; Analysis 2.3).
2.2. Analysis.
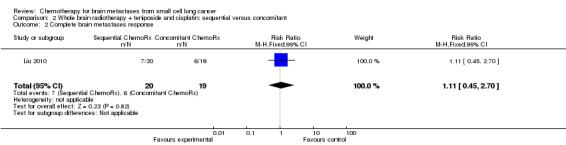
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 2 Complete brain metastases response.
2.3. Analysis.
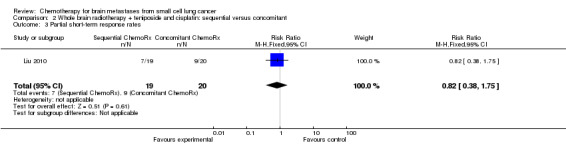
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 3 Partial short‐term response rates.
Secondary outcomes
Postmus 2000 did not find significant differences between groups receiving the combined treatment of teniposide and WBRT or teniposide alone for the following clinical outcomes: clinical response, toxicity, improvement on the ECOG performance status, and improvement in neurologic function score and status after two cycles.
Neuhaus 2009 found no differences in the occurrence of nonhematologic grade 3/4 toxicities between the treatment arms. Hematological toxicities occurred more often in patients exposed to chemoradiotherapy; 25 grade 3/4 hematological adverse events were reported, 24 in patients receiving combined treatment and one in a patient treated with WBRT alone.
Liu 2010 found that myelosuppression and grade 3/4 leukopenia were the most common adverse reactions, particularly in patients receiving sequential treatment compared with those receiving concomitant chemoradiotherapy (RR 8.42, 95% CI 1.16 to 61.10; Analysis 2.4). Other reported adverse events that had statistically significant differences between groups were grade 3/4 leukopenia (Analysis 2.7) and any grade of thrombocytopenia (Analysis 2.8). However, no significant differences were found for grade 3/4 anemia, thrombocytopenia, or nausea and vomiting between groups (Analysis 2.5; Analysis 2.6; Analysis 2.9). In addition, no events occurred in both arms of treatment for other grade 3/4 adverse events such as diarrhea, hepatic, and renal; no patient experienced grade 3/4 stomatitis in the sequential arm while one patient did in the concomitant arm (Analysis 2.10).
2.4. Analysis.
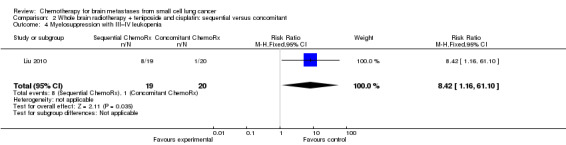
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 4 Myelosuppression with III–IV leukopenia.
2.7. Analysis.
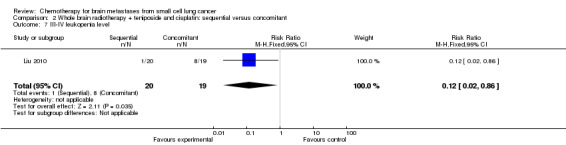
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 7 III‐IV leukopenia level.
2.8. Analysis.
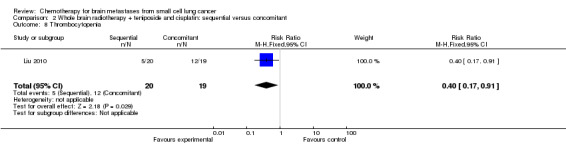
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 8 Thrombocytopenia.
2.5. Analysis.
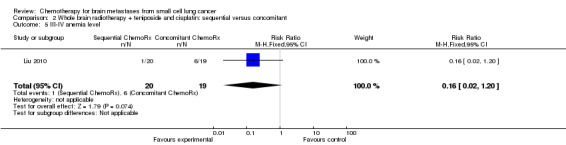
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 5 III‐IV anemia level.
2.6. Analysis.
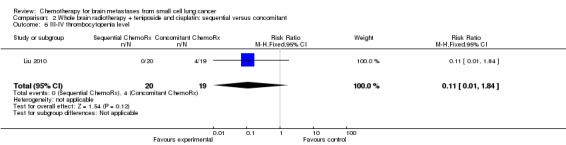
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 6 III‐IV thrombocytopenia level.
2.9. Analysis.
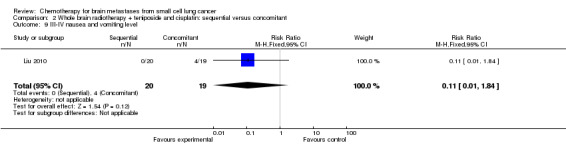
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 9 III‐IV nausea and vomiting level.
2.10. Analysis.
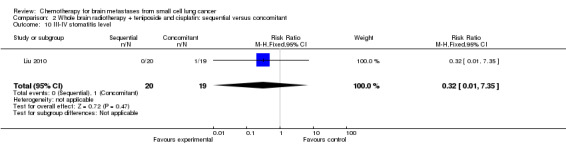
Comparison 2 Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant, Outcome 10 III‐IV stomatitis level.
Discussion
Summary of main results
We have found only one RCT that compared the effectiveness of chemotherapy with no chemotherapy for the treatment of BM due to SCLC, and it included only 96 participants. This study compared topotecan with no chemotherapy ‐ all patients included received WBRT as co‐intervention, without any significant advantage in OS, PFS, and intracranial disease control for concurrent chemoradiotherapy (WBRT + topotecan) when compared to WBRT alone (Neuhaus 2009).
One trial (Postmus 2000) assessed teniposide with or without WBRT. The effectiveness of teniposide with WBRT was comparable to that obtained with the teniposide alone for OS, TTP outside the brain and toxicity. However, TTP in the brain was significantly better in the combined group.
Another trial (Liu 2010) found that both sequential and concomitant chemoradiotherapy had a similar effect on survival and ORR, although patients in the concomitant group had a higher rate of myelosuppression.
Overall completeness and applicability of evidence
The evidence available on the effectiveness and toxicity of systemic chemotherapy for the treatment of patients with BM from SCLC comes from a single study that compared topotecan with no chemotherapy in patients treated with WBRT (Neuhaus 2009). In this study 28 of the 33 patients had recurrent disease, and most of them had also extracerebral progression. Under these circumstances life expectancy is usually less than 12 weeks without treatment, and in platinum‐resistant patients less than 24 weeks. It remains unclear whether the results would be the same in patients not receiving cranial irradiation, in those with genetic abnormalities related to chromosomes 3p and 8q, and in those who were sensitive to platinum. We have found no RCTs comparing the effects of other chemotherapy regimens with no chemotherapy, in patients receiving or not receiving radiotherapy.
For settings in which regular treatment for BM from SCLC involves both chemotherapy and radiotherapy, evidence comes from a single RCT, which compared sequential chemoradiotherapy with concomitant chemoradiotherapy, and included only 39 patients, 22 of them with a single brain metastasis and 24 with also extracerebral metastases (Liu 2010). Although the response rate for the group receiving radiotherapy concomitant with chemotherapy was significantly higher than that for the sequential group, the incidence of III–IV hematologic toxicity, vomiting, and nausea was higher.
In the other RCT the objective was not to assess the effectiveness of chemotherapy but to address the contribution of radiotherapy in 120 patients all treated with chemotherapy (teniposide) (Postmus 2000). The intracranial RR was significantly higher in the combined‐modality arm (57% vs. 22%, P < 0.001); although the TTP in the brain was also significantly longer in the combined‐modality group, no improvement was obtained in OS (Postmus 2000). The median OS was 3.5 months in the combined‐modality arm and 3.2 months in the teniposide‐alone arm (P = 0.087). The majority of patients failed at extracranial sites, affecting any survival advantage that might have been the result of the improved intracranial control with combined WBRT and chemotherapy.
Quality of the evidence
The quality of the evidence available for all comparisons was moderate, mainly because there was only one study for each treatment comparison and main results were imprecise in some cases, as reflected in wide CIs. The Neuhaus 2009 study was closed early because of problems in recruiting patients and the author considered that the number of participants was too low to demonstrate any small advantage for the combined treatment (chemoradiotherapy).
Even though blinding of outcome assessment was not done in two of the RCTs, it is unlikely that this would have biased the outcome measures most relevant for patients, especially survival time. However, other outcomes, such as response rate, could be open to biased assessments. Similarly, the choice of patient made by the recruiter may have been influenced by the lack of allocation concealment.
Given the paucity of robust studies assessing clinical effects of treatments, available evidence is insufficient to judge the effectiveness and safety of chemotherapy for the treatment of BM from SCLC. Published studies are not adequate to address the objectives of this review.
Potential biases in the review process
It is quite unlikely that publication bias could have happened given that all included studies presented results not favorable for the more active interventions. Also the search strategy has been comprehensive, including search in most important clinical trial registers and contact with author of the studies, and data were analyzed and extracted independently by at least two review authors.
All relevant data for the objectives of this review were available from the publications of included studies.
Agreements and disagreements with other studies or reviews
Our review provides evidence derived from three trials using different interventions and duration of treatment. Our results for the main outcome, OS, are similar to the findings from other studies. Mehta 2010 assessed the role of chemotherapy in the management of newly diagnosed BM in a systematic review and evidence‐based clinical practice guidelines. The authors (Mehta 2010) considered that routine use of chemotherapy following WBRT for BM has not been shown to increase survival. In one study, response rate of the group concomitant with chemotherapy was significantly higher than that of the sequential group, however further research is still needed (Liu 2010).
Authors' conclusions
Implications for practice.
There are very few trials assessing the effectiveness and toxicity of systemic chemotherapy for the treatment of patients in whom BM is the only site of progression. The available evidence is insufficient to judge the effectiveness and safety of chemotherapy for the treatment of BM from SCLC. According to our findings, chemotherapy does not improve specific brain PFS and OS in patients with SCLC. The combined treatment of teniposide and WBRT contributed to outcome in terms of increased CR and better TTP (though not OS).
Implications for research.
This systematic review has identified the need for well‐designed, adequately powered RCTs to assess the benefits and harms of chemotherapy for the treatment of BM from SCLC (specifically in patients with only intracranial metastases, including the group treated with prophylactic cranial irradiation). Approximately 60% of SCLC patients will develop BM during the course of the disease (Quan 2004) and overall, the prognosis is poor, with a median OS in the range of 3 to 14 months (van Hazel 1983; Kochhar 1997). Studies frequently exclude SCLC‐related BM, and therefore, advances in the treatment of the disease have been few over the last decades. One of the main reasons why patients with SCLC BM are rarely entered into research protocols is that approximately 60% to 90% of these patients have simultaneous progression at extracranial sites (Glantz 1997).
Many questions remain open. Some important considerations for future research are as follows:
The effects of different chemotherapy agents (including platinum‐based regimens, differences between cisplatin and carboplatin, and combinations including irinotecan);
The effects of chemotherapy agents compared to supportive care;
The effectiveness of WBRT in patients with widespread metastatic disease;
Determining which patients with SCLC BM should be treated with chemotherapy alone.
Acknowledgements
We would like to thank the Cochrane Lung Cancer Group for their assistance. Desiree West (Consumer of the Lung Cancer Group) provided feedback to the Plain Language Summary.
Appendices
Appendix 1. MEDLINE (PubMed, 6 July 2011)
#1 brain[tiab] OR cerebral[tiab] 728755
#2 neoplasm metastasis[mh] 134860
#3 metastas*[tiab] 185221
#4 #1 AND #2 3097
#5 #1 AND #3 10614
#6 #4 OR #5 11820
#7 "Carcinoma, Small Cell"[mh] 15901
#8 SCLC[tiab] 4382
#9 carcinoma*[tiab] OR cancer*[tiab] OR adenocarcinoma*[tiab] OR malignan*[tiab] OR tumor[tiab] OR tumors[tiab] OR tumour*[tiab] OR neoplasm*[tiab] 1859304
#10 small[tiab] AND cell[tiab] 171289
#11 reserve[tiab] AND cell[tiab] 2199
#12 oat[tiab] AND cell[tiab] 1575
#13 #10 OR #11 OR #12 174365
#14 #9 AND #13 75920
#15 lung*[tiab] OR pulmonary[tiab] OR bronchus[tiab] OR brochogenic[tiab] OR bronchial[tiab] OR bronchoalveolar[tiab] OR alveolar[tiab] 717310
#16 #14 AND #15 39524
#17 #7 OR #8 OR #16 46037
#18 #6 AND #17 1432
#19 (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT (humans[mh] AND animals[mh])) 2411998
#20 #18 AND #19 656
Appendix 2. CENTRAL (The Cochrane Library 2011, Issue 6; 6 July 2011)
#1 (brain OR cerebral):ti,ab 15468
#2 MeSH descriptor Neoplasm Metastasis explode all trees 3132
#3 metastas*:ti,ab 4188
#4 (#1 AND #2) 49
#5 (#1 AND #3) 373
#6 (#4 OR #5) 386
#7 MeSH descriptor Carcinoma, Small Cell explode all trees 753
#8 SCLC:ti,ab 551
#9 carcinoma*:ti,ab OR cancer*:ti,ab OR adenocarcinoma*:ti,ab OR malignan*:ti,ab OR tumor:ti,ab OR tumors:ti,ab OR tumour*:ti,ab OR neoplasm*:ti,ab 60011
#10 small:ti,ab AND cell:ti,ab 5891
#11 reserve:ti,ab AND cell:ti,ab 2863
#12 oat:ti,ab AND cell:ti,ab 78
#13 (#10 OR #11 OR #12) 8347
#14 (#9 AND #13) 5333
#15 lung*:ti,ab OR pulmonary:ti,ab OR bronchus:ti,ab OR brochogenic:ti,ab OR bronchial:ti,ab OR bronchoalveolar:ti,ab OR alveolar:ti,ab 33277
#16 (#14 AND #15) 4403
#17 (#7 OR #8 OR #16) 4564
#18 (#6 AND #17) 127 (120 in clinical Trials)
Appendix 3. EMBASE (Ovid, 1980 to 2011 Week 26; 6 July 2011)
1 (brain or cerebral).ti,ab. (796852)
2 exp metastasis/ (285646)
3 metastas*.ti,ab. (207675)
4 1 and 2 (13553)
5 1 and 3 (12516)
6 4 or 5 (15747)
7 exp lung small cell cancer/ (12193)
8 SCLC.ti,ab. (5195)
9 (carcinoma* or cancer* or adenocarcinoma* or malignan* or tumor or tumors or tumour* or neoplasm*).ti,ab. (2029015)
10 (small and cell).ti,ab. (185557)
11 (reserve and cell).ti,ab. (2470)
12 (oat and cell).ti,ab. (1529)
13 10 or 11 or 12 (188771)
14 9 and 13 (87734)
15 (lung* or pulmonary or bronchus or brochogenic or bronchial or bronchoalveolar or alveolar).ti,ab. (778801)
16 14 and 15 (46989)
17 7 or 8 or 16 (50506)
18 6 and 17 (1862)
19 Clinical trial/ (810309)
20 Randomized controlled trials/ (4595)
21 Random Allocation/ (53159)
22 Single‐Blind Method/ (13675)
23 Double‐Blind Method/ (99014)
24 Cross‐Over Studies/ (29973)
25 Placebos/ (180293)
26 Randomi?ed controlled trial$.tw. (61040)
27 RCT.tw. (7053)
28 Random allocation.tw. (1025)
29 Randomly allocated.tw. (15048)
30 Allocated randomly.tw. (1674)
31 (allocated adj2 random).tw. (682)
32 Single blind$.tw. (10777)
33 Double blind$.tw. (115701)
34 ((treble or triple) adj blind$).tw. (237)
35 Placebo$.tw. (155788)
36 Prospective Studies/ (164952)
37 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (1114331)
38 Case study/ (12385)
39 Case report.tw. (201855)
40 Abstract report/ or letter/ (782469)
41 38 or 39 or 40 (992855)
42 37 not 41 (1081476)
43 animal/ not human/ (1233322)
44 42 not 43 (1060563)
45 18 and 44 (443)
Data and analyses
Comparison 1. Teniposide versus teniposide + whole brain radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early death (overall response outside the brain) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.34, 1.48] |
| 2 Clinical response | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.91, 1.62] |
| 3 Complete brain metastases response | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.6 [1.43, 9.07] |
| 4 Partial brain metastases response | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 5 Complete + partial brain metastases response | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.10, 0.47] |
| 6 Improvement on the ECOG performance status after 2 courses | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.74, 1.55] |
| 7 Improved neurologic function score after cycle 2 | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.51] |
| 8 Complete overall response outside the brain | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.91] |
| 9 Nausea and vomiting WHO grade 3‐4 toxicity | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.58] |
| 10 Infection WHO grade 3‐4 toxicity | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.51] |
1.1. Analysis.
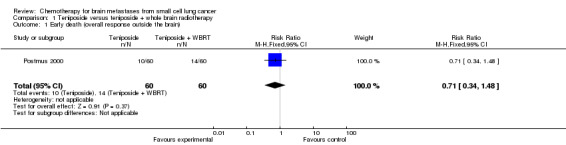
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 1 Early death (overall response outside the brain).
1.2. Analysis.
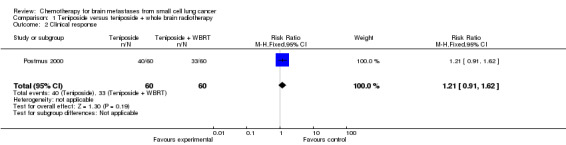
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 2 Clinical response.
1.4. Analysis.
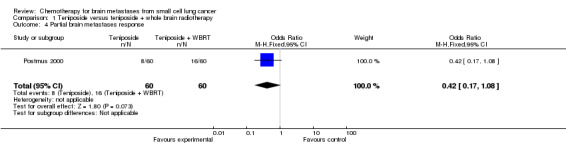
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 4 Partial brain metastases response.
1.5. Analysis.
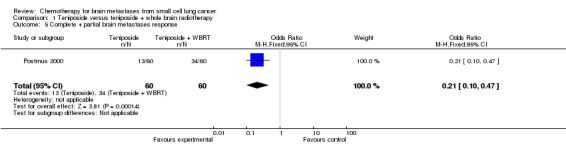
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 5 Complete + partial brain metastases response.
1.6. Analysis.
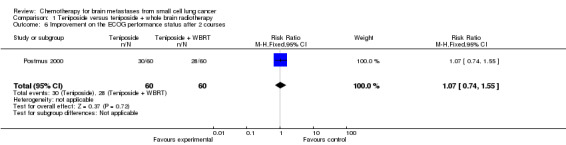
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 6 Improvement on the ECOG performance status after 2 courses.
1.7. Analysis.
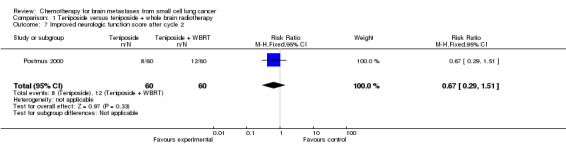
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 7 Improved neurologic function score after cycle 2.
1.8. Analysis.
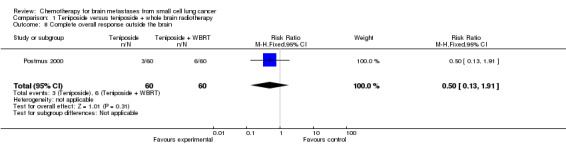
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 8 Complete overall response outside the brain.
1.9. Analysis.
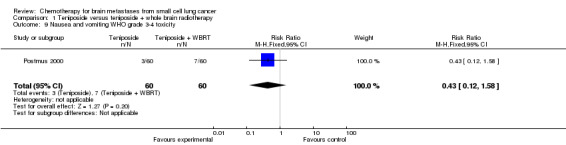
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 9 Nausea and vomiting WHO grade 3‐4 toxicity.
1.10. Analysis.
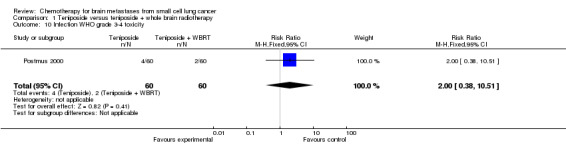
Comparison 1 Teniposide versus teniposide + whole brain radiotherapy, Outcome 10 Infection WHO grade 3‐4 toxicity.
Comparison 2. Whole brain radiotherapy + teniposide and cisplatin: sequential versus concomitant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival at 18 months | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.48, 16.81] |
| 2 Complete brain metastases response | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.45, 2.70] |
| 3 Partial short‐term response rates | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.38, 1.75] |
| 4 Myelosuppression with III–IV leukopenia | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [1.16, 61.10] |
| 5 III‐IV anemia level | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.20] |
| 6 III‐IV thrombocytopenia level | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.84] |
| 7 III‐IV leukopenia level | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.86] |
| 8 Thrombocytopenia | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.17, 0.91] |
| 9 III‐IV nausea and vomiting level | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.84] |
| 10 III‐IV stomatitis level | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Liu 2010.
| Methods | RCT, parallel, open, single center, phase III trial Henan Provincial People’s Hospital, Zhengzhou (China) |
|
| Participants | n = 39 Sex: 26 male, 13 female Mean age: 57 ± 18 years Inclusion criteria:
|
|
| Interventions | Intervention Sequential radiotherapy and chemotherapy group: systemic chemotherapies 2 weeks after WBRT (n = 20) Control Concomitant radiotherapy and chemotherapy group: parallel systemic chemotherapies and WBRT (n = 19) WBRT: 1.8 to 2 Gy/time for 18 to 20 times, and the total dose in 4 weeks was 36 Gy Systemic chemotherapy: teniposide (Vm26) 60 mg/m2, from day 1 to day 3; cisplatin (DDP) 20 mg/m2, from day 1 to day 5. One cycle was defined as a 21‐day therapy duration, with a total of 4 cycles |
|
| Outcomes | Main and secondary outcomes not differentiated:
|
|
| Notes | Publication presents preliminary results Competing interests and information on funding sources not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment. Mentioned as "randomized" but sequence generation process is not explained in a detailed way. Quote: "patients were randomly divided”. Additional information requested to authors but no answer received |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment. Additional information requested to authors but no answer received |
| Blinding of participants and personnel (performance bias) Survival | Low risk | No information was provided. Probably unblinded. Outcomes measures are not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Toxicity and disease related symptoms | Unclear risk | No information was provided. Probably unblinded |
| Blinding of outcome assessment (detection bias) Survival | Low risk | No information was provided. Probably unblinded. Outcomes measures are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) CR, toxicity, quality of life | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) Survival | Low risk | No losses in follow‐up were reported |
| Incomplete outcome data (attrition bias) Toxicity, disease related symptoms, CR | Low risk | No losses in follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | Authors presented results on all outcome measures that were pre‐specified as relevant We searched for protocol information in the ICTRP |
| Other bias | Low risk | The study seems to be free of other sources of bias |
Neuhaus 2009.
| Methods | RCT phase III | |
| Participants | Patients with histologically confirmed lung cancer and intracerebral metastases. Initially only patients with recurrence of lung cancer after first‐line therapy were included in the study. However, due to a slow recruitment, after 1 year an amendment allowing the inclusion of primary diagnosed patients was added. Randomization was performed by considering the parameters SCLC, NSCLC, extracerebral metastases, and a number of BM n = 96; 47 chemoradiotherapy, 49 radiotherapy Sex: 62 male, 34 female Median age: 58/59 years (range: 34 to 75 years) Clinical condition:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Arm A (chemoradiotherapy): topotecan was administered as a 30‐minute infusion with 0.4 mg/m2/day for 5 days over 4 weeks within 2 hours before radiotherapy. WBRT was applied with a fraction size of 2 Gy/day to a total of 40 Gy. Arm B (radiotherapy): WBRT was applied with a fraction size of 2 Gy/day to a total of 40 Gy. Continuation therapy: subsequently, patients with extracerebral cancer lesions from both arms had the option to receive 3 additional cycles of topotecan chemotherapy (1.25 mg/m2/day, days 1 to 5, 4 cycles of 21 days), starting on day 15 after the end of WBRT. Where a patient had not received any kind of chemotherapy or chemoradiotherapy before entering the study, the institutionally preferred chemotherapeutic regimen was allowed to be used instead. Continuation therapy was stopped after 3 cycles or when tumor progression of the extracerebral metastases occurred |
|
| Outcomes | Primary end point:
Secondary end points:
A complete response was defined as a complete disappearance of all evidence of disease in the brain. A partial response was defined as radiologic response > 50% in all BM. Responses in tumor lesions < 50% or increase in size < 25% was defined as stable disease. A progressive disease was defined as either the occurrence of new lesions or an increase in size of > 25% |
|
| Notes | Numerical results for patients with SCLC were not presented in a disaggregated way, but authors reported in a narrative way that in relation with OS and PFS differences between compared groups were not significant either for SCLC and NSCLC Initially only patients with recurrence of lung cancer after first‐line therapy could be included in the study. However, due to a slow recruitment, after 1 year an amendment allowing the inclusion of primary diagnosed patients was added An interim analysis was planned after the death of 150 patients. However, until 6 August 2004, that is, after a study duration of 34 months, only 95 patients in 11 centers had been recruited, and so the interim analysis was performed at that time point. This analysis did not show any benefit of chemoradiotherapy with regard to OS and thus, on the basis of the slow recruitment and the result of the interim analysis, a continuation of the study did no longer appear reasonable. The results described here represent the final analysis, in which 96 patients were included. Study supported by GlaxoSmithKline Competing interests not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment. Mentioned as "randomized" but sequence generation process is not explained in a detailed way. Probably centralized. Quote: "Randomisation was performed by considering the parameters SCLC, NSCLC, extracerebral metastases and a number of brain metastases". Additional information requested from authors but no answer received |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment. Additional information requested from authors but no answer received |
| Blinding of participants and personnel (performance bias) Survival | Low risk | Stated as "open", but main outcome measure (OS) is it not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Toxicity and disease related symptoms | Unclear risk | Stated as "open" |
| Blinding of outcome assessment (detection bias) Survival | Low risk | Stated as "open", but main outcome measure (OS) is it not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) CR, toxicity, quality of life | Unclear risk | Stated as "open" |
| Incomplete outcome data (attrition bias) Survival | Low risk | Data was not presented for the SCLC subgroup No losses in follow‐up reported. Causes for protocol deviations well reported. Quote: "The reasons for protocol deviations are mainly early deaths, haematological toxicities, dosage failure, worsening of general condition and tumour progression. In detail, in arm A the chemotherapy was delayed or reduced in nine patients because of neutropenia, and in six of them G‐CSF was given at least once. Although no patient stopped topotecan because of neutropenia, one patient left the study because of prolonged thrombocytopenia." Quote: "The treatment was stopped as per the patients' wish, by the decision of the physician, tumour progression, severe side effects according to the NCIC CTCG guidelines or non‐compliance of the patient." |
| Incomplete outcome data (attrition bias) Toxicity, disease related symptoms, CR | Low risk | Data were not presented for the SCLC subgroup No losses in follow‐up reported. Causes for protocol deviations well reported Quote: "The reasons for protocol deviations are mainly early deaths, haematological toxicities, dosage failure, worsening of general condition and tumour progression. In detail, in arm A the chemotherapy was delayed or reduced in nine patients because of neutropenia, and in six of them G‐CSF was given at least once. Although no patient stopped topotecan because of neutropenia, one patient left the study because of prolonged thrombocytopenia." Quote: "The treatment was stopped as per the patients' wish, by the decision of the physician, tumour progression, severe side effects according to the NCIC CTCG guidelines or non‐compliance of the patient"] |
| Selective reporting (reporting bias) | Low risk | Authors presented results on all outcome measures that were pre‐specified as relevant Search for protocol in clinical trials registers |
| Other bias | Low risk | Early termination of the study, but according to previously established criteria Quote: "The whole study was to be stopped in case new therapeutic regimens with superior benefit of either therapy arm were published, if the interim analyses showed that the criteria for stopping the study by using the methods of Pocock (Pocock 1978) and O'Brien and Fleming (O'Brien 1979) were reached and when the number of patients recruited was clearly below the expected value." Quote: "until August 6, 2004, that is, after a study duration of 34 months, only 95 patients in 11 centers had been recruited, and so the interim analysis was performed at that time point. This analysis did not show any benefit of chemoradiotherapy with regard to OS and thus, on the basis of the slow recruitment and the result of the interim analysis, a continuation of the study did no longer appear reasonable. The results described here represent the final analysis, in which 96 patients were included." |
Postmus 2000.
| Methods | RCT, parallel, open, multicentric, phase III 11 centers in Europe (EORTC) |
|
| Participants | N = 120; 60 teniposide, 60 teniposide + WBRT Sex: 95 male, 25 female Median age: 60/61 years (range: 38 to 75 years) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention
Control
Teniposide 120 mg/m2 intravenous infusion on days 1, 3, and 5 every 3 weeks. Patients underwent treatment until they had received the maximum number of courses (n = 12) or until the disease had progressed inside or outside the brain. If the WBC count was ≤ 3000 /mL or the platelet count was ≤ 100.000/mL on the day of scheduled retreatment, treatment was delayed. Counts were measured weekly, and treatment was given at full doses when the WBC and platelet counts returned to ≥ 3000 /mL and ≥100.000/mL, respectively. If recovery was still incomplete after 2 weeks, the patient went off study. In the event of WHO grade 4 leukocytopenia, thrombocytopenia, or both during 2 subsequent courses, a 25% dose reduction for subsequent courses was advised WBRT consisted of 30 Gy (midplane dose) in 10 fractions in 2 weeks with parallel opposing fields. Both fields were treated each day. WBRT had to be started within 3 weeks after the start of teniposide and continued during administration of teniposide. All cranial meningeal surfaces, including the anterior, middle, and posterior cranial fossae, were included with a minimum 1‐cm margin. Treatment was given with megavoltage equipment with a minimum source‐to‐skin distance or target‐to‐skin distance of 80 cm. Corticosteroids (dexamethasone 2 mg, 4 times) were given during irradiation and tapered off as soon as possible after WBRT |
|
| Outcomes | Primary end point:
Secondary end points:
|
|
| Notes | Analysis was performed on all eligible patients according to the intent‐to‐treat principle. The analysis of toxicity was based on the treatment patients actually received Competing interests and information on funding sources not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done using minimization techniques with patients stratified according to their institution, number of BM (> 2), and prior chemotherapy (naive/pretreated) |
| Allocation concealment (selection bias) | Unclear risk | No further description in the manuscript. Probably central allocation. Additional information requested to authors but no answer received |
| Blinding of participants and personnel (performance bias) Survival | Low risk | No information was provided, but main outcome measure (duration of survival) is it not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Toxicity and disease related symptoms | Unclear risk | No information was provided |
| Blinding of outcome assessment (detection bias) Survival | Low risk | No information provided, but main outcome measure (duration of survival) is it not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) CR, toxicity, quality of life | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) Survival | High risk | Only 1 participant in the teniposide + WBRT was lost to follow‐up. However, only 6 patients completed all 12 courses of protocol therapy in the combined group compared with 0 in the teniposide group. Reasons for stopping protocol therapy were reported in both groups. Tumor progression was the principal cause for stopping protocol therapy |
| Incomplete outcome data (attrition bias) Toxicity, disease related symptoms, CR | High risk | Only 1 participant in the teniposide + WBRT was lost to follow‐up. However, only 6 patients completed all 12 courses of protocol therapy in the combined group compared with 0 in the teniposide group. Reasons for stopping protocol therapy were reported in both groups. Tumor progression was the principal cause for stopping protocol therapy |
| Selective reporting (reporting bias) | Low risk | Authors presented results on all outcome measures that were prespecified as relevant |
| Other bias | Low risk | The study seems to be free of other sources of bias |
BM: brain metastases; CR: complete remission; CT: computer tomography; ECG: electrocardiogram; ECOG: Eastern Cooperative Oncology Group; ECT: emission computer tomography; G‐CSF: granulocyte colony‐stimulating factor; EORTC: European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative Group; ICTRP: International Clinical Trials Registry Platform; MRI: magnetic resonance imaging; NC: no change; NSCLC: non‐small cell lung cancer; OS: overall survival; PD: progressive disease; PFS: progression‐free survival; PR: partial remission; QoL: quality of life; RCT: randomized controlled trial; RECIST: Response Evaluation Criteria In Solid Tumors; SCLC: small cell lung cancer; TTO: time to progression; WBRT: whole brain radiotherapy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antonadou 2002 | The study did not include disaggregated data on results for small cells lung cancer patients |
| Fietkau 2001 | Not a randomized study |
| Hanna 2002 | The study did not include disaggregated data on results for small cells lung cancer patients |
| Loehrer 1995 | The study did not include disaggregated data on results for small cells lung cancer patients |
| Malacarne 1996 | Not a randomized study |
| Mehta 2003 | Small cells lung cancer patients excluded |
| Meyers 2004 | The study did not include disaggregated data on results for small cells lung cancer patients. Maybe included in "other cancers" category |
| Pandya 2009 | The study did not include disaggregated data on results for patients with BM |
| Postmus 1992 | Non‐randomized study |
| Postmus 1995 | Non‐randomized trial |
| Schiller 2001 | The study did not include disaggregated data on results for patients with BM |
| Suh 2006 | The study did not include disaggregated data on results for small cells lung cancer patients. Maybe included in "other cancers" category |
| Thomas 1990 | Not a randomized study |
| Yue 2004 | Non‐randomized trial |
Differences between protocol and review
Cuello M, Viteri S, and Carrasco E were review authors in the protocol but did not participate in the review.
Contributions of authors
Ludovic Reveiz conceived the review question, developed and coordinated the protocol and the review, completed the first draft of the review, assessed the studies and extracted and analyzed data, approved the final version prior to submission, and is guarantor for the review. Ludovic Reveiz has contributed to this systematic review in a personal capacity and during his spare time. Most of the work was performed before joining the Pan American Health Organization. The Pan American Health Organization does not assume responsibility for the statements contained therein.
José‐Ramón Rueda developed the review, completed the first draft of the review, assessed the studies and extracted and analyzed data, and approved the final version prior to submission. He performed part of the writing/editing of the review.
Andres Felipe Cardona conceived the review question, developed the protocol and the review, completed the first draft of the review, and approved the final version prior to submission.
Sources of support
Internal sources
University of the Basque Country, Spain.
Cochrane Lung Cancer Group, Spain.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Liu 2010 {published data only}
- Liu M, Zhou Y, Han Q, Gao T, Luo Z, Wang W. Whole brain radiotherapy concomitant or sequential Vm26/DDP in treating small cell lung cancer patients with brain metastases. Chinese‐German Journal of Clinical Oncology 2010;9(1):17‐21. [Google Scholar]
Neuhaus 2009 {published data only}
- Neuhaus T, Ko Y, Muller RP, Grabenbauer GG, Hedde JP, Schueller H, et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS‐metastases due to lung cancer. British Journal of Cancer 2009;100(2):291‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Postmus 2000 {published data only}
- Postmus PE, Haaxma‐Reiche H, Smit EF, Groen HJ, Karnicka H, Lewinski T, et al. Treatment of brain metastases of small‐cell lung cancer: comparing teniposide and teniposide with whole‐brain radiotherapy ‐ a phase III study of the European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative Group. Journal of Clinical Oncology 2000;18(19):3400‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Antonadou 2002 {published data only}
- Antonadou D, Paraskevaidis M, Sarris G, Coliarakis N, Economou I, Karageorgis P, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. Journal of Clinical Oncology 2002;20(17):3644‐50. [DOI] [PubMed] [Google Scholar]
Fietkau 2001 {published data only}
- Fietkau R. Controversial treatment of brain metastases of small‐cell lung cancer. Randomized comparison of chemotherapy alone and radiochemotherapy. Strahlentherapie und Onkologie 2001;177(6):314‐6. [PubMed] [Google Scholar]
Hanna 2002 {published data only}
- Hanna NH, Sandier AB, Loehrer PJ Sr, Ansari R, Jung SH, Lane K, et al. Maintenance daily oral etoposide versus no further therapy following induction chemotherapy with etoposide plus ifosfamide plus cisplatin in extensive small‐cell lung cancer: a Hoosier Oncology Group randomized study. Annals of Oncology 2002;13(1):95‐102. [DOI] [PubMed] [Google Scholar]
Loehrer 1995 {published data only}
- Loehrer PJ Sr, Ansari R, Gonin R, Monaco F, Fisher W, Sandler A, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small‐ cell lung cancer: a Hoosier oncology group study . Journal of Clinical Oncology 1995;13(10):2594‐9. [DOI] [PubMed] [Google Scholar]
Malacarne 1996 {published data only}
- Malacarne P, Santini A, Maestri A. Response of brain metastases from lung cancer to systemic chemotherapy with carboplatin and etoposide. Oncology 1996;53(3):210‐3. [DOI] [PubMed] [Google Scholar]
Mehta 2003 {published data only}
- Mehta MP, Rodrigus P, Terhaard CH, Rao A, Suh J, Roa W, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole‐brain radiation therapy in brain metastases. Journal of Clinical Oncology 2003;21(13):2529‐36. [DOI] [PubMed] [Google Scholar]
Meyers 2004 {published data only}
- Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole‐brain radiation and motexafin gadolinium: results of a randomized phase III trial. Journal of Clinical Oncology 2004;22(1):157‐65. [DOI] [PubMed] [Google Scholar]
Pandya 2009 {published data only}
- Pandya KJ, Dahlberg S, Hidalgo M, Cohen RB, Lee MW, Schiller JH, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI‐779) in patients with extensive‐stage small‐cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500). Journal of Thoracic Oncology 2007;2(11):1036‐41. [DOI] [PubMed] [Google Scholar]
Postmus 1992 {published data only}
- Postmus PE, Smit EF, Berendsen HH, Sleijfer DT, Haaxma‐Reiche H. Treatment of brain metastases of small cell lung cancer with teniposide. Seminars in Oncology 1992;19(2 Suppl 6):89‐94. [PubMed] [Google Scholar]
Postmus 1995 {published data only}
- Postmus PE, Smit EF, Haaxma‐Reiche H, Zandwijk N, Ardizzoni A, Quoix E, et al. Teniposide for brain metastases of small‐cell lung cancer: a phase II study. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. Journal of Clinical Oncology 1995;13(3):660‐5. [DOI] [PubMed] [Google Scholar]
Schiller 2001 {published data only}
- Schiller JH, Adak S, Cella D, DeVore RF 3rd, Johnson DH. Topotecan versus observation after cisplatin plus etoposide in extensive‐stage small‐cell lung cancer: E7593 ‐ a phase III trial of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2001;19(8):2114‐22. [DOI] [PubMed] [Google Scholar]
Suh 2006 {published data only}
- Suh JH, Stea B, Nabid A, Kresl JJ, Fortin A, Mercier JP, et al. Phase III study of efaproxiral as an adjunct to whole‐brain radiation therapy for brain metastases. Journal of Clinical Oncology 2006;24(1):106‐14. [DOI] [PubMed] [Google Scholar]
Thomas 1990 {published data only}
- Thomas P, Herkert A, Soyez F, Kleisbauer JP. Chemotherapy of cerebral metastasis of lung cancer. Revue de Pneumologie Clinique 1990;46(1):5‐9. [PubMed] [Google Scholar]
Yue 2004 {published data only}
- Yue X, Zang QC. Sequential treatment of VmP regimen and whole brain radiotherapy for small cell lung cancer patients with brain metastases. Ai Zheng 2004;23(12):1671‐6. [PubMed] [Google Scholar]
Additional references
Glantz 1997
- Glantz MJ, Choy H, Yee L. Prophylactic cranial irradiation in small cell lung cancer: Rationale, results, and recommendations. Seminar Oncology 1997;24:477‐83. [PubMed] [Google Scholar]
Golder 2005
- Golder S, McIntosh HM, Duffy S, Glanville J. Centre for Reviews and Dissemination and UK Cochrane Centre Search Filters Design Group. Developing efficient search strategies to identify reports of adverse effects in MEDLINE and EMBASE. Health Information and Libraries Journal 2006;23(1):3‐12. [DOI] [PubMed] [Google Scholar]
Gril 2010
- Gril B, Evans L, Palmieri D, Steeg PS. Translational research in brain metastasis is identifying molecular pathways that may lead to the development of new therapeutic strategies. European Journal of Cancer 2010;46(7):1204‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grossi 2001
- Grossi F, Scolaro T, Tixi L, Loprevite M, Ardizzoni A. The role of systemic chemotherapy in the treatment of brain metastases from small‐cell lung cancer. Critical Reviews in Oncology/Hematology 2001;37(1):61‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kochhar 1997
- Kochhar R, Frytak S, Shaw EG. Survival of patients with extensive small‐cell lung cancer who have only brain metastases at initial diagnosis. American Journal Clinical Oncology 1997;20:125‐7. [DOI] [PubMed] [Google Scholar]
Kristensen 1992
- Kristensen CA, Kristjansen PE, Hansen HH. Systemic chemotherapy of brain metastases from small‐cell lung cancer: a review. Journal of Clinical Oncology 1992;10(9):1498‐502. [DOI] [PubMed] [Google Scholar]
Lorger 2010
- Lorger M, Felding‐Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. The American Journal of Pathology 2010;176(6):2958‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macdonald 1990
- Macdonald DR, Gaspar LE, Cairncross JG. Successful chemotherapy for newly diagnosed aggressive oligodendroglioma. Annals of Neurology 1990;27(5):573‐4. [DOI] [PubMed] [Google Scholar]
Manríquez 2008
- Manríquez JJ. A highly sensitive search strategy for clinical trials in Literatura Latino Americana e do Caribe em Ciências da Saúde (LILACS) was developed. Journal of Clinical Epidemiology 2008;61(4):407‐11. [DOI] [PubMed] [Google Scholar]
Mehta 2010
- Mehta MP, Paleologos NA, Mikkelsen T, Robinson PD, Ammirati M, Andrews DW, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence‐based clinical practice guideline. Journal of Neuro‐oncology 2010;96(1):71‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathoo 2005
- Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. Journal of Clinical Pathology 2005;58(3):237‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nayak 2011
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Current Oncology Reports 2012;14(1):48‐54. [DOI] [PubMed] [Google Scholar]
Nieder 2006
- Nieder C, Grosu AL, Astner S, Thamm R, Molls M. Integration of chemotherapy into current treatment strategies for brain metastases from solid tumors. Radiation Oncology 2006;27(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nussbaum 1996
- Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996;78(8):1781‐8. [PubMed] [Google Scholar]
O'Brien 1979
- O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35(3):549‐56. [PubMed] [Google Scholar]
Palmieri 2007
- Palmieri D, Chambers AF, Felding‐Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clinical Cancer Research 2007;15(13):1656‐62. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistic in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pocock 1978
- Pocock SJ. Size of cancer clinical trials and stopping rules. British Journal of Cancer 1978;38(6):757‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Postmus 1999
- Postmus PE, Smit EF. Chemotherapy for brain metastases of lung cancer: a review. Annals of Oncology 1999;10(7):753‐9. [DOI] [PubMed] [Google Scholar]
Quan 2004
- Quan AL, Videtic GM, Suh JH. Brain metastases in small cell lung cancer. Oncology (Williston Park) 2004;18(8):961‐72. [PubMed] [Google Scholar]
Schuette 2004
- Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 2004;45(Suppl 2):S253‐7. [DOI] [PubMed] [Google Scholar]
Seute 2004
- Seute T, Leffers P, Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer 2004;100(4):801‐6. [DOI] [PubMed] [Google Scholar]
Seute 2005
- Seute T, Leffers P, Velde GP, Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer 2005;104(8):1700‐5. [DOI] [PubMed] [Google Scholar]
Seute 2006
- Seute T, Leffers P, Wilmink JT, Velde GP, Twijnstra A. Response of asymptomatic brain metastases from small‐cell lung cancer to systemic first‐line chemotherapy. Journal of Clinical Oncology 2006;24(13):2079‐83. [DOI] [PubMed] [Google Scholar]
Siegers 1990
- Siegers HP. Chemotherapy for brain metastases: recent developments and clinical considerations. Cancer Treatment Reviews 1990;17(1):63‐76. [DOI] [PubMed] [Google Scholar]
Soffietti 2005
- Soffietti R, Costanza A, Laguzzi E, Nobile M, Rudà R. Radiotherapy and chemotherapy of brain metastases. Journal of Neuro‐oncology 2005;75(1):31‐42. [DOI] [PubMed] [Google Scholar]
Stewart 1984
- Stewart DJ, Richard MT, Hugenholtz H, Dennery JM, Belanger R, Gerin‐Lajoie J, et al. Penetration of VP‐16 (etoposide) into human intracerebral and extracerebral tumors. Journal of Neuro‐oncology 1984;2(2):133‐9. [DOI] [PubMed] [Google Scholar]
Stewart 1993
- Stewart DJ, Feun LG, Maor M, Leavens M, Burgess MA, Benjamin RS, et al. Weekly cisplatin during cranial irradiation for malignant melanoma metastatic to brain. Journal of Neuro‐oncology 1983;1(1):49‐51. [DOI] [PubMed] [Google Scholar]
Stewart 1994
- Stewart DJ. A critique of the role of the blood‐brain barrier in the chemotherapy of human brain tumors. Journal of Neuro‐oncology 1994;20(2):121‐39. [DOI] [PubMed] [Google Scholar]
Van den Bent 2003
- Bent MJ. The role of chemotherapy in brain metastases. European Journal of Cancer 2003;39(15):2114‐20. [DOI] [PubMed] [Google Scholar]
van Hazel 1983
- Hazel GA, Scott M, Eagan RT. The effect of CNS metastases on the survival of patients with small cell cancer of the lung. Cancer 1983;51:933‐937. [DOI] [PubMed] [Google Scholar]


